Early-phase of learning enhances communication between brain hemispheres
Abstract
In the somatosensory system, inputs from one side of the body are only transmitted to the contralateral primary somatosensory cortex, but both sides of the body representation can interact via interhemispheric connections. These interactions depend on the behavioural requirements of the animal and its level of arousal. During the process of learning, alertness and attention may modify the responsiveness of neuronal pathways. We functionally mapped the brains of mice by using [14C]2-deoxyglucose (2DG) autoradiography during the first and the third session of a classical conditioning paradigm, involving whiskers stimulation on one side of the muzzle paired with an aversive or appetitive unconditioned stimulus. During the first pairing session, an increased 2DG uptake was seen in the barrel cortex of both hemispheres, independently of the type of applied unconditioned stimulus. In the third session of the sensory pairing, activation of the barrel cortex was solely contralateral, as expected after unilateral whisker stimulation. Thus, sensory stimulation directed to one cerebral hemisphere during the initial stages of Pavlovian conditioning activates the primary sensory area in both hemispheres. These results suggest that during the early phase of conditioning, when alertness is presumably strongest, the interhemispheric interactions are enhanced.
Introduction
Although tactile information from the ipsilateral body surface is only relayed to the contralateral primary somatosensory cortex, bilateral interactions via the corpus callosum are necessary for a unitary perception of the body surface across the midline (Pidoux & Verley, 1979; Olavarria et al., 1984). Callosal transfer of somatosensory information is also supported by results of electrophysiological studies in which ipsilateral activation of somatosensory cortex was eliminated by transection of the corpus callosum (Pidoux & Verley, 1979; Shuler et al., 2001; Wiest et al., 2004). In the somatosensory pathway from vibrissae to cortical barrels, unilateral lesions of the barrel cortex have been shown to influence spontaneous activity, responses to whisker stimulation and plasticity in the contralateral cortex (Rema & Ebner, 2003). However, a bilateral response to unilateral whisker activation was not observed in the brains of awake rodents mapped with 2-deoxyglucose (2DG) (Hand, 1982; Chmielowska et al., 1986; Sharp et al., 1988; Ginsberg et al., 1989), suggesting that the ipsilateral signal was not robust enough to be reflected in a significant elevation of 2DG uptake.
It has been reported previously that commissural integration can be modulated by the performance of a movement (Leocani et al., 2000), by the complexity of a behavioural task (Weissman & Banich, 2000; Passarotti et al., 2002) and by the level of arousal or alertness of the animal (Banich, 1998). Alertness is the initial response of the animal to salient stimuli during the early phase of conditioning. New stimuli evoke a general readiness that primes the brain in preparation for detecting and attending to biologically significant stimuli and initiating an appropriate response (Pavlov, 1927; Posner, 1978). ‘Arousal’ is defined by Sarter & Bruno (2000) as the activation of the forebrain in order to mediate processing of emotional, novel and/or stress-related information, and the initiation of adaptive responses. Arousal-induced attention is typically initiated by a very salient external stimulus (novelty, threat), triggering ‘low-level’ pre-attentional responses, including orientation responses, defensive reflexes and global search. We subjected mice to training in a Pavlovian conditioning paradigm involving stimulation of vibrissae and examined if the brain activation in the barrel field region (Woolsey & Van der Loos, 1970) in the initial stage of conditioning, when the stimuli are novel, differs from activation in the late phase of conditioning, when the stimuli are expected. Brain activation was estimated from 2DG autoradiograms (Sokoloff et al., 1977). We have shown previously that classical conditioning involving unilateral activation of vibrissae as a conditioned stimulus (CS) paired with an unconditioned stimulus (UCS) such as a mild tail shock results in an enlargement of the contralateral cortical area activated in response to vibrissae stimulation, accompanied by development of conditioned bradycardia (Siucinska & Kossut, 1996; Skibinska et al., 2001). We show here that the pattern of metabolic brain activation visualized with 2DG autoradiography changes in the course of training, with bilateral activation of the barrel cortex in response to unilateral whisker stimulation in the early phase of training changing to unilateral activation during the last session of sensory pairing.
Materials and methods
Fifty-two young adult (7–8 weeks old during training) Swiss albino and ten C57BL/6 mice were used in the present study. The mice were accustomed to a neck restraint by being placed in the apparatus for 10 min a day for 2–3 weeks prior to behavioural training. During this customization period, 10–15 droplets of water sweetened with sugar were given to the animal in the restrainer in order to accustom the animal to drink the sweet droplet even under this initially stressful context.
During training, all whiskers on one side of the snout were stroked (CS) with a hand-held fine brush in a posterior to anterior direction. The CS lasted 9 s and consisted of three strokes each one lasting 3 s. During the third second of the last deflection the appetitive (a droplet of sweetened water applied to the mouth with a syringe) or an aversive (a mild electric shock of 0.5 mA for 0.5 s to the tail) UCS was delivered. After a 6-s interval the trial was repeated. The training consisted of one or three sessions, one session per day. In 3-day training, session no. 1 and session no. 2 lasted for 10 min each and the third session lasted 20 min. In 1-day training the session lasted 20 min. Thus, mice whose brain activity was mapped during the 1st training session received 80 CS + UCS pairings and mice whose brain activity was mapped during the 3rd session received 40 + 40 + 80 = 160 total pairings. The session during which brain activity was mapped had to last 20 min, because this was the time needed for 2DG incorporation into the brain tissue.
During either the first (appetitive UCS, n = 5; aversive UCS, n = 5) or the third (appetitive UCS, n = 5; aversive UCS, n = 6) training session, [14C]2-deoxy-d-glucose (American Radiochemcial, specific activity 55 mCi/mmol), 10 mCi per mouse, was injected (i.p.) immediately before the first stimulation. The sessions lasted 20 min, and then the mice were killed with an overdose (0.2 mL per mouse) of Vetbutal (Biowet, Pulawy) and briefly perfused with 4% paraformaldehyde in phosphate buffer. Brains were removed, frozen in isopentane and coronally sectioned at 25 µm with a cryostat. The sections were collected onto gelatinized glass slides, dried on a hot plate and then exposed to X-ray Kodak mammography film together with sets of [14C] standards. After obtaining the autoradiograms, the sections were counterstained with cresyl violet in order to aid in the identification of the brain structures.
Additionally, mapping was performed during the first (n = 5) and third (n = 5) session of pseudoconditioning (unpaired presentation of stimuli) or during only the application of either the CS (1st session, n = 6; 3rd session, n = 5) or UCS (n = 5) (tail shock). Naïve controls were placed in the restraining apparatus for 20 min but received no stimulation (n = 5).
The autoradiograms were analysed with a computer-controlled image analysis system (Visionetics). The software allowed us to display on a computer screen the image of a stained section from which the autoradiogram was obtained, and to mark the outlines of regions of interest, which were superimposed on the autoradiogram so that the relationships between the labelled regions of the morphological structures could be accurately determined. The software measured the optical density (OD) within delineated regions of labelling determined by the experimenter. The files were coded and the experimenter did not know the identity of the sample.
OD measurements from the barrel field region and from reticular thalamic nucleus were obtained from seven sections per brain. The ratios of the OD values obtained for contralateral barrel cortex to ipsilateral barrel cortex (in control mice: right/left barrel cortex) were calculated for each section. A mean ratio was calculated for all sections. Additionally, the OD values for the white matter (fibres of thalamic radiation in both hemispheres, white matter of cerebral peduncles) and cortex situated away from the barrel field (frontal pole) were measured and used as a background intensity reference to normalize the data between experimental groups. All three reference structures yielded the same pattern of results and showed the same OD values in both hemispheres. We decided to use the OD values of the thalamic radiation, as they came from the same sections as the barrel fields. The results were expressed as a ratio of the structure of interest OD/reference structure OD. Statistical significance was determined using the Kruskal–Wallis non-parametric anova followed by the Mann–Whitney U-test.
Although the mice were placed in the neck-restraining apparatus during the training so they could not touch anything with their vibrissae, they could move their whiskers freely in the air. The time spent whisking was analysed and counted by video-recording the intensity of whisker movements during the first (n = 5) and the third (n = 5) sessions of CS + UCS (UCS − tail shock) training, and the first (n = 5) and third (n = 5) sessions using only the CS.
The behaviour of mice conditioned with appetitive UCS was video-recorded during all three sessions of the training and then analysed in order to monitor any characteristics of behaviour revealing a CS–UCS association.
Additionally, the heart rate was monitored during the training in three mice conditioned with aversive UCS and in the mice which received only UCS or no stimulation at all. Heart rate was measured for 30 s before the first trial in each session when the mouse was in the experimental set-up and during CS applications. Heart rate values were averaged for blocks of ten trials.
All experimental procedures were approved by the First Ethical Commission in Warsaw, Poland, and were in agreement with the UK Animals (Scientific Procedures) Act, 1986.
Results
Stimulation of all whiskers on one side of the muzzle (CS alone) resulted in an increased 2DG uptake in the contralateral barrel cortex; labelling was significantly higher than in ipsilateral barrel cortex (P < 0.01) and in control mice that did not receive whisker stimulation (Table 1). Following the unilateral stimulation of vibrissae the ratio of 2DG uptake in the barrel cortex/white matter was 12% higher (P < 0.01) than in the unstimulated controls.
| Group of mice | Optical density of 2DG uptake in the barrel cortex | |
|---|---|---|
| Ipsilateral hemisphere | Contralateral hemisphere | |
| No stimulation | 1.60 ± 0.16 | 1.60 ± 0.15 |
| UCS only | 1.56 ± 0.10 | 1.57 ± 0.10 |
| CS only, 1st session | 1.67 ± 0.06 | 1.79 ± 0.09 *** |
| CS only, 3rd session | 1.65 ± 0.03 | 1.77 ± 0.04 *** |
| Tail shock, 1st session | 1.89 ± 0.11 ***### | 1.88 ± 0.09 ***# |
| Tail shock, 3rd session | 1.61 ± 0.08 | 1.77 ± 0.16 * |
| Sweet water, 1st session | 1.82 ± 0.04 ***### | 1.83 ± 0.07 *** |
| Sweet water, 3rd session | 1.62 ± 0.08 | 1.72 ± 0.10 * |
| Pseudoconditioning, 1st session | 1.74 ± 0.11 *# | 1.76 ± 0.15 ** |
| Pseudoconditioning, 3rd session | 1.77 ± 0.14 **## | 1.85 ± 0.18 ** |
- * P < 0.05, vs. unstimulated control;
- ** P < 0.01, vs. unstimulated control;
- *** P < 0.001, vs. unstimulated control;
- # P < 0.05, vs. CS only, corresponding session;
- ## P < 0.01, vs. CS only, corresponding session;
- ### P < 0.001, vs. CS only, corresponding session.
During the first session of CS–UCS an increased 2DG uptake was seen in the barrel cortex of both the contralateral (C) and ipsilateral (I) sides (Fig. 1); it can be seen that either UCS (aversive or appetitive) gave similar results (Table 1). The ratio of barrel cortex/reference structure labelling for the tail shock group was: I, 1.89 ± 0.11; C, 1.88 ± 0.09 and for the sweetened water group: I, 1.82 ± 0.04; C, 1.82 ± 0.07. Although there were no significant differences between I and C barrel fields (Fig. 2) both hemispheres were significantly (P < 0.01) different from the unstimulated controls. The intensity of 2DG labelling during the first training sessions was significantly enhanced compared with sessions when just CS was applied (P < 0.01).
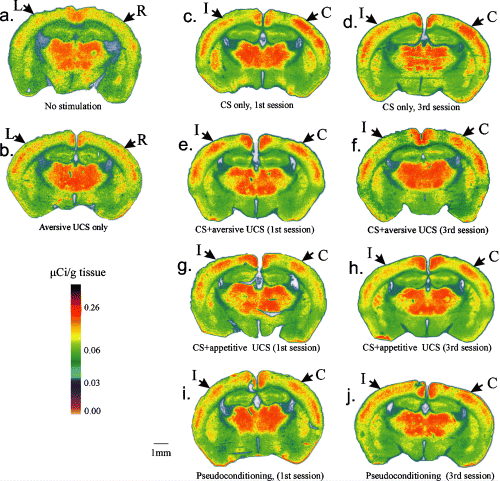
Functional activation of the barrel cortex during training. Digitized, pseudocolored autoradiograms are shown of the distribution of 2DG uptake on representative brain sections from all experimental groups. (a) Control mice without whisker stimulation; (b) application of tail shock only (UCS); (c) the 1st session of only vibrissae stimulation (CS) without UCS; (d) the 3rd CS session without UCS; (e) the 1st session of CS paired with a tail shock; (f) the 3rd session of CS–UCS (tail shock); (g) the first session of CS–UCS with a droplet of sweetened water; (h) the 3rd CS–UCS session (sweetened water); (i) the 1st session of pseudoconditioning; (j) the 3rd session pseudoconditioning. Arrows point to somatosensory barrel cortex; I, ipsilateral to stimulated vibrissae; C, contralateral to stimulated vibrissae; L, left hemisphere; R, right hemisphere.
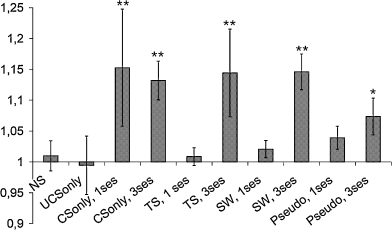
Ratios of the optical density of 2DG uptake in the contralateral/ipsilateral barrel cortex in all experimental groups. NS, no stimulation; TS, tail shock; SW, sweetened water; pseudo, pseudoconditioning. Mean ± SD, **P < 0.01, *P < 0.05, Mann–Whitney U-test. Significant differences denote unequal activation of the barrel cortex in the two hemispheres.
During the third training session the 2DG uptake differed from that observed during the first session (Fig. 1). In contrast to the first session, the intensity of 2DG labelling in the contralateral barrel field was significantly higher than that on the ipsilateral side, both with aversive or appetitive UCS; for the tail shock group the difference of 2DG uptake between contralateral and ipsilateral barrel field amounted to 14% (P < 0.01) and for the sweetened water group it was 15% (P < 0.01) (Fig. 2). The third training session produced slightly less activation in both barrel fields than the first session, irrespective of the type of UCS applied.
Application of the UCS alone did not produce enhanced labelling of the barrel cortex in either hemisphere (left or right) (ratio of 2DG uptake in the barrel field/reference structure was 1.56 ± 0.1 for the left and 1.57 ± 0.1 for the right hemisphere), and was similar to the labelling intensity in control, non-stimulated mice (right, 1.60 ± 0.15; left, 1.60 ± 0.16) (Table 1, Fig. 1).
Figure 2 shows the ratios of labelling in the contralateral to ipsilateral barrel field of OD readings within the same brain section. The contralateral/ipsilateral values were close to 1 in mice during the first session of sensory pairing (tail shock, 1.01 ± 0.01; sweetened water, 1.02 ± 0.01) demonstrating that labelling intensity in both hemispheres was equally strong. These ratios were greater than 1 for the mice during the third session of conditioning (tail shock, 1.14 ± 0.07; sweetened water, 1.15 ± 0.03); for the mice with unilateral whiskers stimulation without UCS (one session, 1.15 ± 0.09; three sessions, 1.13 ± 0.03) and for the mice during the third session of pseudoconditioning (1.07 ± 0.03). We found that they all were significantly higher than the ratio found in control, unstimulated animals (P < 0.05, Mann–Whitney U-test).
Random application of CS and UCS (pseudoconditioning) did not result in significantly stronger contralateral activation during either the first or the third pairing (normalized data: I, 1.74 ± 0.11; C, 1.76 ± 0.15 and I, 1.77 ± 0.14; C, 1.85 ± 0.18, respectively). In both sessions these ratios were significantly higher (P < 0.05) than for the unstimulated controls and for sessions when just CS was applied (see Table 1).
The non-normalized ratio of contralateral vs. ipsilateral barrel field 2DG labelling obtained for animals during the first session of pseudoconditioning (1.04 ± 0.02) was not significantly higher than for the control groups of mice (0.1 > P > 0.05; Mann–Whitney U-test) or for 2DG uptake during the third pseudoconditioning session (1.07 ± 0.03). The ratio of C/I labelling found for the third pseudoconditioning session differed significantly from that of the control, unstimulated mice (P < 0.05), but it was significantly lower (P < 0.05) than the C/I ratio in the third conditioning session.
2DG uptake in the reticular thalamic nucleus was measured to provide an index of activation in structures involved in attentional processes. Labelling intensity of the thalamic reticular nucleus was significantly higher in the contralateral hemisphere than in the ipsilateral hemisphere in all groups of animals except control groups (unstimulated control and application of UCS alone) (P < 0.05, Wilcoxon test).
Average 2DG uptake in the thalamic reticular nucleus (Rt) of both hemispheres was significantly lower during the 3rd session than the 1st session of both appetitive (1st session, 1.44 ± 0.06; 3rd session, 1.38 ± 0.02) and aversive conditioning (1st session, 1.56 ± 0.06; 3rd session, 1.45 ± 0.1) (P < 0.05, Mann–Whitney U-test) (Fig. 3). Labelling intensity was significantly lower in control mice (unstimulated control, 1.29 ± 0.05; application of UCS alone, −1.32 ± 0.05) in comparison with other groups of mice and also significantly higher during the 1st training session with aversive UCS in comparison with the remaining groups of animals (Fig. 3).
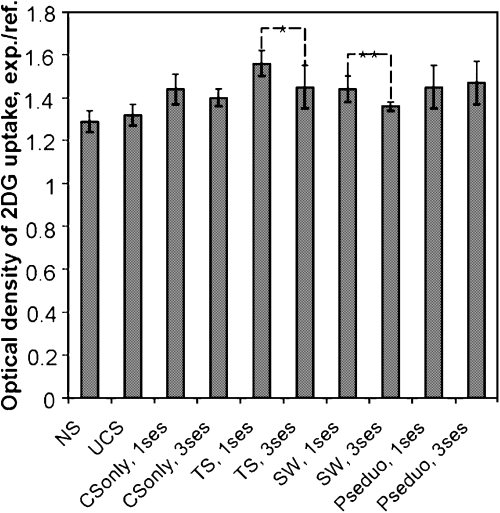
Ratios of optical density of 2DG uptake in the reticular thalamic nucleus/reference structure in groups of animals conditioned with appetitive and aversive UCS in one or three sessions. NS, no stimulation; TS, tail shock; SW, sweetened water; pseudo, pseudoconditioning. Significant differences between sessions are marked. Mean ± SD, **P < 0.01, *P < 0.05, Mann–Whitney U-test.
There were no statistically significant differences in the percentage time spent whisking (Fig. 4) between all analysed groups of mice (P > 0.1; anova), so we can exclude the possibility that increased whisker mobility during the first pairing session contributed to the higher metabolic labelling of ipsilateral barrel field.
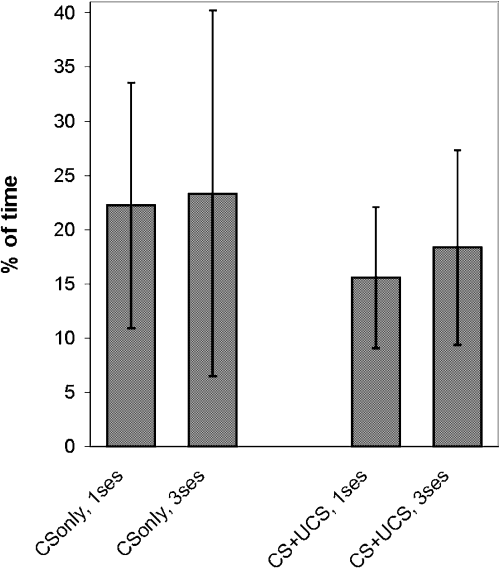
Percentage of training session time spent whisking. No statistically significant differences were seen between any of the analysed groups of mice (P > 0.1; anova). No statistically significant differences were observed between mice that were conditioned (CS + UCS) and mice that were exposed to CS only.
In the group of mice that received appetitive UCS, behaviour during the first pairing session varied considerably – one mouse bit the syringe, two readily turned their head towards it, and two did so only occasionally (in 15% of trials). In the progress of training, mice became calmer and turned their heads towards the stimulation or towards the syringe, especially during the last 3 s of CS application as if they predicted the occurrence of UCS. During the third training session head turning towards the syringe was observed on average in 60% of trials. All of the mice licked the offered drops of sweetened water until the end of the last phase of training.
Monitoring of heart rate in the group of animals trained with aversive UCS showed that during the first ten pairings of stimuli, heart rate increased (on average by 4.7 ± 1.9%) comparing with that immediately before the training session. In the course of training, during the second or third session, slowing of the heart rate during application of the CS was observed. In comparison with the heart rate taken before each training session the average deceleration was 5 ± 2%. The mice that received only UCS or no stimulation at all showed only an acceleration of heart rate.
Discussion
We have shown that during the first CS–UCS pairing session the barrel cortex was activated strongly and equally in both hemispheres by unilateral whisker stimulation. In addition, pairing of vibrissae stimulation with different unconditioned stimuli (appetitive or aversive) caused the same result. This effect was present during the first session only. By contrast, during the third pairing session contralateral 2DG uptake was much stronger than that observed ipsilaterally. Application of an aversive UCS (tail shock) alone did not produce activation of the barrel cortex in either hemisphere. As there were no differences in the proportion of time spent whisking during the first and third sessions of conditioning, we can exclude the possibility that the bilateral cortical activation is related to an increase in whisking behaviour. The behaviour of mice conditioned with the appetitive stimulus, indicating that they learned to predict the occurrence of the UCS, and also the conditioned bradycardia observed in mice trained with an aversive stimulus – an observation confirming previous results obtained in our laboratory (Siucinska & Kossut, 1996) – seems to suggest that the CS–UCS associations actually developed during the training. Thus, we have observed a transient phenomenon, associated with information processing in the brain during the first conditioning session.
The bilateral activation of the barrel cortex at the beginning of sensory association training was surprising, in view of the numerous previous reports with 2DG documenting only contralateral activation of the barrel cortex after unilateral whisker stimulation (Hand, 1982; Chmielowska et al., 1986; Sharp et al., 1988; Ginsberg et al., 1989; Siucinska & Kossut, 1996). It should be stressed, however, that in contrast to previous studies we mapped brain activity during conditioning and not after conditioning (Siucinska & Kossut, 1996) or during simple sensory stimulation (Chmielowska et al., 1986; Sharp et al., 1988). Electrophysiological studies have demonstrated the existence of ipsilateral activation of infragranular cortical layers, albeit with a longer latency and lower amplitude, which could be eliminated by sectioning of the corpus callosum (Pidoux & Verley, 1979; for rat see Shuler et al., 2001; Wiest et al., 2004). Intracellular recordings from layer V pyramidal cells also showed that ipsilateral stimulation evoked postsynaptic potentials of smaller and longer latency than contralateral stimulation (Manns et al., 2004). In contrast to the interhemispheric inhibition observed in the motor cortex (Warbrooke & Byblow, 2004), callosal transmission in the somatosensory cortex appears to be predominantly excitatory (Innocenti et al., 1972; Shin et al., 1997). Callosal axons make excitatory synapses in the opposite sensory cortex (Sloper & Powell, 1979), some of which project upon inhibitory interneurons (Somogyi et al., 1983), so the corpus callosum is a source of tonic input to inhibitory interneurons. Excitatory callosal effects were shown to be direct (monosynaptic) and inhibitory effects to be indirect (disynaptic) (Feeney & Orem, 1971; Innocenti, 1980). We have shown that during the initial phase of learning, during the first conditioning session, interhemispheric activation is significantly enhanced. The function of such interhemispheric interactions has been related to the ability to transfer information and learning between the hemispheres. The transfer of tactile learning to whisker position on the opposite side of the snout via callosal projections was shown in a barrel cortex-dependent task (Harris & Diamond, 2000). Cortical integration of ipsilateral and bilateral whisker stimuli is also crucial for solving a tactile discrimination task, which requires the integration of left and right whisker-mediated information (Shuler et al., 2001). It has been shown that monkeys with commissural transection fail to transfer hand tasks learned with the other hand, whereas normal monkeys successfully perform such tests (Ebner & Myers, 1962). Semmes & Mishkin (1965) reported that unilateral cortical ablation of the sensorimotor region in monkeys impairs ipsilateral, as well as contralateral, sensory functions tested by tactile discriminations.
In psychological studies it was postulated that interhemispheric interactions are more enhanced while performing attentionally demanding complex tasks than when performing simple tasks and are used as a general strategy to increase computational resources (Banich, 1998; Passarotti et al., 2002). Within-hemispheric processing is thought to become less efficient than across-hemisphere processing as task complexity increases, regardless of the modality (tactile, auditory or visual) in which information is presented (Weissman & Banich, 2000; Passarotti et al., 2002). Additionally, results obtained with electrophysiological recordings suggest that neural activity in primary somatosensory cortex is under the direct influence of attentional processes (Iriki et al., 1996). Our results seem to be in accordance with these observations. Classical conditioning involving unilateral whisker stimulation, which is an attentionally demanding task, evoked bilateral barrel cortical activity in the early stages of learning, whereas the less complex situation involving unilateral CS whisker stimulation caused only unilateral cortical activity. The transition from bilateral to unilateral activation of the barrel cortex (sessions 1–3) occurs with either an aversive or an appetitive UCS, indicating that the emotional context of learning does not seem as important to this process as the influence of attention and high level of alertness during the first training session. We found that the first session produced a significantly higher density of contralateral 2DG labelling than that observed with just the CS. This was not just due to the addition of the UCS, but also to a behavioural situation created by pairing the sensory stimuli, because the UCS alone did not activate the barrel cortex. When the association is well established, the level of alertness lessens.
The pseudoconditioning experiments were carried out in order to estimate the importance of the association between CS and UCS to interhemispheric interactions. Pseudoconditioning is a non-associative modification of behaviour in which application of a UCS changes the quality of responses to stimuli other than the UCS, transforming these responses into ones resembling the unconditioned response (Rescorla, 1967). Thus, pseudoconditioning is a kind of generalized sensitization and an elicited response that appears to be a conditioned response is actually the result of sensitization rather than conditioning.
We found that both the first and the third session of pseudoconditioning activated the contralateral and ipsilateral barrel field, in contrast to the change from strong to weak bilateral activation seen in the CS + UCS groups. It is very likely that the effect we ascribe to arousal was reflected in the results during both pseudoconditioning and the initial CS–UCS conditioning. The lack of laterality seen with the pseudoconditioning may reflect the fact that the animals cannot form an association between the stimuli and therefore maintain an attentional window that is similar to the initial learning process. Learning the sensory association may direct attention towards the signalling stimulus.
Measurements of labelling of the reticular nuclei of the thalamus were obtained in order to analyse activation during the training of a structure that is known to be influenced by attention. 2DG uptake in the thalamic reticular nucleus in our study was significantly lower during the third session compared with the first session of both appetitive and aversive conditioning. The thalamic reticular nucleus receives collaterals from both thalamocortical and corticothalamic fibres (Jones, 1975; Guillery & Harting, 2003) and therefore it is in a position to gate the flow of information between thalamus and cortex. It is involved in the processing of sensory information (Hartings et al., 2000) but this processing is modulated by attention (for a review see Guillery et al., 1998). McAlonan et al. (2000) argued that the reticular thalamic nucleus acts as an attentional gate or filter and in that view our Rt labelling results support our presumption that alertness and attentional processing are a possible basis for the different barrel cortex activation patterns in early and late sessions of the training.
Cortical responses to somatosensory stimuli are often suppressed when the sensory information (SI) is irrelevant to a given task (sensory gating), or facilitated when relevant for task performance. Staines et al. (2002) found that both contralateral and ipsilateral SI activity levels depend on stimulus relevancy: task-relevant stimuli increased activation in the contralateral SI compared with passive stimuli. Within ipsilateral SI, activation in the homologous region diminishes during task-relevant stimulation. Meyer et al. (1991) provided evidence for attention-induced enhancement of activity in regions of human primary sensory cortex. It was also shown using positron emission tomography that there was a significant decrease in ipsilateral somatosensory cortex blood flow of the human brain to anticipated toe stimulation (Drevets et al., 1995). This phenomenon might be related to the lack of ipsilateral cortical activation observed in the third session of conditioning, when the initially unimportant stimulus becomes predictive for the UCS, compared with the bilateral activation during the first session.
The effect observed in our experiment during the initial stage of learning, when the animal is very alert and expectant, is no longer observable later, after sensory association is established. The early steps of arousal-induced attentional processes differ from the processing that underlies the more habitual, well-practised attentional performance (for a review see Sarter & Bruno, 2000). Similarly, transient effects of alertness were recently described for sensory adaptation during learning (Castro-Alamancos, 2004). During learning of a behavioural task and processing sensory inputs, sensory adaptation is mostly absent. After learning appears and the task becomes routine, the level of alertness lessens and sensory adaptation becomes present (Castro-Alamancos, 2004). It is known from human neurorehabilitation studies that alertness exerts a powerful influence upon the interhemispheric coordination; for example, non-spatial phasic alerting can overcome deficits in spatial awareness in patients with unilateral neglect caused by injury to one hemisphere (Robertson et al., 1998).
Our results, showing a transient modification of activity within a sensory pathway that is involved in the learning process, demonstrate that remodelling of the pattern of interhemispheric integration can be influenced by behavioural contingencies.
Acknowledgements
This study was supported by McDonnel Pew Award no. 99-56 and grants from the Foundation for Polish Science and State Committee for Scientific Research to M.K. We thank Prof. Colin Blakemore in whose lab some of the experiments were conducted and Mrs Renata Zakrzewska for skilled technical assistance.
Abbreviations
-
- 2DG
-
- 2-deoxyglucose
-
- CS
-
- conditioned stimulus
-
- OD
-
- optical density
-
- Rt
-
- thalamic reticular nucleus
-
- SI
-
- sensory information
-
- UCS
-
- unconditioned stimulus