Kindling-induced alterations in GABAA receptor-mediated inhibition and neurosteroid activity in the rat piriform cortex
Abstract
The piriform cortex makes strong interconnections with limbic structures (amygdala, entorhinal cortex and hippocampus) that are involved in memory processing. These connections have also been implicated in the development of temporal lobe epilepsy. However, little is known about how neurones in this region may change during seizure genesis. Here we tested the hypothesis that in the kindling model of temporal lobe epilepsy GABAA receptor-mediated inhibition is altered in the piriform cortex. To do this we performed whole-cell patch-clamp recordings in piriform cortex brain slices obtained from non-kindled and amygdala-kindled adult rats. We found that kindling coincided with an increase in the amplitude and duration of miniature inhibitory post-synaptic currents (mIPSCs) recorded from non-pyramidal neurones, whereas the mIPSCs occurring on pyramidal (excitatory) cells did not change. Non-stationary noise analysis of mIPSCs occurring on the non-pyramidal neurones showed that inferred unitary conductance of synaptic channels were the same before and after kindling, implying that the channel number increased significantly. Immunocytochemical analysis of the inhibitory innervation showed that it was also unaltered by seizure induction. We also found that the effect of the positive modulator tetrahydrodeoxycorticosterone was reduced on the pyramidal neurones after kindling. In contrast, the potentiating effects of tetrahydrodeoxycorticosterone on non-pyramidal cells were about the same after kindling as in control (sham) rats. These data indicate that amygdala kindling causes a shift in the inhibition ‘balance’ between the pyramidal and non-pyramidal cells, perhaps leading to the disinhibition of pyramidal cells.
Introduction
Epilepsy is one of the most common neurological conditions in humans, with a prevalence of about 0.6% and a high incidence in young children and the elderly (Sundqvist, 2002). The epileptic seizure is by definition a paroxysmal and excessive electrical neuronal discharge in the brain that results from too much excitation or too little inhibition in the area in which the abnormal discharge starts (McIntyre et al., 2002b), and subsequently propagates throughout the brain. Although most areas of the brain can have seizure foci, the limbic region of the brain that includes the hippocampus, entorhinal, piriform and perirhinal cortices and their underlying structure (e.g. endopiriform nucleus) was recognized early on as being highly seizurogenic (for review see McIntyre & Poulter, 2001). These areas (particularly the hippocampus) have been scrutinized by researchers for over 30 years as being central to partial complex seizure generation. Because of its intrinsic properties (relatively simple neuronal layer structure, vast connection to other limbic structures, broad responses/afterdischarges as a result of limbic kindling and early presence of interictal discharges) the piriform cortex represents an important candidate for studying and understanding the mechanisms of kindled induced epilepsy (for review see Loscher & Ebert, 1996).
Different animal models of epilepsy reflect certain kinds of epilepsy, albeit with varying accuracy, and no model seems to entirely recapitulate all aspects of seizures seen in humans. Kindling, the model used here, is a widely accepted model as it produces complex partial seizures that secondarily generalize, as in temporal lobe epilepsy. Kindling represents the propagation of the epileptic discharge to other sites and the possible recruitment of those sites into the discharge, leading to enhanced sensitivity to the focal electrical stimulation.
GABA acting at GABAA receptors is the principal inhibitory neurotransmitter that mediates fast inhibition. GABAA receptors are constructed from a repertoire of at least 19 subunit types: α1−6, β1−3, γ1−3, δ, ε, π, θ and ρ1−3 (Wafford, 2005). The reorganization of GABAA receptor subunit expression due to seizures or epilepsy has been investigated in several laboratories and alterations in expression have been implicated in the generation of seizures (Titulaer et al., 1995; Brooks-Kayal et al., 1998, 1999; Nusser et al., 1998; Sperk et al., 1998; Fritschy et al., 1999; Loup et al., 2000; Maguire et al., 2005). Two studies have shown that inhibitory transmission is increased in the hippocampus on excitatory neurones (Nusser et al., 1998; Cohen et al., 2003). At present there is no study that has investigated the hypothesis that kindling may differentially alter the inhibitory transmission on pyramidal vs. non-pyramidal cortical neurones. To test this hypothesis we performed recordings from the piriform cortex, an area that is highly epileptogenic (de Guzman et al., 2004), before and after amygdala kindling. As tetrahydrodeoxycorticosterone (THDOC) is a potent positive allosteric modulator of GABAA receptors with anticonvulsive and anxiolytic properties (Lambert et al., 1995; Reddy, 2003) we also investigated the hypothesis that the efficacy of THDOC may also change, as changes in the action of this naturally occurring compound may mitigate (promote or ameliorate) seizurogenesis (Leroy et al., 2004).
Materials and methods
All experiments were conducted in accordance with the guidelines of the Canadian Council on Animal Care and protocols approved by Carleton University Animal Care Committee.
Animals and surgery
Male Sprague Dawley rats weighing 200 g at the time of the initial surgery were used. They were housed individually with free access to food and water under a continuous 12-h/12-h light/dark cycle.
Electrode implantation
Animals were anaesthetized with pentobarbital (60 mg/kg, i.p.) and implanted with two bipolar stimulating/recording electrodes bilaterally in the basolateral amygdala with the following coordinates: 2.6 mm posterior to Bregma, 4.5 mm lateral to midline and 8.0 mm ventral (Paxinos & Watson, 1986). The electrodes were constructed of two twisted strands of 0.127-mm diameter Diamel-insulated Nichrome wire and were attached to male Amphenol pins. The electrodes were implanted and secured to the skull with jeweller's screws. The electrode assembly was fixed to the skull by dental acrylic cement (McIntyre & Molino, 1972).
Kindling and slice preparation
The rats were allocated to two groups. A control group (sham) of rats was implanted but never kindled and an experimental group was kindled 1 week after the surgery. The afterdischarge threshold (ADT) was determined in each amygdala by delivering a 2-s 60-Hz sine wave stimulus of progressively increasing intensity (15, 25, 35, 50, 75, 100, 150, 200, 250, 300 and 350 µA) until an ADT was triggered (McIntyre & Plant, 1993). The rats were stimulated daily until six generalized stage 5 convulsions were elicited. Seizure severity and duration were recorded daily during the kindling acquisition. At 5 days after the last stage 5 seizure the ADT was determined again (post-kindling ADT) using the same protocol as for pre-kindling ADT.
Brain slices were isolated from the sham rats or from those which had been fully kindled and allowed to recover for a minimum of 2 weeks after the last seizure. Rats were deeply anaesthetized with sodium pentobarbital (60 mg/kg, i.p.) and then perfused through the heart with an ice-cold Ringer's solution in which sodium was replaced by choline [containing (in mm): choline Cl, 110; KCl, 2.5; NaH2PO4, 1.2; NaHCO3, 25; CaCl2, 0.5; MgCl2, 7; Na pyruvate, 2.4; ascorbate, 1.3; dextrose, 20] (as described in McIntyre et al., 2002b).
After perfusion, the brain was rapidly removed and the temporal lobe area was excised as a block. The block was sliced coronally with a Vibratome (300-µm-thick sections). The slices were obtained from 1.5 to −0.3 mm relative to bregma. The slices were incubated at 37 °C for 30 min and subsequently moved to a room-temperature (22 °C) bath for at least 45 min. Slicing, incubation and storage were all performed in the choline solution. The Ringer's solution used during electrical recordings was similar to the choline solution except that pyruvate and ascorbate were removed, equimolar NaCl replaced the choline Cl and MgCl2 was used at a concentration of 2 mm. In order to slow the miniature inhibitory post-synaptic current (mIPSC) frequency, CaCl2 was present at 0.5 mm. All solutions were maintained at pH 7.4 and bubbled with 5% CO2/95% O2 (carbogen).
Electrophysiology
Patch electrodes were pulled from borosilicate glass capillaries and filled with KCl solution having a composition (in mm) of: KCl, 145; NaCl, 10; CaCl2, 2; EGTA, 10; MgATP, 2; dextrose, 10 and HEPES, 10 (300–320 mOsm, pH 7.3–7.4). The resistance of these electrodes was 3–8 MΩ.
Recordings from neurones in layers II and III of the anterior piriform cortex were made with an EPC-9/2 amplifier (HEKA, Lambrecht, Germany). In the kindled rats recordings were made in the piriform cortex of both the ipsilateral (IPS) and contralateral (CLS) hemisphere relative to the amygdala which was kindled. Series resistance compensation was performed in all recordings. The initial access was < 20 MΩ and compensated by 50–70%. All experiments reported here were performed at 32 °C.
Individual neurones were visually identified by using differential phase contrast optics (Dodt; Zeiss, Jena, Germany) on a modified Zeiss Axioscope upright microscope. Voltage-gated currents and the excitability of the cell were monitored by means of voltage-clamp and current-clamp protocols. Spontaneously occurring mIPSCs were recorded after blocking of Na+, N-methyl-d-aspartate, α-amino-3-hydroxy-5-methylisoxazole-4-propionate and kainate channels with a low Ca2+ Ringer's solution (described above) containing 250 nm tetrodotoxin (Sigma-Aldrich, Oakville, Ontario, Canada), 10 µm dinitroquinoxaline-2,3-dione (Research Biochemicals, Natick, MA, USA) and 20 µm 2-amino phosphonovaleric acid (Research Biochemicals). The neurosteroid THDOC (Sigma-Aldrich) was dissolved in 100% ethanol at a concentration of 10 mm. The final concentration in Ringer's solution was performed on the day of the experiment. THDOC data were collected after a minimum of 10–20 min after the perfusion started (this is twice the length of time required for the blocking Ringer's solution to suppress the sodium current).
Analysis of miniature inhibitory post-synaptic currents
In order to evaluate the overall effect of kindling and/or drug treatment on the summed inhibition, all mIPSCs having the attributes described below were averaged. These averaged events are denoted by the term mIPSCav and are almost invariably biphasic. We also calculated the total charge transfer from mIPSCav (McIntyre et al., 2002a). The attributes of these events (amplitude and time constants) were analysed as appropriate by a paired and unpaired t-test or by one-way anova and displayed as ‘combined’ in Tables 1–3.
| Group | ||||||
|---|---|---|---|---|---|---|
| Non-kindled | Contralateral | Ipsilateral | ||||
| Cell type | Cell type | Cell type | ||||
| Pyramidal(n = 20) | Non-pyramidal(n = 14) | Pyramidal(n = 12) | Non-pyramidal(n = 19) | Pyramidal(n = 8) | Non-pyramidal (n = 28) | |
| Monoexponential mIPSCs | ||||||
| τ1 (ms) | 7.3 ± 0.4 | 6.5 ± 0.2 | 7.3 ± 0.2 | 7.5 ± 0.3 | 8.0 ± 0.4 | 8.4 ± 0.1** |
| Current (pA) | 50.8 ± 2.0 | 44.9 ± 2.1 | 52.8 ± 1.9 | 53.5 ± 2.8* | 50.3 ± 1.7 | 62.2 ± 2.4** |
| Biexponential mIPSCs | ||||||
| τ1 (ms) | 2.8 ± 0.1 | 2.5 ± 0.1 | 2.2 ± 1.3 | 2.4 ± 0.2 | 2.3 ± 0.3 | 2.2 ± 0.1 |
| τ2 (ms) | 20.2 ± 1.5 | 17.8 ± 1.2 | 17.7 ± 0.7 | 17.7 ± 0.6 | 19.4 ± 0.8 | 18.4 ± 0.4 |
| Current (pA) | 58.8 ± 2.0 | 53.7 ± 2.4 | 61.6 ± 2.7 | 60.4 ± 2.9* | 57.5 ± 2.1 | 71.3 ± 2.6** |
| Combined, averaged mIPSCs | ||||||
| τ1 (ms) | 2.4 ± 0.1 | 2.2 ± 0.1 | 2.4 ± 0.1 | 2.5 ± 0.1 | 2.5 ± 0.2 | 2.5 ± 0.1 |
| τ2 (ms) | 13.2 ± 0.6 | 14.3 ± 1.1 | 15.0 ± 0.9 | 17.2 ± 1.1 | 15.7 ± 1.3 | 20.0 ± 1.0* |
| Current (pA) | 52.2 ± 1.6 | 50.9 ± 1.7 | 53.8 ± 0.9 | 56.8 ± 1.9* | 52.3 ± 2.1 | 63.2 ± 1.9** |
- * P < 0.05 and
- ** P < 0.001, compared with NK sham. mIPSC, miniature inhibitory post-synaptic current.
| mIPSC type | ||||||||
|---|---|---|---|---|---|---|---|---|
| Monoexponential | Biexponential | Combined | ||||||
| mIPSC attributes | mIPSC attributes | mIPSC attributes | ||||||
| τ1 (ms) | Current (pA) | τ1 (ms) | τ2 (ms) | Current (pA) | τ1 (ms) | τ2 (ms) | Current (pA) | |
| NK (n = 11) | 9.1 ± 0.3** | 41.6 ± 1.9* | 2.7 ± 0.1 | 25.7 ± 0.9** | 49.9 ± 2.0* | 2.6 ± 0.1 | 20.7 ± 2.1* | 47.8 ± 3.2 |
| CLS (n = 9) | 9.5 ± 0.3* | 46.0 ± 2.8* | 2.9 ± 0.1 | 26.2 ± 1.0** | 53.5 ± 2.7* | 3.0 ± 0.2 | 25.2 ± 1.5** | 49.4 ± 1.8 |
| IPS (n = 16) | 9.8 ± 0.4* | 55.7 ± 2.4* | 2.4 ± 0.1 | 26.1 ± 1.2** | 65.9 ± 3.4* | 2.7 ± 0.1* | 26.5 ± 2.4** | 56.3 ± 2.4* |
- * P < 0.05 and
- ** P < 0.01, compared with matched control. mIPSC, miniature inhibitory post-synaptic current.
| mIPSC type | ||||||||
|---|---|---|---|---|---|---|---|---|
| Monoexponential | Biexponential | Combined | ||||||
| mIPSC attributes | mIPSC attributes | mIPSC attributes | ||||||
| τ1 (ms) | Current (pA) | τ1 (ms) | τ2 (ms) | Current (pA) | τ1 (ms) | τ2 (ms) | Current (pA) | |
| NK (n = 16) | 9.5 ± 0.6* | 46.9 ± 2.4* | 2.8 ± 0.1 | 28.0 ± 1.9* | 52.8 ± 3.9* | 2.8 ± 0.2 | 17.3 ± 1.1* | 49.4 ± 1.2 |
| CLS (n = 9) | 9.6 ± 0.6** | 49.0 ± 1.1* | 2.7 ± 0.3 | 27.6 ± 1.8** | 56.4 ± 1.5* | 2.7 ± 0.1* | 22.5 ± 1.3** | 48.3 ± 1.1** |
| IPS (n = 6) | 9.4 ± 0.8* | 45.9 ± 1.8* | 2.6 ± 0.1 | 26.0 ± 1.0** | 51.6 ± 1.8* | 2.6 ± 0.1 | 18.8 ± 1.4* | 46.8 ± 1.2*,*** |
- * P < 0.05 and
- ** P < 0.01, compared with matched control.
- *** P < 0.05, compared with NK. mIPSC, miniature inhibitory post-synaptic current.
As the mIPSCav is composed of both mono- and biexponential mIPSCs these different kinds of events were also evaluated individually. For illustrative purposes averages of these events are shown in all figures. All mIPSCs included in the analysis were temporally resolved and had very fast 10–90% rise times (< 0.3 ms) to prevent the influence of dendritic filtering distortions on mIPSC kinetics. These selected events had their deactivation phases individually fitted with exponential functions. The residual deviations were then compared to decide which of the fits to retain, as we have described previously (Hutcheon et al., 2000). After categorization, 90–150 mIPSCs randomly selected from each recording were used for further offline analysis. From the individual fits five different parameters were collected and compared as appropriate: (i) time constant of monoexponential mIPSCs; (ii) amplitude of monoexponential mIPSCs; (iii) and (iv) fast and slow time constants of deactivation of biexponential mIPSCs; and (v) amplitude of biexponential mIPSCs. The total charge transfer of the mono- and biexponential mIPSCs was calculated from an average of about 90–150 of these events.
As the attributes of the individual mIPSCs are not normally distributed, for statistical analysis the median values describing the amplitudes and deactivation kinetics of the selected mono- and biexponential mIPSCs from each cell were calculated in each group and then averaged to generate an overall representation of the group (thus the values stated in the text are the averages of medians). As these average values are normally distributed, comparisons within the groups (pyramidal vs. non-pyramidal) were made using a t-test. Comparisons between the groups [e.g. pyramidal mIPSC attributes comparison between non-kindled (NK), CLS and IPS groups] were made by using anova and a Tukey multiple comparison post-hoc test as appropriate. Drug effects were compared by averaging the change in the median values for a particular parameter before and after drug treatment. The average difference between these values was then compared using a paired t-test. Significance of the differences was set at P < 0.05.
As tonic inhibition via extrasynaptic GABAA receptors could be potentiated by our compound (see review by Lambert et al., 2003), the holding current shift was monitored in all recordings, before and after neurosteroid (THDOC) application (as described in Schwabe et al., 2005).
Peak-scaled non-stationary fluctuation analysis of GABAA receptor-mediated mIPSCs was performed here in a manner analogous to other studies on dentate gyrus granule cells (Otis et al., 1994), CA1 pyramidal neurones (Wierenga & Wadman, 1999), cerebellar interneurones (Nusser et al., 2001) or cultured visual cortical neurones (Kilman et al., 2002). By using the non-stationary fluctuation analysis provided in mini analysis software (Synaptosoft Inc., Leonia, NJ, USA), we derived the channel kinetics and conductance of GABAA receptor synaptic currents recorded from both sham and kindled tissue. Briefly, this was performed by aligning 50–100 events/cell (by their fastest rate of rise), averaging and scaling them and calculating the variance of the current for 30 amplitude bins. Estimates of the single channel parameters were generated by plotting the variance vs. current amplitude and fitting this relationship for the best parabola (var = iI − I2/N, where i is the unitary current, I is the mean current and N the number of channels generating the mIPSC).
Histochemistry and quantitative image analysis
In order to reconstruct the morphology and understand where the recordings were made, patch electrodes included 0.3% Lucifer Yellow. After the completion of a recording, the slice was removed from the microscope chamber and fixed in Lana's solution (4% paraformaldehyde and 14% picric acid in phosphate-buffered saline, pH 6.9) for 24–48 h. The sections were rinsed in phosphate-buffered saline and then mounted on microscope slides for viewing on a confocal microscope. Under the appropriate illumination and emission, individual confocal images were gathered and then projected onto a single two-dimensional image to show the morphology of the cell. We used previously described (McIntyre et al., 2002a; Schwabe et al., 2005) criteria for the classification of pyramidal vs. non-pyramidal cells that were based on: (i) soma morphology; (ii) projection of the axon to deeper layers for pyramidal cells vs. non-projecting non-pyramidal cells; (iii) presence of dendritic spines for pyramidal cells; as well as (iv) spiking properties. On this basis, the neurones were classified as pyramidal and non-pyramidal. A few cells that were spiny non-pyramidal were also found but not included in the analysis. In order to ascertain the level of inhibitory innervation in the layer where recordings were performed, immunohistochemistry was performed in a separate set of rats (n = 8 sham and 8 kindled). Rats were perfused under deep anaesthesia (sodium pentobarbital 40 mg/kg, i.p.) first with phosphate-buffered saline and then with a mixture of acetone : methanol (70 : 30). Brains were then removed and post-fixed for 3–5 days in acetone : methanol (70 : 30) and then transferred to a cryprotectant of 20% sucrose in phosphate-buffered saline until sectioning. The sections (12 µm) were mounted on Superfrost slides and used for immunohistochemical analysis. We used an antibody raised against the GABA plasma membrane transporter type 1 (GAT-1) (Chemicon, Mississauga, Ontario, Canada) that is highly expressed in inhibitory nerve terminals and to some extent in glial processes (Ribak et al., 1996). Confocal images were taken using a 60 × 1.35-NA objective on an Olympus IX 60 inverted microscope outfitted with a Perkin Elmer Spinning Disk attachment. Cells were counterstained using Sytox green (shown in blue), GAT-1 immunoreactivity was visualized using a secondary antibody conjugated to Alexa 647 (shown in green) whereas calbindin-immunoreactive interneurones were visualized using a secondary antibody conjugated to Texas Red (shown in red). Images were quantitatively analysed using iplab software (Scanalytics Inc., Fairfax, VA, USA). In this analysis the program was asked to indicate and count the total number of pixels that were two times above background. This number was expressed as a percentage of the total number of pixels in the field.
Results
Whole-cell patch-clamp recordings were taken from neurones located in the second and third layers of the piriform cortex. Here we show the analysis of 101 recordings that met the criteria stipulated in the Materials and methods with regard to low-access resistance, appropriate resistance compensation and morphological identification. In the presence of tetrodotoxin (250 nm), 2-amino phosphonovaleric acid (20 µm) and dinitroquinoxaline-2,3-dione (10 µm), spontaneously occurring mIPSCs were collected for later offline analysis. First, we analysed the attributes of the mIPSCs in the pyramidal and non-pyramidal cells ipsilateral and contralateral to the kindled site as well as in sham brain slices (electrode-implanted but unstimulated rats). Second, we compared the activity of the neurosteroid THDOC on the different populations of neurones.
As reported previously, we have identified two different populations of mIPSCs that can be characterized by their amplitudes and kinetic attributes (Hutcheon et al., 2000; McIntyre et al., 2002b). One population is small in amplitude and deactivates at a monoexponential rate whereas the other has a larger amplitude and decays with two exponential phases. Thus, in addition to the more usual analysis where all suitable mIPSCs are averaged together to ascertain the global attributes of synaptic transmission, we also sorted and analysed (averaged) these two differing populations of events for each cell type (see Materials and methods for a complete description).
In NK rats, the attributes of the mIPSCav, monophasic and biphasic mIPSCs on pyramidal and non-pyramidal neurones were indistinguishable (Fig. 1). The amplitudes of the mIPSCav were about 52 pA and the deactivation could be described by the sum of exponentials having time constants of about 2 and 15 ms (see Table 1 for exact values). The monophasic mIPSCs were about 45 pA in amplitude and decayed with a half time of 7 ms whereas the biphasic mIPSCs were 55 pA and deactivated at rates of 2.5 and 18 ms. Parenthetically we should add that these rates are faster than we have previously reported (McIntyre et al., 2002b), where experiments were performed at room temperature.
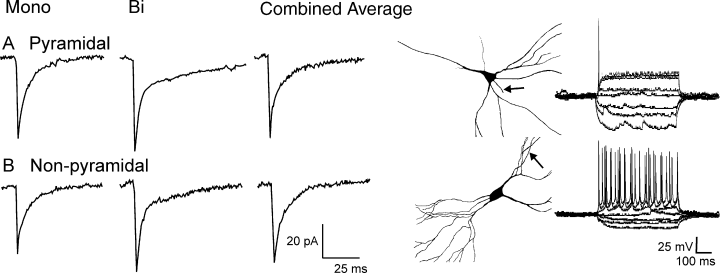
Miniature inhibitory post-synaptic currents in pyramidal (A) and non-pyramidal (B) neurones are indistinguishable in sham rats. Pictures of neurones show typical morphologies of cells classified as pyramidal (top) and non-pyramidal (bottom). Arrows indicate the axon; layer I is on the left side of the panel. Current–voltage relations are shown from each cell type.
After kindling, ipsilateral to the kindling site, we found that the amplitudes of the averaged mIPSCs of non-pyramidal cells were larger than those recorded from sham tissue (P < 0.001, Fig. 2A; Table 1). This change was due to a 45 and 27% increase in the amplitude of mono- and biexponential mIPSCs, respectively (P < 0.001 for both). Additionally the deactivation time constants of monoexponential mIPSCs of ipsilateral non-pyramidal neurones were also prolonged by 28% in comparison to naive non-pyramidal neurones (Table 1, P < 0.001). However, the time course of the biexponential population was unchanged. The impact of these changes in the averaged time course was primarily on amplitude, which increased by 17 ± 3.7 pA with a small reduction in the time course of deactivation. Thus, the overall effect of kindling was to increase the inhibitory charge transfer on the non-pyramidal neurones.
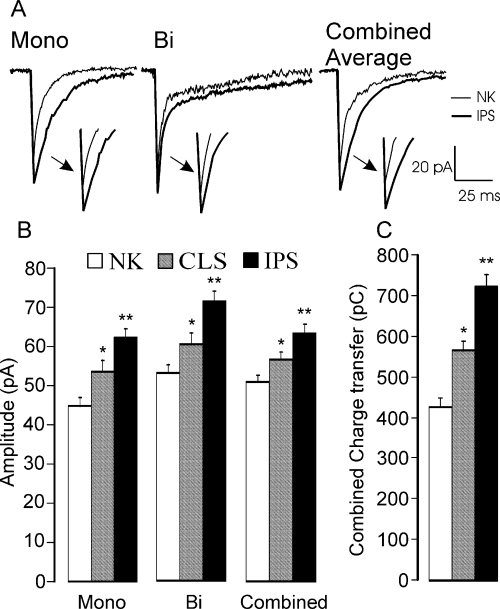
Kindling differentially affects mono- and biexponential events on non-pyramidal (NP) neurones. Monoexponential miniature inhibitory post-synaptic currents (mIPSCs) were prolonged in duration and became larger whereas the deactivation of biexponential mIPSCs was unchanged but their amplitudes were increased (A). No effect was observed on pyramidal cells. Comparison of the amplitudes (B) and the total charge transfer (C) in NP cells located on the contralateral (CLS) and ipsilateral (IPS) sides relative to the kindling site. The amplitude of both types of mIPSCs (mono- and biexponential) as well as that of the combined [averaged mIPSC (mIPSCav)] was enhanced in both CLS and IPS groups compared with the non-kindled (NK) group. This increased the estimated charge transfer (mIPSCav) by 33% in CLS and 69% in IPS non-pyramidal cells, respectively. *P < 0.05; **P < 0.01.
It has been observed that, contralateral to the kindling site in the temporal cortex, the brain behaves as though it is partially kindled (McIntyre & Poulter, 2001). Thus, it was of interest to compare and contrast the status of the inhibition in the contralateral piriform cortex in order to ascertain whether or not concomitant changes in inhibition had occurred in this region. Again, the mIPSCs from the pyramidal cells were indistinguishable from those recorded from either non-kindled control or ipsilateral brain slices. The time constants of the contralateral non-pyramidal mIPSCavs were not statistically different from the control mIPSCs (Table 1, P = 0.3). However, the mIPSCs were on average about 11% larger than those in the control group (Table 1, P < 0.05). Figure 2B shows the upward trend in all mIPSCs after kindling.
Next, we calculated the change in the total charge transfer that would occur due to these changes in the amplitude and time course of the mIPSCs. In control animals the average charge transfer for a monoexponential mIPSC was 295 ± 16 pC. This value increased significantly to 524 ± 24 pC (P < 0.001) in non-pyramidal cells on the ipsilateral side, an increase of nearly 80%. For the biexponential mIPSCs the increase observed in the non-pyramidal IPS group was less dramatic, increasing only by 35% (542 ± 45 pC in NK vs. 732 ± 28 pC in kindled IPS group; P < 0.05). Smaller yet statistically significant increases in charge transfer were also observed in non-pyramidal neurones contralateral to the kindling site. The charge transfer due to monoexponential events increased to 387 ± 17 pC (P < 0.05), whereas the charge transfer due to biexponential mIPSCs was significantly enhanced to 601 ± 27 pC (P < 0.05). In Fig. 2C we summarize the effect of these changes on the charge transfer of the mIPSCav.
The increase in total charge transfer for these currents can be due to increased channel conductance, enhanced open state probability and/or increased channel number. Both channel conductance and the number of channels opened at the peak (γ*N) were estimated by non-stationary fluctuation analysis of mIPSCs recorded from non-pyramidal cells in both non-kindled and kindled brain slices. In control recordings the unitary currents were estimated to be 2.7 ± 0.1 and 2.6 ± 0.1 pA for mono- and biexponential mIPSCs (n = 10), respectively. After kindling, these values were virtually identical; the unitary currents of the two-mIPSC populations (n = 15) were 2.6 ± 0.1 pA (monoexponential; P = 0.6) and 2.4 ± 0.1 pA (biexponential; P = 0.5; Fig. 3). However, we found that the total number of channels opened at the peak increased significantly after kindling, from 14.6 ± 0.7 to 17.5 ± 0.9 for monoexponential events and from 22.5 ± 0.9 to 27.3 ± 1.0 for biexponential population (P < 0.01 and P < 0.001, respectively).
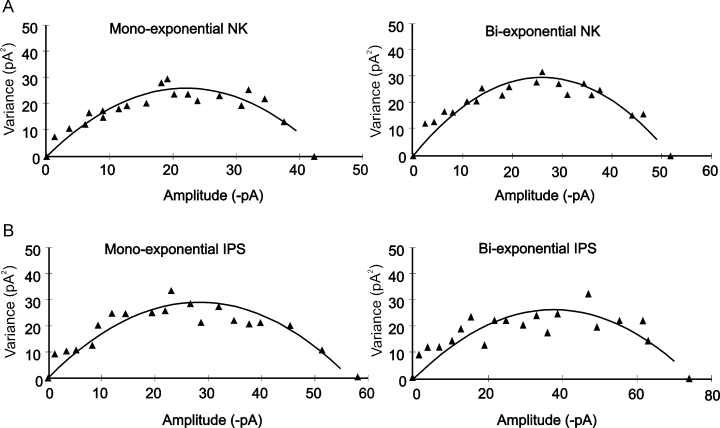
Non-stationary fluctuation analysis of non-pyramidal miniature inhibitory post-synaptic currents (mIPSCs) before (A) and after (B) kindling indicated the same estimated unitary conductance. (A) Mono- and biexponential mIPSC analysis in non-kindled (NK) group. (B) Analysis of the events after kindling in the ipsilateral (IPS) group (ipsilateral to the kindling site). Estimated channel amplitude was about 2.7 pA in all conditions, corresponding to an estimated mean channel conductance of 45 pS.
In order to find whether kindling differentially modulates the inhibition on non-pyramidal population subtypes, we further analysed the mIPSC amplitudes recorded on bitufted and multipolar cells found in layers II and III of the piriform cortex. The cells were classified as bitufted or multipolar on the basis of anatomical traits (Ekstrand et al., 2001). Bitufted cells displayed elongated soma with long dendritic tufts emerging vertically from the top and bottom of the soma, whereas multipolar cells were characterized by round or ovoid somata with dendrites extended radially (not shown). We did not find a significant effect of kindling on these two subtypes of non-pyramidal neurones. Thus, in the kindled group (ipsilateral to the kindled amygdala), the comparison of mono- and biexponential mIPSC amplitude between bitufted and multipolar non-pyramidal cells showed non-significant differences (P = 0.3 for monoexponential and P = 0.4 for biexponential comparison; bitufted cell, 57.2 ± 4.2 pA for monoexponential and 66.2 ± 4.1 pA for biexponential mIPSC amplitude, n = 11; multipolar cell, 61.1 ± 1.1 pA for monoexponential and 70.5 ± 3.2 pA for biexponential events, n = 12).
We also found that there was no change in the mIPSC frequency for both non-pyramidal (1.3 ± 0.4 Hz in the control group and 1.6 ± 0.3 Hz in the kindled group; P = 0.8) and pyramidal (control, 1.8 ± 0.5 Hz; kindled, 1.4 ± 0.3 Hz; P = 0.5) cells.
Effect of kindling on inhibitory nerve terminal expression
Monitoring the mIPSC frequency (before and after kindling) cannot provide information about the inhibitory terminal number, thus we chose a more direct method (immunohistochemistry) to ascertain the level of inhibitory innervation in the layer where recordings were performed. GAT-1 immunohistochemistry was performed in a separate set of rats (n = 8 sham and 8 kindled). Consistent with another report that showed GAT-1 staining in the piriform cortex (Ekstrand et al., 2001) we found that GAT-1 formed discreet puncta around cells in layer II as well as into layer III. Our staining pattern was consistent with this previous report with a distinct loss of staining in the most superficial area of layer I. These puncta have been shown to be associated with GAD67 staining (Ekstrand et al., 2001) and are thought to be nerve terminals (although a small proportion of low intensity staining is undoubtedly glial in origin). In order to determine if this pattern of staining changes after kindling we used quantitative image analysis that permitted us to count GAT-1 immunoreactivity (see Materials and methods). Our examination showed that there was no increase in GAT-1 immunoreactivity (Fig. 4A and B shows that about 7% of the area of layer II was intensely stained with GAT-1 but this is not significantly different in kindled tissue; control, 6.5 ± 0.8%; kindled, 4.7 ± 1.0% mean ± SEM, n = 8 and 8, P = 0.14). Thus, the inhibitory innervation of this cell layer does not appear to change after kindling.
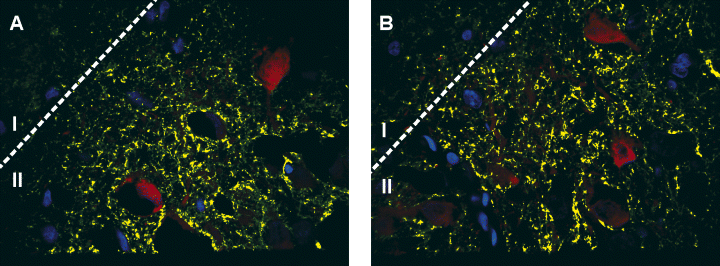
Analysis of GABA plasma membrane transporter type 1 (GAT-1) expression in layer II of the piriform cortex. In A (control) the yellow segmented pixels indicate GAT-1 immunoreactivity that is 2× or greater above the background immunofluorescence. (B) The equivalent image after kindling. Quantitative analysis of the total amount of pixels that are above this level revealed that there was no difference in the total amount of GAT-1 immunoreactivity. Cells stained in red are calbindin-positive interneurones and blue indicates the nuclei of all of the cells in the field. Qualitatively, it should be noted that there was also no obvious change in the pattern of staining with most immunoreactivity being distributed over the cell body layer in both control and kindled tissue. Cells were counterstained using Sytox green (shown in blue), GAT-1 immunoreactivity was visualized using a secondary antibody conjugated to Alexa 647 (shown in green) whereas calbindin-immunoreactive interneurones were visualized using a secondary antibody conjugated to Texas Red (shown in red). Demarcation between layers I and II is shown. The GAT-1 density was measured in layer II.
Characterization of the effect of tetrahydrodeoxycorticosterone on miniature inhibitory post-synaptic currents in pyramidal and non-pyramidal cells of the piriform cortex
In the next set of experiments, we were interested to determine if the neurosteroid THDOC would differentially affect the non-pyramidal vs. pyramidal populations in non-kindled, ipsilateral and contralateral side groups.
In 10–12 recordings 50 nm THDOC was without effect in both kindled and non-kindled tissue. Therefore, we used 100 nm THDOC, a concentration that is considered to be the upper limit of what is thought to be ‘physiological’ (Cooper et al., 1999). At 100 nm THDOC had significant effects on non-pyramidal neurones in the NK, CLS and IPS slices (Table 2). Figure 5A shows an example of the effects of THDOC on mIPSCs recorded from IPS non-pyramidal neurones. THDOC significantly prolonged the monoexponential time constant and slow component of the biexponential decay but had no effect on the time course of the fast component of the biexponential population.
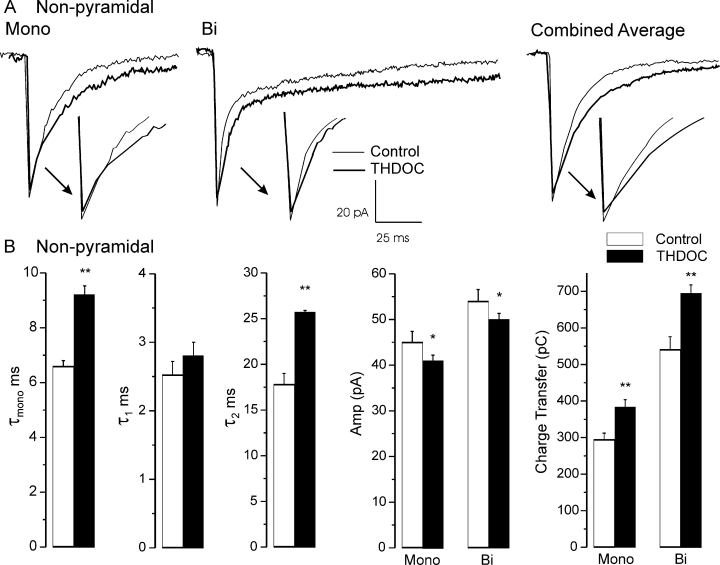
Summary of the effects of tetrahydrodeoxycorticosterone (THDOC) (100 nm) on miniature inhibitory post-synaptic currents recorded from non-pyramidal neurones in kindled brain, ipsilateral to the kindling site (A), and non-kindled brain (B). The effects of THDOC on both sham and kindled brain were the same. *P < 0.05; **P < 0.001.
In non-pyramidal cells, there was little or no change in the contribution of the fast component to the deactivation after kindling. The fast deactivation contributed to about 55% of the decay in all groups. As this component is due primarily to desensitization, kindling had no effect on this attribute. As expected (Zhu & Vicini, 1997), THDOC increased the fast deactivation by 5% (P < 0.05) in two cell groups, IPS non-pyramidal and CLS pyramidal. It also caused a small reduction in the amplitude of both mono- and biexponential mIPSCs. Nevertheless, the charge transfer in both non-kindled and kindled slices was still significantly increased due to the prolongation of the slow deactivation (P < 0.001, Fig. 5B).
In sham rats, mIPSCav on pyramidal cells was enhanced by a prolongation of the deactivation with a small change in amplitude (Table 3; Fig. 6A). In slices obtained from kindled brain ipsilateral to the kindling site, THDOC produced a small prolongation in the deactivation but there was also a significant reduction in mIPSC amplitude (Table 3; Fig. 6B, P < 0.05 compared with sham). This reduction in amplitude by THDOC also appeared on the contralateral side but this failed to reach statistical significance in comparison to sham controls (P = 0.1).
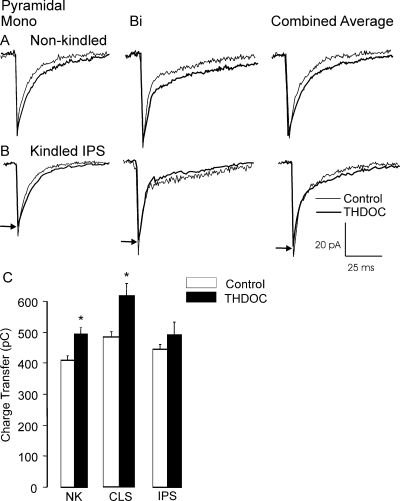
The effects of tetrahydrodeoxycorticosterone (THDOC) on pyramidal neurones are muted by kindling. In non-kindled (NK) rats, THDOC effects on the time course of miniature inhibitory post-synaptic currents (mIPSCs) recorded from pyramidal neurones (A) produced about a 20% increase in charge transfer. Similar effects on mIPSCs recorded from brain slices contralateral (CLS) to the kindling site were also observed. However, ipsilateral (IPS) to the kindling site, THDOC enhanced the deactivation but also further reduced the amplitude of mIPSCs (B). Thus overall, the charge transfer was not enhanced after kindling (C). Arrows indicate amplitude in the presence of THDOC. *P < 0.05.
Although increasing the deactivation time constant would tend to enhance inhibition, the reduction in mIPSC amplitude tends to decrease it. In order to assess the overall impact of these conflicting effects we calculated the average charge transfer for the individual events in each cell and compared these values with those obtained in the presence of THDOC. As shown in Fig. 6C, kindling in IPS eliminates the expected increase in charge transfer that THDOC produces in NK and CLS.
Finally, we analysed the shift in the tonic holding current after the application of THDOC. Although THDOC induced a small and reversible increase in holding current, it was not statistically significant. Thus, the activity of THDOC was the same with regard to holding current (Fig. 7, P > 0.05). In both non-pyramidal the mean change in holding current was: sham, −14.6 ± 10.3 pA (n = 16); kindled contralateral, −29.1 ± 21.1 pA (n = 9); and kindled ipsilateral, −25.4 ± 14.62 pA (n = 6). For the pyramidal population the mean change was: sham, −19.1 ± 10.7 pA (n = 11); kindled contralateral, – 33.3 ± 16.9 pA (n = 9); and kindled ipsilateral, −31.3 ± 21.1 pA (n = 16).
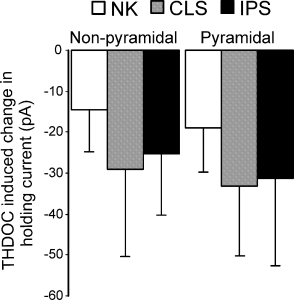
Kindling had no effect on the magnitude of the tonic current shift induced by tetrahydrodeoxycorticosterone (THDOC) in both pyramidal and non-pyramidal cells. CLS, contralateral; IPS, ipsilateral; NK, non-kindled.
Discussion
The development of seizures through kindling has been studied for many years. Although it has been a widely held view that there must be some sort of imbalance between excitation and inhibition, evidence that this is so is scant. Although evidence of inhibitory plasticity due to kindling has been shown in the dentate granule cells of the hippocampus (Nusser et al., 1998), in highly seizurogenic regions like the piriform cortex (de Guzman et al., 2004) no information on how the synaptic activity may change has been reported. The data reported here show that the inhibitory synapses on interneurones are uniquely plastic and that this plasticity is best explained by an increase in channel number. Thus, seizures are inducing mechanisms that traffic and maintain receptor localization at synaptic sites. The most obvious outcome of this increase is an enhancement of charge transfer in non-pyramidal cells (Fig. 2C) and perhaps enhanced disinhibition. It also seems that the system does not compensate for these increases, as the GABAergic innervation is unchanged. Finally, a more surprising endpoint was that THDOC efficacy was reduced on pyramidal neurones after kindling (Fig. 6C). As tonic inhibition was not altered by kindling and/or THDOC in both non-pyramidal and pyramidal cell populations (Fig. 7), overall these data suggest that seizures may be supported by increased inhibitory synaptic strength on interneurones and that endogenous steroid activity may be ineffective in counteracting this outcome on pyramidal cells.
Several possible mechanisms could account for the alterations in mIPSC attributes: (i) enhanced channel conductance; (ii) increased open state probability; (iii) increased channel number and (iv) increased GABAergic quantal size. As the channel conductance is unchanged after kindling (as revealed by non-stationary noise analysis), the parameters that could explain the amplitude increase are the open state probability and the total number of channels. Owing to the fact that we recorded from many synapses of unknown heterogeneity we could not estimate the open state probability here. However, in other studies this was possible [the open state probability has been found to be relatively high (> 0.9)] (Borst et al., 1994; Auger & Marty, 1997; Nusser et al., 1998). If we assume a similar high probability then any further increase would be unlikely to explain the increased amplitude after kindling. This assumption is concordant with other studies that failed to detect changes in the open state probability (Otis et al., 1994; Nusser et al., 1998). In the latter study (Nusser et al., 1998), an increased receptor density was also found. Similar, albeit indirect measures of increased receptor density have been suggested in another study (Cohen et al., 2003), as well as in others where benzodiazepine binding sites have been shown to increase after kindling in the amygdala (Tuff et al., 1983) and dentate gyrus (Shin et al., 1985; Clark et al., 1994). An increased amount of GABA per vesicle could account for the increased mIPSC amplitude in non-saturating transmitter concentration conditions (Frerking et al., 1995; Hajos et al., 2000). However, under control circumstances, if the synapses were non-saturating then THDOC should augment their amplitude (as it increases affinity). This was never observed in this study nor in our previous study (Schwabe et al., 2005) and so it would appear that these synapses before kindling are saturating. Thus, an increase in the GABA release by a single vesicle seems unlikely as a mechanism that would account for the increased amplitude. This interpretation agrees with a similar kindling study that failed to detect an increase in GABAergic quantal size (Nusser et al., 1998).
As we analysed only very fast events (fast rise time mIPSCs) that were therefore electrically close, alterations in mIPSC amplitude and time course are probably not accounted for by factors that are responsible for changes in dendritic arborization or increases in input resistance. We have also found that GABA receptor subunit expression increases on interneurones in this model (Meguro et al., 2004) and so the most likely explanation is that increased receptor density accounts for the increased amplitude of the mIPSCs. How these changes are reflected in the evoked IPSCs (many release sites) is not entirely clear but it seems unlikely that these changes would have no impact. Thus, evoked IPSCs would be predicted to become larger and slower decaying and therefore to have an impact on the network activity. This speculation precludes alterations in dendritic filtering that could alter IPSCs, independent of changes at the release site.
An increase in mIPSC amplitude after seizure induction has been reported before on excitatory cells in both kindling and pilocarpine models of epilepsy (Otis et al., 1994; Nusser et al., 1998; Cohen et al., 2003). This increase might be compensatory and limit the spread of synchronous activity but an alternative view suggested that increased inhibition would better synchronize the dentate neurones permitting larger and more effective volleys of network activity that spread seizure activity (Nusser et al., 1998). However, this is difficult to resolve and to date these two conflicting interpretations have not been examined. Here, we show an increase in mIPSC amplitude on inhibitory neurones in the piriform cortex, a region that is highly excitable and forms strong connections with other temporal lobe structures (such as the perirhinal cortex) that facilitate the generalization of seizures (de Guzman et al., 2004). However, our immunocytochemical data indicated that there are about the same amount of inhibitory nerve terminals in kindled and non-kindled groups (Fig. 4), suggesting no compensation for the increased synaptic strength. Furthermore, the prolonged time constants of the mIPSCs suggest that the inhibition of the interneuronal network would tend to summate more effectively. This would have the effect of permitting the pyramidal neurones to burst fire, perhaps accounting for ictal activity. Importantly, these changes in inhibition seem to follow the progression of seizures, as on the contralateral side the increased mIPSC-mediated charge transfer was intermediate to the sham and fully kindled piriform cortex.
Kindling also altered the apparently matched kinetic attributes of mIPSCs occurring on pyramidal and non-pyramidal neurones. Thus, another type of imbalance was created between pyramidal and non-pyramidal cells after kindling. This observation is similar to those that we reported in a previous study (McIntyre et al., 2002b) where we found that in seizure-prone rats the inhibition of the interneurones was longer lasting than that on pyramidal neurones, opposite to normal and seizure-resistant rat strains where the attributes of mIPSCs within each strain were indistinguishable. Although it is not clear what importance these equivalent inhibitory profiles may have, it seems possible that altering the apparent balance could have a deleterious impact on how the network synchronizes. Also, synaptic noise has been implicated in controlling the firing patterns of pyramidal cells (Prescott & De Koninck, 2003) and thus it is possible that the firing patterns of the inhibitory neurones may be altered in such a way that the circuit is mistimed, either favouring (causing) or attenuating (compensating) the kindling stimulus.
Kindling induced selective changes in the attributes of the two kinds of synaptic activity (mono- and biphasic) that we categorized. Although both types of inhibition increased in amplitude, only the deactivation kinetics of the monoexponential mIPSCs were prolonged. The change in deactivation suggests a selective change in the identity of the subunit expression at these synaptic sites, in addition to the increased receptor density. The former observation agrees with numerous studies that have shown that seizures are associated with changes in GABAA receptor subunit expression (Kamphuis et al., 1994; Kokaia et al., 1994; Titulaer et al., 1995; Brooks-Kayal et al., 1998, 1999; Nusser et al., 1998; Sperk et al., 1998; Fritschy et al., 1999; Bouilleret et al., 2000; Loup et al., 2000; Redecker et al., 2000; Meguro et al., 2004; Maguire et al., 2005). In general, these studies show that subunit plasticity is very heterogeneous and the factors that control where these changes may or may not take place are not well understood. In a recent study (Meguro et al., 2004), we reported (using the same model employed here) that α2 and α5 GABAA subunit expression is increased in the perirhinal and piriform cortices whereas, in the hilus of the dentate gyrus, the expression is dramatically increased for α2, α5 and α3 subunits. These changes occurred in only one of the two interneurone populations, i.e. calbindin- and not parvalbumin-immunoreactive neurones. Thus, only one type of neurone was plastic under these conditions.
A particularly surprising result was that the mIPSC amplitude was suppressed by THDOC. Although present to some degree in sham rats, this reduction was enhanced by kindling in the pyramidal neurones. This counterbalanced the potentiating effects of the slowed deactivation, thus eliminating an enhancement of charge transfer. As THDOC may enhance the tonic or the rate of entry into desensitization (Zhu & Vicini, 1997), and desensitization is controlled to some degree by phosphorylation (Jones & Westbrook, 1997), it is possible that kindling alters the phosphorylation state which in turn alters the drug efficacy. Indeed, neurosteroid activity has been shown to vary with phosphorylation state (Brussaard et al., 2000; Fancsik et al., 2000; Tasker, 2000; Brussaard & Koksma, 2002; Koksma et al., 2003). For example, phosphorylation increases THDOC activity on recombinant receptors (Leidenheimer, 1996) while preventing allopregnanolone activity in the supraoptic nucleus (Brussaard et al., 2000) and magnocellular neurones of the hypothalamus (Fancsik et al., 2000). Moreover, inhibition of either protein kinase A or C reduced the sensitivity of CA1 pyramidal cell synaptic GABAA receptors to 5β-pregane 3α-ol-20-one (Harney et al., 2003). This treatment had no effect on pentobarbitone potentiation. Interestingly, in dentate gyrus granule cells, the activation of protein kinase C made mIPSCs sensitive to a previously ineffective concentration of 5β-pregnane 3α-ol-20-one. Thus, phosphorylation may differentially modulate the neurosteroid activity in various cell types. Kindling has been shown to cause very large changes in kinase and phosphatase activity (see review by McIntyre et al., 2002b). The differential sensitivity may have something to do with the relative ease with which phosphorylation is induced in pyramidal and non-pyramidal populations.
Studies using recombinant receptors have shown that neurosteroids have some selectivity based on subunit expression (for review see Lambert et al., 2003). For example, the potency of neurosteroids may be greater on α2- than on α1-containing receptors (Maitra & Reynolds, 1998, 1999), suggesting that this subunit may decline in the pyramidal neurones, opposite to that seen in the calbindin-immunoreactive interneurones (Meguro et al., 2004). In contrast, the effect of THDOC on the mIPSCs recorded from non-pyramidal neurones in kindled brain was about the same as in sham tissue. Thus, a change in subunit expression does not seem to have an impact here although our recent findings have shown that α2 subunit expression is up-regulated in calbindin-immunoreactive interneurones in this and adjacent temporal lobe regions (Meguro et al., 2004).
In conclusion, we have shown in this study that kindling selectively induces changes in monosynaptic inhibitory events on non-pyramidal (inhibitory) neurones, whereas the mIPSCs on pyramidal neurones are unchanged. At the simplest level of interpretation, these changes predict that the inhibitory interneurones receive increased inhibition. However, it is not clear how these changes play a role in controlling the activity of the piriform cortex. Increased inhibition of these cells predicts increased disinhibition that may account for the kindling phenomena but it is not clear how these neurones ‘wire’ the pyramidal cells and other interneurones, as they may make reciprocal connections with each other. Thus, the outcome of these synaptic changes may be more complex. The increased inhibition may alter the intrinsic oscillatory properties of the network. This may reflect an underlying cause of the kindling phenomena or it may be an attempt to attenuate the seizurogenesis. Finally, the activity of THDOC may further enhance seizures as it loses its effectiveness to increase charge transfer while still increasing (the already enhanced) inhibition on the interneurones. Overall these data predict greatly altered network activity and its modulation by this endogenous compound. Further study where network activity is stimulated in this model should clarify how these mechanisms may play a role in kindling and perhaps human epilepsy.
Acknowledgements
This work is supported by a Canadian Institutes of Health Operating Grant for M.O.P. The authors would also like to thank D.C. McIntyre for helpful comments and discussion.
Abbreviations
-
- ADT
-
- after-discharge threshold
-
- CLS
-
- contralateral
-
- GAT-1
-
- GABA plasma membrane transporter type 1
-
- IPS
-
- ipsilateral
-
- mIPSC
-
- miniature inhibitory post-synaptic current
-
- mIPSCav
-
- averaged miniature inhibitory post-synaptic currents
-
- NK
-
- non-kindled
-
- THDOC
-
- tetrahydrodeoxycorticosterone