Irreversible blockade of monoamine oxidases reveals the critical role of 5-HT transmission in locomotor response induced by nicotine in mice
Present address: Department of Pharmacology, College of Medicine, University of California, Irvine, CA 92697, USA
Abstract
Although nicotine is generally considered as the main compound responsible for addictive properties of tobacco, some experimental data indicate that nicotine does not exhibit all the characteristics of other substances of misuse such as psychostimulants and opiates. For example, nicotine generally fails to induce locomotor response in mice and self-administration of nicotine is difficult to obtain in rats. We have shown recently that a pretreatment with mixed irreversible monoamine oxidase inhibitors (MAOIs), such as tranylcypromine, triggers a locomotor response to nicotine in mice and induces a robust self-administration of nicotine in rats. We show here that when mice were pretreated with enhancers of extracellular levels of noradrenaline, dopamine or serotonin (d-amphetamine, GBR12783 or para-chloro-amphetamine, respectively) and injected with nicotine (1 mg/kg), only those animals pretreated with para-chloro-amphetamine exhibited a specific locomotor response to nicotine. These data indicate a critical role of serotonin in nicotine-induced locomotor activity in mice. This was further confirmed in microdialysis experiments showing that nicotine induces an increase in extracellular serotonin levels in the ventral striatum in mice pretreated with tranylcypromine. This effect of nicotine on extracellular serotonin levels was absent in mice lacking the β2-subunit of the nicotinic acetylcholine receptor. Our data suggest that mixed irreversible MAOIs contained in tobacco facilitate the effects of nicotine on serotonin release, thus allowing the locomotor and rewarding effects of nicotine.
Introduction
Drugs of misuse, such as d-amphetamine, cocaine, morphine and heroin, share the ability to cause addiction in humans and to increase release of dopamine (DA) in the nucleus accumbens (Di Chiara & Imperato, 1988; Vezina et al., 1989; Pontieri et al., 1995; Koob, 1998; Robbins & Everitt, 1999). The same drugs induce locomotor hyperactivity, behavioural sensitization, conditioned place preference or self-administration in rodents (Vezina et al., 1994; Pontieri et al., 1995; Biala & Langwinski, 1996; Darracq et al., 1998; Carboni et al., 2003; Li et al., 2003). Tobacco is also a potent reinforcing agent in humans, and nicotine is generally considered as the major compound responsible for tobacco addiction (Dani & Heinemann, 1996; Balfour et al., 2000; Di Chiara, 2000). However, animal experiments indicate some discrepancies between the effects of nicotine and those of other drugs of misuse. For example, the stimulation of DA release in the nucleus accumbens after several nicotine administrations remains controversial (Vezina et al., 1992; Balfour et al., 1998; Di Chiara, 2000). These differences may depend upon the site(s) where measurements are made, i.e. the shell or the core of the nucleus accumbens, and also whether delivery is contingent or not upon a response (Di Chiara, 2002). However, repeated nicotine treatments in rats induce a behavioural sensitization that vanishes quicker than for other drugs of misuse (Ksir et al., 1985; Villégier et al., 2003). Similarly, although self-administration of nicotine is observed in rodents (Corrigall & Coen, 1989; Donny et al., 1995; Martellotta et al., 1995; Shoaib et al., 1997; Caggiula et al., 2001) and monkeys (Goldberg et al., 1981; Wakasa et al., 1995), its development seems slower and weaker than for other drugs of misuse (Manzardo et al., 2002), and often needs facilitation by the use of food restriction (Corrigall et al., 2002; Rauhut et al., 2002) or cocaine pretreatment (Picciotto et al., 1998). The importance of environmental stimuli in the rate of self-administration of nicotine has also been emphasized (Caggiula et al., 2001). Altogether, these differences suggest either that nicotine possesses both aversive and rewarding properties (Risinger & Brown, 1996; Shoaib et al., 2002), or that the addictive effects of tobacco are not only due to nicotine.
One of the most striking differences between effects of nicotine and those of other drugs of misuse concerns its locomotor effects. Indeed, although psychostimulants and opiates induce an important locomotor hyperactivity both in rats and in mice, nicotine generally fails to do so in mice whatever the dose (Marks et al., 1983; Kita et al., 1988; Damaj & Martin, 1993; Sparks & Pauly, 1999). Recently, we have shown that a pretreatment with an irreversible monoamine oxidase inhibitor (MAOI), such as tranylcypromine, not only triggered locomotor response to nicotine in mice, but also allowed a robust self-administration of nicotine in rats (Villégier et al., 2005). This suggested that nicotine induced locomotor and rewarding effects only when the extracellular level(s) of one or more monoamines [DA, noradrenaline (NA) and/or serotonin (5-HT)] were increased by an additional pharmacological treatment. The aim of the present study was to understand better how MAOIs, such as tranylcypromine, enhance nicotine's locomotor effects in mice. First, to assess whether one of the monoamines (DA, NA or 5-HT) could be self-sufficient to improve the locomotor effects of nicotine, locomotor response was recorded in mice following the co-injection of nicotine with GBR12783, d-amphetamine or para-chloro-amphetamine (PCA), three compounds that, respectively, increase extracellular DA, NA and 5-HT levels (Bonnet & Costentin, 1986; Florin et al., 1994; Vanderwolf et al., 1997; Drouin et al., 2001; Salomon et al., 2006). Then, because data indicated that locomotor effects of nicotine were enhanced by PCA, microdialysis experiments were conducted to monitor extracellular 5-HT levels in the ventral striatum following tranylcypromine and nicotine injections. Extracellular DA levels were also monitored for comparison. Finally, to assess the specificity of nicotine-induced effects, microdialysis experiments were reproduced using mice knockout (KO) for the β2-subunit of the nicotinic acetylcholine receptor (nAChR), one of the most important nAChR subunits in the central nervous system (Picciotto et al., 1998).
Materials and methods
Subjects
Animals were adult male mice, on genetic background C57Bl6, weighing 25–35 g when experiments took place. Generation of β2-nAChRs-KO mice was as described previously by Picciotto et al. (1995). Mice were maintained on a 12-h light/12-h dark cycle (lights on between 07:00 and 19:00 h) at constant temperature (22 °C), with food and water available ad libitum. Animals were housed by groups of four and were habituated to their home cages for at least 1 week before the experiments. Mice were treated in accordance with the Guide for Care and Use of Laboratory Animals established by the European Community Council Directive 86/609/EEC.
Drugs
(–)-Nicotine hydrogen tartrate, tranylcypromine hydrochloride, d-amphetamine sulphate and PCA were from Sigma Aldrich (France). They were dissolved in saline (NaCl, 0.9%). pH of the solutions was adjusted to 7.4 with 1 n NaOH. GBR12783 was synthesized by Pr. Robba (Caen, France; see Bonnet & Costentin, 1986) and was dissolved in DMSO (< 5%). Doses are expressed as salts for all compounds except for nicotine which is expressed as base. Nicotine was injected subcutaneously (1.5 mL/kg per injection), whereas other treatments were injected intraperitoneally (3 mL/kg per injection).
Measurement of locomotor activity
Mice were introduced to a circular corridor (4.5 cm width, 17 cm external diameter) crossed by four infrared beams (1.5 cm above the base) placed at every 90° (Imetronic, Pessac, France). Locomotor activity was counted when animals interrupted two successive beams and thus had travelled a quarter of the circular corridor. A total of 56 mice were used for this experiment. In each session, the mouse was placed in the locomotor apparatus for 90 min to record spontaneous activity before drug or vehicle treatment. To study the locomotor effects of the association between nicotine and GBR12783, d-amphetamine or PCA, mice were injected at T = 0 with the following treatments: nicotine (1 mg/kg) or vehicle, co-administrated with d-amphetamine (2 mg/kg), GBR12783 (14 mg/kg) or PCA (7 mg/kg) or with their respective vehicle. Then locomotor response was recorded during a subsequent 120-min period. Tests were performed between 12:00 and 18:00 h in stable conditions of temperature and humidity.
Surgery
Mice were anaesthetized with sodium pentobarbital (60 mg/kg; Sanofi Santé Animale, France) and placed in a stereotaxic frame (Kopf Instruments). The head was positioned by means of a mouse nose clamp adaptator (Kopf model 922) supplemented by rat ear bars placed lightly in the external auditory meatus. Unilateral permanent cannula (CMA/7 guide cannula, Microdialysis AB, Sweden) was implanted into the ventral striatum and was secured on the skull with screw and dental cement. The coordinates for the guide cannula tip were antero-posterior: +1.3 relative to bregma, medio-lateral: +0.8, and dorso-ventral: −2.4 mm from dura (Paxinos & Franklin, 2001). The conditions were identical to those previously described and shown in Auclair et al. (2002). After histological examination, only animals correctly implanted (see photograph in Auclair et al., 2002) were kept for analysis. In fact, the relative sizes between dialysis membranes and mouse nucleus accumbens did not allow differentiation between the core and the shell. Our data should therefore be considered as the result of the dialysis of both subdivisions of the structure that we define as the ventral striatum.
After surgery, mice were placed in individual plastic cages and allowed to recover for at least 4 days.
Microdialysis experiment
A total of 34 mice were used for this experiment. On the day of the experiment, the microdialysis probe was inserted (CMA/7, membrane length 2 mm and diameter 0.24 mm, cut-off: 6000 Da, Microdialysis AB, Sweden). Artificial CSF (in mm: NaCl, 147; KCl, 3.5; CaCl2, 1; MgCl2, 1.2, NaH2PO4, 1; NaHCO3, 25, pH = 7.6) was perfused with a CMA100 microinjection pump through the probe at a rate of 1 µL/min via an FEP catheter (internal diameter 0.12 mm) connected to a fluid swivel. An adequate steady state of 5-HT or DA levels in perfusate samples was reached 2 h 20 min after probe insertion, and samples were collected in 300-µL vials placed into a refrigerated computer-controlled fraction collector (CMA/170). Samples of 20 µL taken every 20 min were collected over a period of 1 h 40 min to determine basal extracellular DA or 5-HT values. Then, tranylcypromine (10 mg/kg) or saline was injected and, 100 min later, nicotine (1 mg/kg) or saline. Altogether, samples were collected for 7 h and analysed on the day of the experiment or kept at −80 °C for later analysis.
Biochemistry
Dialysate samples were completed to 30 µL with the mobile phase and placed into a refrigerated automatic injector (Triathlon, Spark Holland, Emmen, The Netherlands). Twenty-five microlitres of the sample was injected through a rheodyne valve in the mobile phase circuit. High-performance liquid chromatography was performed with a reversed-phase column (80 × 4.6 mm, 3 µm particle size, HR-80, ESA inc., Chelmsford, MA, USA). Mobile phase (NaH2PO4 75 mm, EDTA 20 µm, octane sulphonic acid 2.75 mm, triethylamine 0.7 mm, acetonitrile 6%, methanol 6%, pH = 5.2, for DA and 5-HT) was delivered at 0.7 mL/min by an ESA-580 pump. Electrochemical detection was performed with an ESA coulometric detector (Coulochem II 5100A, with a 5014B analytical cell; Eurosep, Cergy, France). The conditioning electrode was set at −0.175 V, and the detecting electrode was set at +0.175 V, allowing a good signal-to-noise ratio of the DA and 5-HT oxidation current. External standards were regularly injected to determine the stability of the sensitivity (0.3 and 0.6 pg for DA and 5-HT, respectively).
Data analysis
Results are presented as means ± SEM. Data were analysed using analysis of variance (anova) for repeated measures on time (10-min intervals over 60, 120 and 120 min for locomotor activity in the presence of d-amphetamine, GBR and PCA, respectively, and 20-min intervals over 220 min for microdialysis). A shorter time was chosen for d-amphetamine because its locomotor effect lasted for less than for the two other compounds. According to the number of parameters statistically relevant, one-way to three-way anovas were performed with repeated measures on time. Time was analysed as a within-subject factor and pharmacological treatments and genotype correspond to independent groups of animals and were analysed as between-subject factors. Significant main effects or interactions were tested separately with anovas and Bonferroni- or Dunnett's-corrected post-hoc comparisons. All data analyses were performed using GraphPad Prism 4.0 software (San Diego, CA, USA) or systat 10 statistical software. Statistical significance was set at P < 0.05.
Results
Effects of GBR12783, PCA or d-amphetamine on nicotine-induced locomotor activity
As previously described (Villégier et al., 2005), administration of nicotine alone had no significant effect on locomotor activity when compared with saline (F1,14 = 2.684, P = 0.124). GBR12783, a specific inhibitor of DA reuptake (Bonnet & Costentin, 1986; Drouin et al., 2001), induced a significant increase of locomotor response as shown by a significant GBR12783 effect (F1,28 = 35.191, P < 0.001) and a significant GBR12783–time interaction (F11,308 = 3.536, P < 0.001) (Fig. 1A). Nicotine co-administration did not significantly modify locomotor activity induced by GBR12783 (absence of nicotine effect: F1,28 = 0.016, P = 0.901; and absence of nicotine–time interaction: F11,308 = 0.718, P = 0.721). PCA, a compound known to release 5-HT (Vanderwolf et al., 1997), significantly increased locomotor activity (PCA effect: F1,26 = 71.28, P < 0.001; and PCA–time interaction: F11,286 = 15.54, P < 0.001) (Fig. 1B). A significant nicotine effect (F1,26 = 6.802, P = 0.015) and nicotine–time interaction (F11,286 = 3.076, P = 0.001) were also found. Interestingly, a significant nicotine–PCA interaction (F1,26 = 4.76, P = 0.038) and nicotine × PCA–time interaction (F11,286 = 5.102, P < 0.001) were found (Fig. 1B). Nicotine significantly increased locomotor activity in PCA-pretreated mice (+87%: F1,12 = 4.874, P = 0.047). Finally, Fig. 1C shows that d-amphetamine induced a significant increase of locomotor activity, as indicated by a significant d-amphetamine effect (F1,22 = 50.653, P < 0.001) and a significant d-amphetamine–time interaction (F5,110 = 6.966, P < 0.001). A significant nicotine–time interaction (F5,110 = 10.915, P < 0.001) was found. A significant nicotine × d-amphetamine interaction (F1,22 = 14.69, P = 0.001) and nicotine × d-amphetamine–time interaction (F5,110 = 3.173, P = 0.01) were found (Fig. 1C). Nicotine significantly decreased locomotor response in d-amphetamine-pretreated mice (−47%; F1,8 = 5.398, P = 0.049).
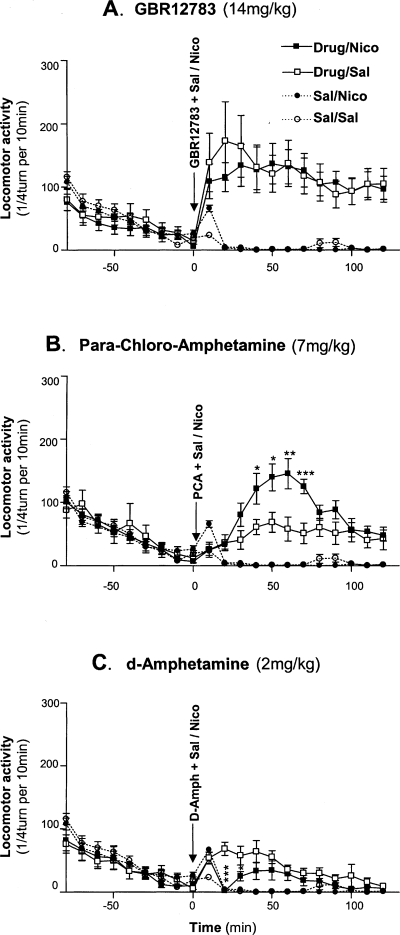
Effects of monoamine extracellular enhancers on locomotor response to nicotine. After habituation to the locomotor apparatus, animals were pretreated with either (A) GBR12783, (B) PCA or (C) d-amphetamine. Each drug was co-administered with saline or nicotine (1 mg/kg) and locomotor activity was recorded for 120 min. *P < 0.05; **P < 0.01; ***P < 0.001 significantly different between saline and nicotine-treated animals. n = 5–8 mice per group.
Effects of tranylcypromine on nicotine-induced locomotor activity and increases in extracellular 5-HT and DA levels in the ventral striatum
Figure 2A has been included for comparison. This figure has already been published (Villégier et al., 2005), and shows that pretreatment with tranylcypromine significantly potentiates locomotor response to nicotine. The same conditions were used to assess the effect of this treatment on extracellular 5-HT and DA levels in the ventral striatum (Fig. 2B and C, respectively).
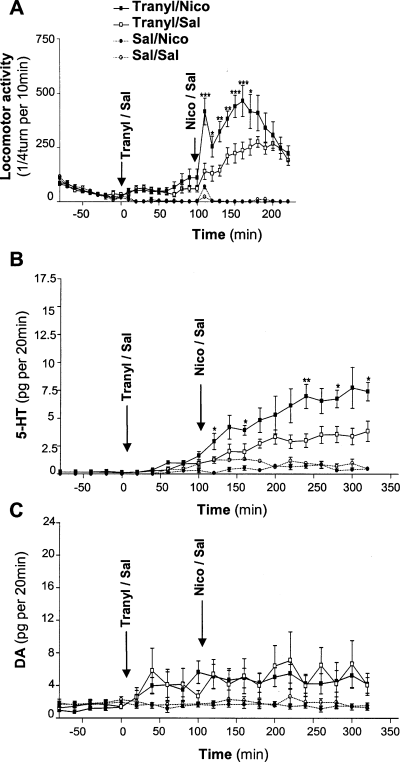
Effects of tranylcypromine on nicotine-induced locomotor response and extracellular 5-HT and DA levels in WT mice ventral striatum. (A) Locomotor response to nicotine in mice pretreated with tranylcypromine or saline is shown for comparison (Villégier et al., 2005). Animals were pretreated with tranylcypromine (10 mg/kg) or saline and received an injection of either saline or nicotine (1 mg/kg) 100 min later. *P < 0.05; **P < 0.01; ***P < 0.001 significantly different between tranylcypromine/nicotine- and tranylcypromine/saline-treated animals. n = 8–10 mice per group. (B) Effects of tranylcypromine and nicotine on extracellular 5-HT levels in the ventral striatum. Animals were treated as in A and extracellular 5-HT levels were monitored. These are expressed as the mean ± SEM in pg 5-HT per 20 min. Basal extracellular 5-HT values were at the level of our detection limit, ∼0.6 pg 5-HT/20 min. *P < 0.05; **P < 0.01 significantly different between tranylcypromine/nicotine- and tranylcypromine/saline-treated animals. n = 4–6 mice per group. (C) Effects of tranylcypromine and nicotine on extracellular DA levels in the ventral striatum. Animals were treated as in A and extracellular DA levels were monitored. These are expressed as the mean ± SEM in pg DA per 20 min. Basal extracellular DA levels were 1.8 ± 0.2 pg DA/20 min. No significant effect of nicotine treatment was detected. n = 4–6 mice per group.
As shown in Fig. 2B, tranylcypromine significantly increased extracellular 5-HT levels, as shown by a significant tranylcypromine effect (F1,16 = 31.185, P < 0.001) and a significant tranylcypromine–time interaction (F11,176 = 5.747, P < 0.001). Interestingly, a significant nicotine–tranylcypromine interaction (F1,16 = 7.054,, P = 0.017) was found. Nicotine significantly decreased extracellular 5-HT levels in saline-pretreated mice (F1,6 = 12.823 P = 0.012) and significantly increased it in tranylcypromine-pretreated mice (F1,10 = 7.912, P = 0.018).
In the same experimental conditions, extracellular DA levels were significantly increased by tranylcypromine, as shown by the significant tranylcypromine effect (F1,15 = 7.597, P = 0.015) (Fig. 2C). However, this increased level was not significantly affected by the subsequent injection of nicotine (absence of nicotine effect: F1,15 = 0.053; P = 0.820; and absence of nicotine–tranylcypromine interaction: F1,15 = 0.002, P = 0.966).
Effects of nicotine in mice KO for the β2 nAChR subunit
To verify that locomotor effects of nicotine were related to the stimulation of nAChRs, locomotor activity was monitored in the presence of 10 mg/kg tranylcypromine in mice KO for the β2 subunit of nAChRs. As previously found (Villégier et al., 2005), tranylcypromine alone induced a significantly higher locomotor response in β2-nAChR-KO mice than in wild-type (WT) littermates and nicotine did not increase locomotor activity in β2-nAChR-KO mice pretreated with tranylcypromine (Fig. 3A). By contrast, nicotine decreased locomotor activity in β2-nAChR-KO tranylcypromine-treated animals during the 20 min following its injection (Fig. 3A; Villégier et al., 2005). Interestingly, the locomotor hyper-reactivity to tranylcypromine observed in β2-nAChR-KO compared with WT mice was paralleled by a hyper-reactivity to tranylcypromine for extracellular 5-HT levels, as shown by a significant tranylcypromine–genotype interaction (F1,12 = 7.742, P = 0.017) and tranylcypromine × genotype–time interaction (F11,132 = 1.894, P = 0.045) (comparison between 2, 3). Analysis of modifications in extracellular 5-HT levels in β2-nAChR-KO mice (Fig. 3B) indicated that a significant tranylcypromine effect was obtained (F1,10 = 14.338, P = 0.004), but no nicotine effect (F1,10 = 0.012, P = 0.915) or nicotine–tranylcypromine interaction (F1,10 = 0.024, P = 0.0879). Similarly, extracellular DA levels were significantly increased by tranylcypromine in β2-nAChR-KO mice (Fig. 3B), as shown by a significant tranylcypromine effect (F1,8 = 8.573, P = 0.019), but no nicotine effect (F1,8 = 0.053, P = 0.824) or nicotine–tranylcypromine interaction (F1,8 = 0.048, P = 0.832). This tranylcypromine-induced increase in extracellular DA levels occurred immediately after the tranylcypromine injection in KO mice, but appeared 30 min before occurring in WT mice, another indication of the hyper-reactivity of β2-nAChR-KO mice (Villégier et al., 2004, 2005). The great variability in the 5-HT and DA responses to tranylcypromine observed in β2-nAChR-KO mice (Fig. 3B and C) is probably related to a dysregulation of monoamine cell reactivity induced by the genetic deletion. Indeed, the basal extracellular 5-HT and DA levels were significantly higher in β2-nAChR-KO mice than in WT mice over 80 min (F1,32 = 87.88, P < 0.001; F1,30 = 41.44, P < 0.001, respectively).
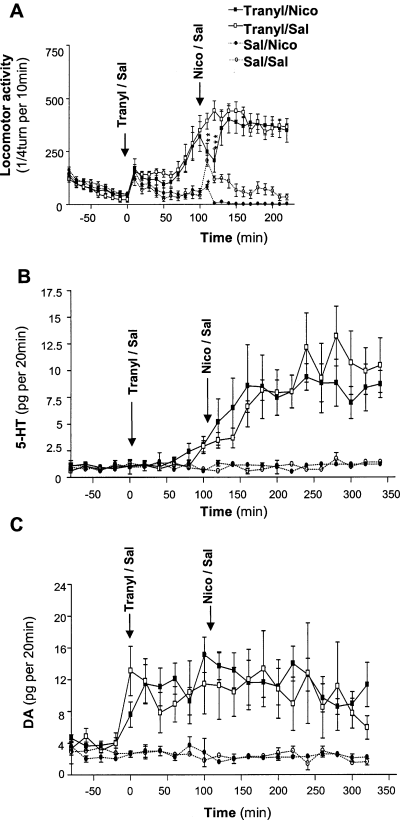
Effects of tranylcypromine on nicotine-induced locomotor response and extracellular 5-HT and DA levels in β2-nAChR-KO mice ventral striatum. (A) Locomotor response to nicotine in β2-nAChR-KO mice pretreated with tranylcypromine or saline is shown for comparison (Villégier et al., 2005). Animals were pretreated with tranylcypromine (10 mg/kg) or saline and received an injection of either saline or nicotine (1 mg/kg) 100 min later. *P < 0.05; **P < 0.01; ***P < 0.001 significantly different between tranylcypromine/nicotine- and tranylcypromine/saline-treated animals and between saline/nicotine- and saline/saline-treated animals. n = 8–9 mice per group. (B) Effects of tranylcypromine and nicotine on extracellular 5-HT levels in the ventral striatum. Animals were treated as in A and extracellular 5-HT levels were monitored. They are expressed as the mean ± SEM in pg 5-HT per 20 min. Basal extracellular 5-HT levels were 1.2 ± 0.2 pg 5-HT/20 min. No significant effect of nicotine treatment was detected. n = 4–6 mice per group. (C) Effects of tranylcypromine and nicotine on extracellular DA levels in the ventral striatum. Animals were treated as in A and extracellular DA levels were monitored. They are expressed as the mean ± SEM in pg DA per 20 min. Basal extracellular DA levels were 3.9 ± 0.4 pg DA/20 min. No significant effect of nicotine treatment was detected. n = 4–6 mice per group.
Discussion
This study indicates a critical role of 5-HT release for the development of a locomotor response to nicotine in mice. We show that, among three compounds which increase extracellular monoamine levels, only the one which releases 5-HT, i.e. PCA, triggers a locomotor response to nicotine.
GBR12783 is a specific inhibitor of DA reuptake that induces locomotor hyperactivity in mice (Bonnet & Costentin, 1986; Drouin et al., 2001). However, nicotine had no effect on this locomotor hyperactivity, indicating that DA is not a limiting factor for the absence of nicotine-induced locomotor response in mice. When animals were pretreated with d-amphetamine, nicotine induced an inhibition of locomotor hyperactivity. d-amphetamine is generally considered to be a DA releaser. However, it has been shown that d-amphetamine releases NA more potently than DA (Rothman et al., 2001). In our experimental conditions, we observed that, in mice, 2 mg/kg d-amphetamine increases extracellular NA levels by 300% in the frontal cortex, whereas the same dose of d-amphetamine increases extracellular DA levels by less than 50% in the nucleus accumbens (Auclair et al., 2002; Salomon et al., 2006). Moreover, d-amphetamine-induced locomotor hyperactivity and reward were shown to be dependent on noradrenergic signalling in mice (Drouin et al., 2002; Ventura et al., 2003). Therefore, we suggest that the inhibition of locomotor response induced by nicotine in amphetamine-treated animals is due to an excessive nicotine-induced depolarization of noradrenergic neurons (‘hyperdepolarization block’). Indeed, nicotine is known to be an enhancer of noradrenergic neuron activity (Engberg, 1989). Regardless, even if d-amphetamine releases both NA and DA, our data exclude NA as a limiting factor for the absence of nicotine-induced locomotor response in mice. Accordingly, the effect obtained with PCA and nicotine on locomotor activity strongly suggests that the release of 5-HT is a limiting factor in obtaining a nicotine-induced locomotor response. Nicotine is commonly considered to be a monoamine releaser (Summers & Giacobini, 1995; Summers et al., 1996) that increases serotonergic neuron firing (Li et al., 1998; Marubio et al., 1999; Olausson et al., 2001a, b, 2002). This increased release of 5-HT – in the absence of MAOI – is, however, transient. Indeed, an immediate inhibitory retro-control blocking of the firing of serotonergic raphe neurons through the stimulation of somato-dendritic 5-HT1A receptors has been described (Li et al., 1998; Mihailescu et al., 1998; Engberg et al., 2000). This may explain why, in the present study, injection of nicotine alone transiently decreases extracellular 5-HT levels in the ventral striatum. We therefore propose that PCA, because of its enhancing effect on extracellular 5-HT levels, compensates for the consequences of this indirect inhibition of serotonergic cells by nicotine. Indeed, there is some indication that the interruption of serotonergic transmission through 5-HT2A receptors can block the locomotor response to d-amphetamine and morphine (Sorensen et al., 1993; Moser et al., 1996; McMahon & Cunningham, 2001; Auclair et al., 2004).
The second finding of this study is that nicotine increases extracellular 5-HT levels in the ventral striatum in tranylcypromine-pretreated mice. Interestingly, this effect seems specific to extracellular 5-HT levels as no effect of nicotine on extracellular DA levels in the ventral striatum in animals pretreated with tranylcypromine was observed. However, our experimental conditions did not allow us to obtain reliable data regarding nicotine effects on extracellular NA levels in the ventral striatum, even in the presence of tranylcypromine. The absence of an effect of nicotine on extracellular 5-HT levels in β2-nAChR KO mice pretreated with tranylcypromine confirms that the effect we observe is linked to the stimulation of nAChRs. As found previously, the locomotor activity induced by tranylcypromine is increased in β2-nAChR KO mice when compared with WT mice. It cannot therefore be excluded that, in WT mice, nicotine desensitizes nAChRs containing β2 subunits. To date, 12 neuronal nAChR subunits have been cloned in mammals, eight of which (α3–7, β2–4) are expressed in rat dopaminergic nuclei neurons (Le Novere et al., 1996; Charpantier et al., 1998; Klink et al., 2001; Champtiaux et al., 2002). Three main types of heteromeric nAChRs (α4β2, α6β2 and α4α6β2) have been identified in dopaminergic terminal fields, whereas (non-α6)α4β2 nAChRs represent the majority of functional heteromeric nAChRs on dopaminergic neuronal somas and dendrites (Champtiaux et al., 2003). Finally, homomeric α7 and heteromeric α4β2 nAChRs located in the ventral tegmental area, respectively, on glutamatergic and GABAergic nerve somas and terminals were proposed to control the electrophysiological response to nicotine of mesencephalic dopaminergic neurons (Mansvelder & McGehee, 2000). Our data therefore indicate that at least β2-containing nAChRs are involved in nicotine-induced stimulant effects.
Nicotine by itself did not induce any increase in extracellular 5-HT and DA levels in the ventral striatum in WT and β2-nACh KO mice. Therefore, our data on DA levels disagree with those of Picciotto et al. (1998) and Maskos et al. (2005) who indicated a doubling of the basal extracellular DA value in mice ventral striatum following 0.5 mg/kg nicotine. However, their basal extracellular DA values were similar between WT and β2-nACh KO mice and were about five-fold higher than those found here. These discrepancies may be due to different experimental conditions. Indeed, these authors performed their experiments in mice under anaesthesia, whereas our microdialysis data were obtained in freely moving mice. It should also be recalled that, in freely moving rats, Cadoni & Di Chiara (2000)and Iyaniwura et al. (2001) have shown that nicotine does not increase extracellular DA levels in the core of the nucleus accumbens. It is therefore possible that our probe was largely located within the core of the ventral striatum.
Conclusion
Following a previous demonstration that mixed irreversible MAOIs allow locomotor and rewarding responses to nicotine, the present study indicates that these MAOI effects are due to a facilitation of the increase in extracellular 5-HT levels. Our data suggest a mechanism by which MAOIs contained in tobacco smoke could act in synergy with nicotine to induce addiction. Finally, the involvement of serotonergic systems in the stimulant and reinforcing effects of the association nicotine + MAOIs may improve our understanding of tobacco addiction mechanisms and suggest new therapeutic strategies to aid those wishing to quit smoking.
Acknowledgements
We thank Dr Sylvie Granon and Professor Jean-Pierre Changeux for their advice and their help in obtaining the KO mice.
Abbreviations
-
- 5-HT
-
- serotonin
-
- DA
-
- dopamine
-
- KO
-
- knockout
-
- MAOI
-
- monoamine oxidase inhibitor
-
- NA
-
- noradrenaline
-
- nAChR
-
- nicotinic acetylcholine receptor
-
- PCA
-
- para-chloro-amphetamine
-
- WT
-
- wild-type