Lack of localization of 5-HT6 receptors on cholinergic neurons: implication of multiple neurotransmitter systems in 5-HT6 receptor-mediated acetylcholine release
Abstract
The involvement of the cholinergic system in learning and memory together with the cognitive enhancing properties of 5-HT6 receptor antagonists led us to study the relationship between 5-HT6 receptors and cholinergic neurotransmission. A selective cholinergic lesion, induced by injection of the immunotoxin 192-IgG-Saporin into the nucleus basalis magnocellularis, failed to alter the density of 5-HT6 receptor mRNA or protein expression in the deafferentated frontal cortex, suggesting that 5-HT6 receptors are not located on cholinergic neurons. The 5-HT6 receptor antagonist SB-357134 (0.001–1 µm) induced a concentration-dependant K+-evoked [3H]acetylcholine (ACh) release in vitro in rat cortical and striatal slices, which was blocked by tetrodotoxin. SB-357134, up to 1 µm, stimulated glutamate release in cortical and striatal slices. In the cortex, riluzole (1 µm) blocked the SB-357134-induced K+-stimulated [3H]ACh release, and simultaneous administration of MK-801 (1 µm) and SB-357134 (0.05 µm) elicited an increase in K+-evoked ACh release. In the striatum, SB-357134, 1 µm, decreased dopamine release, and the increase in K+-evoked [3H]ACh release induced by 5-HT6 receptor blockade was reversed by the D1 receptor antagonist, SCH23390 (1 µm). In both the frontal cortex and striatum, bicuculline, 1 µm, showed no effect on SB-357134-evoked [3H]ACh. These results are discussed in terms of neurochemical mechanisms involved in 5-HT6 receptor functions.
Introduction
The 5-HT6 receptor is the most recently identified of the serotonin (5-HT) receptor superfamily (Monsma et al., 1993; Ruat et al., 1993). 5-HT6 receptor expression is widespread, with the highest levels found within the olfactory tubercle, striatum, nucleus accumbens, cerebral cortex and hippocampus (Monsma et al., 1993; Ward et al., 1995). These receptors are present within 5-HT projection fields and not in serotonergic neurons of the raphe, indicating a probable postsynaptic role for these receptors (Ward et al., 1995; Gérard et al., 1997).
Although controversial (Lindner et al., 2003), a role for 5-HT6 receptors in memory and learning is emerging. In the Morris water-maze task, 5-HT6 receptor antagonists, such as Ro 046790, SB-271046 and SB-357134, significantly improved cognitive function (Rogers & Hagan, 2001; Woolley et al., 2001; Stean et al., 2002). Furthermore, Ro 046790 reverses scopolamine-induced deficits in a passive-avoidance task (Bos et al., 2001) and in an operant autoshaping task (Meneses, 2001).
It has been suggested that the effects of 5-HT6 receptor antagonists in cognition could be mediated, at least in part, by a modulation of the cholinergic activity (Woolley et al., 2004). Although the localization of 5-HT6 receptors on cholinergic terminals has not been demonstrated yet, it has been shown that 5-HT6 receptor antagonists increased acetylcholine (ACh) release in vivo both in the rat hippocampus (Shirazi-Southall et al., 2002) and cortex (Riemer et al., 2003).
Other neurotransmitter systems have also been shown to be associated with 5-HT6 receptor function, such as the glutamatergic and dopaminergic system (Dawson et al., 2000, 2001; Lacroix et al., 2004), which may be linked with the cognitive effects of 5-HT6 receptor antagonists. In addition, atypical anti-psychotics with high affinities for 5-HT6 receptors enhance glutamate levels in the frontal cortex (Daly & Moghaddam, 1993), and this neurochemical effect has been proposed to contribute, at least in part, to the efficacy of these drugs to treat the cognitive dysfunction of schizophrenia (Tollefson, 1996). It has also been suggested that 5HT6 receptors may be expressed on γ-aminobutyric acid (GABA)ergic spiny neurons of the striatum (Gérard et al., 1997). Recent data showed co-localization of glutamic acid decarboxylase immunoreactivity with 5-HT6 receptors in the cerebral cortex and hippocampus (Woolley et al., 2004). Together, these data suggest that 5-HT6 receptor antagonists may modulate cholinergic and/or glutamatergic systems via disinhibition of GABAergic neurons.
The present work aims to further investigate the relationship between 5-HT6 receptors and cholinergic neurotransmission. (1) To check a purported localization of 5-HT6 receptors on cholinergic neurons, we have measured changes in the density of 5-HT6 receptor mRNA and protein expression in the frontal cortex of rats following a selective cholinergic lesion. (2) Considering the suggested involvement of the glutamatergic, dopaminergic or GABAergic systems in 5-HT6 receptor-mediated cholinergic effects, we have measured glutamate, dopamine (DA) and GABA release after 5-HT6 receptor blockade in slices from rat frontal cortex and striatum. The effect of antagonists acting at N-methyl-d-aspartate (NMDA), AMPA, D1, D2 and GABAA receptors on the 5-HT6 receptor-mediated effects on ACh release was also studied.
Materials and methods
Materials
SB-357134 (N-2, 5-dibromo-3-fluorophenyl-4-methoxy-3-piperazin-1-ylbenzenesulphonamide) and SB-271046 (5-chloro-N-(4-methoxy-3piperazin-1-yl-phenyl)-3-methyl-2-benzothiophenesulphon-amide) and [125I]-SB-258585 (4-iodo-N-[4-methoxy-3-(4-methylpiperazin-1-yl)phenyl]benzene-sulphonamide) were generously provided by GlaxoSmithKline, Harlow, UK. 192-IgG-Saporin was from Chemicon International (USA). SCH23390 [R(+)-7-chloro-8-hydroxy-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride], NBQX (2,3-dioxo-6-nitro-1,2,3,4-tetrahydrodibenzo[f]quinoxaline-7-sulphonamide), MK801 (dizolcipine), riluzole and tetrodotoxine citrate were from Tocris, UK. Haloperidol was obtained from Syntex-latino, Spain. Bicuculline, hemicholinium-3, acetyltiocholine iodide, o-phthalaldehyde and α-mercaptoethanol were from Sigma, USA. [Methyl-3H] choline chloride (86 Ci/mmol) was purchased from Perkin Elmer, USA. αS[35S]dATP was from Amersham Biosciences, UK. Terminal deoxynucleotide transferase was from Roche, Germany. Inorganic salts and other reagents were from Panreac and Merck.
Animal housing
Male Wistar rats weighing 230–250 g were used. Animals were kept at constant room temperature (21 ± 1 °C) and relative humidity (55 ± 5%), with a 12 h light : dark cycle (dark from 20.00 h) and free access to food and water. All the experiments were carried out in strict compliance with the recommendations of the EU (DOCE L 358/1/18/2/1986) for the care and use of laboratory animals, and were approved by the Ethical Committee of the University of Navarra.
Surgery and characterization of cholinergic lesions
All surgical procedures were conducted as previously reported (Gil-Bea et al., 2004). Briefly, rats anaesthetized with a mixture of ketamine and xylazine were placed in a stereotaxic frame (Kopf, USA), with the incisor bar set 3.5 mm below the interaural line. One microlitre of the immunotoxin 192-IgG-Saporin (0.067 µg/µL/hemisphere) was infused bilaterally into the nucleus basalis magnocellularis (NBM) of the basal forebrain at the following coordinates (from bregma): AP −0.9 mm, ML ± 2.9 mm, DV −6.5 mm, according to the atlas of Paxinos & Watson (1982). Post-lesion survival times were established at 7 days.
To assess the extent of the lesion, acetylcholinesterase (AChE) and choline acetyltransferase (ChAT) activities were measured in the frontal cortex as described in Gil-Bea et al. (2004).
In situhybridization for 5-HT 6 receptor mRNA
The oligonucleotide complementary to the coding sequence of rat 5-HT6 mRNA used was: 5′-GGTGGCCCTGGTGGGTGGCGGGGGGTCCCGTGTGGCCTCTCCAG-3′ (Sigma Genosys, UK). The probe was 3′-tail labelled with αS[35S]dATP (specific activity > 1000 Ci/mmol). Negative controls including sense oligonucleotide showed minimal background signals. Sections were exposed to Biomax MR film (Kodak) for 21 days at 4 °C The relative abundance of 5-HT6 mRNA was determined by densitometry quantification of autoradiograms using the Microcomputer Imaging Device (Imaging Research, St Catherines, Ontario, Canada) corrected for non-specific signals. Densitometric values from three sections of each animal were averaged and expressed as nCi/g tissue.
5-HT6 receptor autoradiography
5-HT6 receptor quantitative autoradiography studies were performed as described by East et al. (2002); the rats were killed by cervical dislocation and brain sections of 14 µm at the level of the frontal cortex and striatum were used. The 5-HT6 receptor was labelled with 1 nm of the selective radioligand [125I]-SB-258585 according to previous binding studies (Hirst et al., 2000). Adjacent sections were used to measure non-specific binding, in the presence of 10 µm methiothepin. Slides were apposed to [3H]-Hyperfilm (Amersham, Little Chalfont, UK) for 4 days at 4 °C with [125I] standards. Autoradiograms were quantified using an MCDI image analysis system (Imaging Research), and densitometric measurements of the frontal cortex and striatum were taken. The average value of the duplicate sections was taken and the non-specific signal subtracted. Results were expressed as nCi of [125I] per mg of wet tissue.
ACh release in vitro
K+-evoked [3H]ACh efflux was measured as previously described (Ramírez et al., 1996). Briefly, the animals were killed by cervical dislocation, frontal cortex and striatum were dissected out and cross-chopped into 350-µm slices (frontal cortex), or cut sagittally into 500-µm slices (striatum) using a McIlwain tissue chopper (The Mickle Laboratory Engineering Co.). Slices were incubated with gassed (95% O2/5% CO2) Krebs–Ringer bicarbonate buffer (KRB) made up of (in mmol/L): NaCl, 113; KCl, 4.75; CaCl2, 2.52; MgSO4, 1.19; NaH2PO4, 1.18; NaHCO3, 25; glucose, 10; pH, 7.4. The KRB was replaced by one containing 40 mm KCl (osmolarity was maintained by reducing the NaCl concentration) and slices were shaken at 37 °C for 20 min, to induce a massive endogenous neurotransmitter release. Slices were labelled by incubation for 40 min at 37 °C with [3H]choline (3 µL/mL, 81 Ci/mmol) in KRB.
Aliquots (100 µL) of packed slices (frontal cortex) or three–four slices (striatum) were immediately added to each chamber of a Brandel Superfusion-1000 apparatus. Tissues were perfused continuously with oxygenated (95% O2/5% CO2) KRB containing 1 µm hemicholinium-3 to inhibit reuptake of [3H]choline during the experiment, at a rate of 0.35 mL/min. After a 40-min equilibration period, fractions were collected at 3-min intervals for a total of 60 min.
[3H]ACh release was estimated from the outflow of tritium into the superfusate, as it is generally accepted that under the present experimental conditions, tritium overflow reflects the actual [3H]ACh released (Beani et al., 1984).
At 12 min (S1) and 45 min (S2) after the equilibration period, the slices were depolarized by changing the superfusion fluid for 6 min to a KRB solution containing 20 mm KCl. All drugs to be tested were added 15 min before S2. Tritium content was assayed by a liquid scintillation spectroscopy (Rackbeta 1214, LKB-Wallack, Finland). S1 and S2 were calculated as K+-stimulated tritium increase over basal efflux. Results are expressed as S2/S1 ratio.
Glutamate, DA and 5-HT release in vitro
Cortical and striatal slices were prepared as described above. Slices were allowed to equilibrate for 20 min at room temperature in gassed KRB. Tissue was then transferred into superfusion chambers (Brandel Superfusion-1000 apparatus) and continuously superfused with continuously oxygenated KRB at a rate of 0.35 mL/min. After a 40-min equilibration period, fractions were collected at 5-min intervals for a total of 40 min (eight samples). The first four samples were used to assess a stable basal neurotransmitter release (B1–B4). Slices were depolarized by changing the superfusion fluid for 5 min (S1) to a KRB solution containing 30 mm KCl. The 5-HT6 receptor antagonist SB-357134 was added 10 min before S1. Neurotransmitter levels were measured in the superfusate fractions.
Glutamate and GABA were measured using high-performance liquid chromatography (HPLC) with electrochemical detection (Waters Spheribor® 5µ ODS2 4.6 × 150 mm) including pre-column derivatization with o-phthaldehyde and β-mercaptoethanol (Roettger & Goldfinger, 1991). The mobile phase consisted of 72 : 28 (v/v) mixture of buffer (NaH2PO4 0.1 m, pH 5.5) and methanol; the mixture was filtered and degassed through a 0.22-µm nitrocellulose membrane. Glutamate and GABA content were calculated by comparing with a 2 ng standard. The limit of detection was 20 pg/10 µL for glutamate and 50 pg/10 µL for GABA content.
DA content was also measured by HPLC with electrochemical detection (Waters Spheribor® S10 0DS2 4.6 × 150 mm). The mobile phase consisted of 80 : 16 (v/v) mixture of buffer (KH2PO4 · 2H2O 0.1 m, citric acid 0.1 m, EDTA 1 mm and octanosulphonic acid 0.74 mm; pH 3) and methanol; the mixture was filtered and degassed through a 0.22-µm nitrocellulose membrane. DA content was calculated by comparing with a 0.5 ng standard. The limit of detection was 1 pg/10 µL.
5-HT levels were determined by HPLC with electrochemical detection. 5-HT content was calculated by comparing with a 1 ng standard. The limit of detection was 1 pg/10 µL.
Data analysis
Different concentrations of the 5-HT6 receptor antagonists were tested in their ability to release [3H]ACh in vitro. The concentration–response curves were constructed, and the EC50 values calculated using a non-linear regression program to fit the data to a sigmoid curve (Graph Pad Prism v3.0).
K+-evoked glutamate and DA release after 5-HT6 receptor blockade was expressed as amount measured in the superfusate fraction with the highest value sampled during K+ stimulation.
Normality was checked by Shapiro–Wilk's test (P > 0.05) before any other statistical analysis. Statistical differences were determined by Student's t-test or one-way anova followed by Tukey's test.
Unless stated, data are presented as mean ± SEM of n = 8–10.
Results
Effect of a cholinergic lesion on 5-HT6 receptor density in the frontal cortex
Injection of 192-IgG-Saporin into the NBM caused an overall decrease in the activity of the cholinergic markers in the frontal cortex. AChE and ChAT levels were significantly reduced to 52.89 ± 4.25% and 50.96 ± 4.40% of controls (n = 10–15, one-way anova, F3,33 = 19.46, P < 0.001, and F3,117 = 109.46, P < 0.001, respectively).
As illustrated in Fig. 1, both 5-HT6 receptor mRNA and protein expression in the frontal cortex was not affected by a selective cholinergic in the NBM. Thus, densitometry analysis of data revealed no significant differences (Student's t-test) in 5-HT6 mRNA expression in lesioned animals in the frontal cortex (182.95 ± 5.30 vs. 212.71 ± 18.70 nCi/mg, mean ± SEM, n = 4–6). Similarly, [125I]-SB-258585 binding in the frontal cortex of 192-IgG-Saporin-lesioned rats was not significantly (Student's t-test) different from controls (16.26 ± 4.38 and 17.75 ± 5.46 nCi/mg, respectively, mean ± SEM, n = 3–4). The density of 5-HT6 receptors in the striatum, an area not affected by the lesion, was not modified either (data not shown).
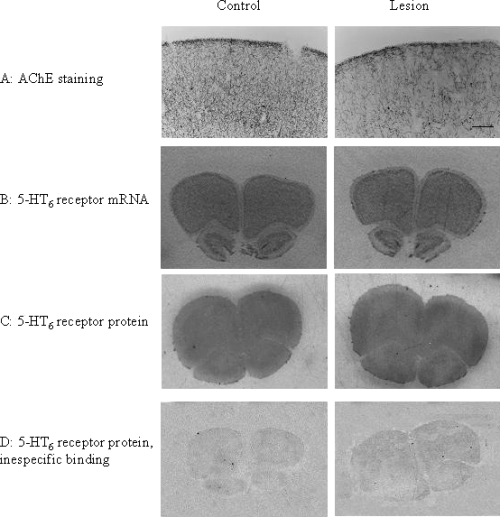
Effects of a cholinergic lesion on the levels of 5-HT6 receptor mRNA and protein expression, and acetylcholinesterase (AChE) staining in the frontal cortex. (A) AChE staining showing the cholinergic deafferentation in the lesioned animals (right panel) compared with controls (left panel). Quantification of AChE activity, see Results, indicated this reduction to be approximately 50%. (B–D) Autoradiograms of sections hybridized with 5-HT6 antisense mRNA probes (B), [125I]-SB-258585 binding site densities (C), and non-specific binding (D) in the frontal cortex of control (left panel) and cholinergic lesioned (right panel) rats. No differences in the density of 5-HT6 receptor mRNA or protein expression were found. Scale bar in A, 400 µm (A); 1.3 mm (B–D).
Effect of 5-HT6 receptor blockade on K+-evoked [3H]ACh release from striatal and cortical slices
In slices from rat frontal cortex and striatum, the 5-HT6 receptor antagonist SB-357134 (0.01–1 µm) produced a significant (one-way anovaF4,60 = 2.542, P < 0.05, and F4,59 = 2.589, P < 0.05, respectively) concentration-dependent increase in K+-evoked [3H]ACh efflux. EC50 values were estimated from the linear ascending portion of the concentration–response curves (Fig. 2), and were 0.05 µm (frontal cortex) and 0.01 µm (striatum).
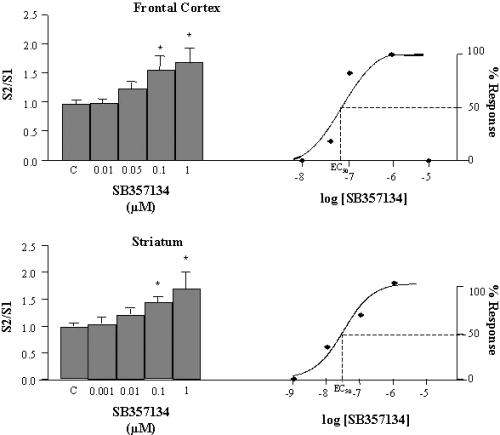
Effect of the 5-HT6 receptor antagonist SB-357134 on the K+-evoked [3H]ACh release in slices from rat frontal cortex (upper panel) and striatum (lower panel). The EC50 values have been calculated using a non-linear regression program to fit the data to a sigmoid curve. C, control. Values are means ± SEM from 12–15 experiments. *Significant difference (P < 0.05) vs. control.
To confirm that the effects of SB-357134 on ACh release were receptor and not compound dependent, the effect of SB-271046, another 5-HT6 receptor antagonist, was also studied. In slices from rat frontal cortex and striatum, SB-271046 (0.01–1 µm) was able to significantly (one way anova, F3,60 = 2.554, P < 0.05, and F3,51 = 3.192, P < 0.05, respectively) increase the K+-evoked [3H]ACh efflux in a concentration-dependent manner (S2/S1 ratio was 0.92 ± 0.05, 0.98 ± 0.12, 1.27 ± 0.16 and 1.39 ± 0.20 for control and SB-271046, 0.01, 0.1 and 1 µm, respectively, in the cortex and 1.00 ± 0.05, 1.15 ± 0.15, 1.38 ± 0.12 and 1.36 ± 0.13 in the striatum). The EC50 value estimated from the concentration–response curves was 0.05 µm for both the frontal cortex and striatum.
Tetrodotoxin (TTX, 1 µm), completely blocked the K+-evoked, SB-357134 (present at EC50 concentrations)-mediated [3H]ACh release in cortical (one-way anova, F3,72 = 14.871, P < 0.01, n = 12–15) and striatal slices (one-way anova, F3,43 = 10.586, P < 0.01, n = 12–15). In the frontal cortex, the S2/S1 ratio was 1.06 ± 0.13, 0.66 ± 0.10, 1.20 ± 0.10 and 0.58 ± 0.03 for control, TTX, SB-357134 and TTX + SB-357134, respectively; and 0.93 ± 0.17, 0.71 ± 0.15, 1.39 ± 0.10 and 0.8 ± 0.09 in the striatum.
Effect of 5-HT6 receptor blockade on K+-evoked release of glutamate, GABA, DA and 5-HT from striatal and cortical slices
In slices of rat frontal cortex and striatum, the 5-HT6 receptor antagonist SB-357134 (0.01 and 1 µm) produced significant increases in K+-evoked glutamate release compared with control values (one-way anova, F2,22 = 4.341, P < 0.05 and one-way anova, F2,26 = 7.132, P < 0.01, respectively).
GABA levels were not modified by application of SB-357134 (0.01 and 1 µm) in the frontal cortex. SB-357134 (0.01 and 1 µm) induced a concentration-dependent increase of the K+-evoked GABA release in striatal slices (one-way anova, F2,16 = 3.405, P < 0.05).
DA levels could not be detected in cortical slices except at the highest concentration of SB-357134 (1 µm) used. In the striatum, using this 1 µm concentration of SB-357134, DA release was significantly inhibited compared with the control value (one-way anova, F3,48 = 14.850, P < 0.01).
All these results are shown in Table 1.
| Glutamate(pg/10 µL) | GABA(pg/10 µL) | DA (pg/100 µL) | |
|---|---|---|---|
| Frontal cortex | |||
| Control | 1244.56±150.72 | 773.85±103.40 | n.d. |
| SB357134 | |||
| 0.05 µm | 2071.25±355.54* | 1009.21±111.80 | n.d. |
| 1 µm | 1901.14±317.70* | 822.56±111.80 | 14.3±2.7 |
| Striatum | |||
| Control | 452.23±91.70 | 319.80±52.16 | 370.83±57.30 |
| SB357134 | |||
| 0.05 µm | 605.94±100.27 | 570.62±80.8* | 362.90±89.16 |
| 1 µm | 750.42±160.10* | 661.12±117.42* | 79.01±19.06* |
- Values are expressed amount measured in the superfusate fraction with the highest value sampled during K+ stimulation (means ± SEM from 10–12 experiments. *P < 0.05, or better, vs. Control. n.d, non detectable neurotransmitter release.
Basal levels of 5-HT were 36.1 ± 4.1 and 25.7 ± 2.7 pg/100 µL of efferent perfusion efflux in the cortex and striatum, respectively. SB-357134 (0.01 and 1 µm) did not modify K+-evoked 5-HT release (data not shown).
Involvement of the glutamatergic system in K+-evoked [3H]ACh release induced by SB-357134 in the frontal cortex and striatum
In the frontal cortex, the glutamate release inhibitor riluzole, 1 µm, fully blocked the K+-evoked [3H]ACh release induced by SB-357134, 1 µm: the S2/S1 ratio was 0.91 ± 0.05, 0.61 ± 0.05, 1.59 ± 0.24 and 0.89 ± 0.19 for control, riluzole, SB-357134 and SB-357134 + riluzole, respectively (one-way anovaF3,44 = 4.063, P = 0.012, n = 8–10).
Simultaneous application of the selective channel blocker of the NMDA receptor, MK801, 1 µm, and SB-357134, at the EC50 concentration, caused an increase in K+-evoked [3H]ACh release, whereas each of these compounds given alone was ineffective (one-way anova, F3,63 = 3.311, P < 0.05, n = 8–10). The AMPA receptor antagonist NBQX, 1 µm, had no effect on K+-evoked [3H]ACh release in cortical slices, but significantly blocked the SB-357134 (1 µm)-mediated [3H]ACh release (one-way anova, F3,59 = 4.026, P < 0.05, n = 8–10). These results are shown in Fig. 3.
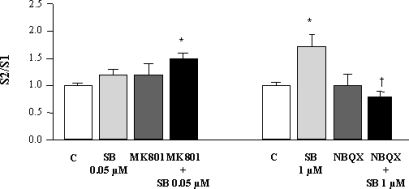
Effect of antagonists acting at glutamatergic receptors on the enhancement by SB-357134 of K+-evoked [3H]ACh release in slices from rat frontal cortex. MK801 and NBQX were used at 1 µm. C, control; MK, MK801; SB, SB-357134; SCH, SCH23390. Values are means ± SEM from 12–15 experiments. *Significant difference (P < 0.05 or better) vs. control; †significant difference (P < 0.05) vs. SB-357134.
In the striatum, riluzole was ineffective in modifying K+-evoked [3H]ACh release induced by SB-357134, 1 µm, and the S2/S1 ratio values were 1.00 ± 0.05, 0.85 ± 0.05, 1.70 ± 0.30 and 1.50 ± 0.22 for control, riluzole, SB-357134 and SB-357134 + riluzole, respectively (n = 10).
Influence of antagonists acting at GABAA receptors on the K+-evoked [3H]ACh release induced by SB-357134 in the frontal cortex and striatum
Both in the frontal cortex and striatum, bicuculline, the typical GABAA receptor antagonist, showed no intrinsic effect on K+-evoked [3H]ACh. In the two brain regions studied, the releasing effect of SB-357134, 1 µm, was not affected by bicuculline: the S2/S1 ratio was 1.20 ± 0.10 and 1.30 ± 0.20 for SB-357134 and SB-357134 + bicuculline, respectively, in the frontal cortex, and 1.30 ± 0.10 and 1.20 ± 0.10 for SB-357134 and SB-357134 + bicuculline in the striatum (n = 10).
Influence of antagonists acting at dopaminergic receptors on the K+-evoked [3H]ACh release induced by SB-357134 in the frontal cortex and striatum
As shown in Fig. 4, the D1 receptor antagonist SCH23390, 1 µm, showed an intrinsic effect on ACh release in the frontal cortex. This was blocked by 0.05 µm SB-357134 (one-way anova, F3,57 = 4.341, P < 0.01). Haloperidol, 1 µm, the typical D2 receptor antagonist, had no intrinsic effect in cortical slices, but significantly reversed the SB-357134 (1 µm)-mediated [3H]ACh release (one-way anova, F3,57 = 4.331, P < 0.01).
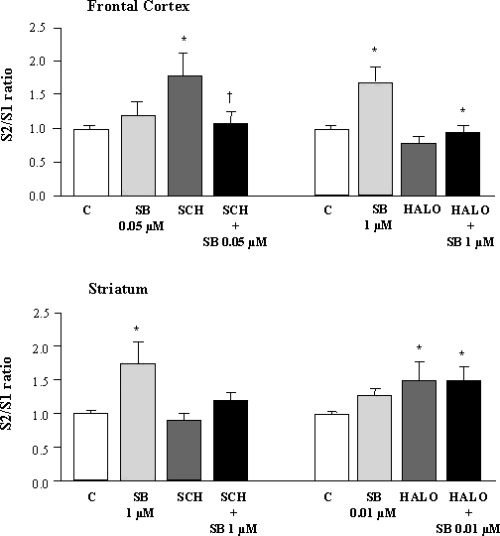
Effect of antagonists acting at dopaminergic receptors on the enhancement by SB-357134 of K+-evoked [3H]ACh release in slices from rat frontal cortex and striatum. SCH23390 and haloperidol were used at 1 µm. C, control; HALO, haloperidol; SB, SB-357134; SCH, SCH23390. Values are means ± SEM from 12–15 experiments. *Significant difference (P < 0.05 or better) vs. control; †significant difference (P < 0.05) vs. SB-35713.
In the rat striatum, in contrast to the cortex, SCH23390, 1 µm, superfusion did not alter [3H]ACh release, but significantly blocked the SB-357134 (1 µm)-induced [3H]ACh release (one-way anova, F3,55 = 3.151, P < 0.05). Haloperidol was able to significantly enhance K+-evoked [3H]ACh. Similar significant releasing effects were found after concomitant perfusion with SB-357134 (0.01 µm) release (one-way anova, F3,31 = 3.953, P < 0.05). These results are depicted in Fig. 4.
Discussion
In the present work we have studied the relationship between 5-HT6 receptors and cholinergic neurotransmission. In vivo experiments with structurally distinct 5-HT6 receptor antagonists have demonstrated increases in hippocampal ACh release (Riemer et al., 2003), supporting the hypothesis that this is a 5-HT6 receptor-mediated effect. In our hands, SB-357134 was able to significantly increase ACh release in vitro in a concentration-dependent manner in both frontal cortex and striatum, two brain areas previously demonstrated to express 5-HT6 receptors (Monsma et al., 1993; Ruat et al., 1993; Gérard et al., 1997). SB-271046, another structurally distinct 5-HT6 receptor antagonist, was also able to increase ACh release in a similar manner, strengthening the hypothesis of an effect related to blockade of 5-HT6 receptors rather than a compound related effect. These observations are in good agreement with 5-HT6 receptor antagonist-mediated reversal of cholinergic receptor blockade-induced cognitive deficits (Meneses, 2001; Bos et al., 2001; Foley et al., 2004), and corroborates previous behavioural studies suggesting that blockade of the 5-HT6 receptor could facilitate cholinergic-mediated effects (Bourson et al., 1998; Bentley et al., 1999).
A note of caution has to be taken at the time of analysing the present results, due to the limitations of in vitro experiments when compared with ex vivo or in vivo studies. The 5-HT6 receptor antagonists used in the present work are silent antagonists regarding neurochemical effects and, therefore, it is likely that the observed increase in ACh release originates from a blockade of tonic serotonergic inhibition of cholinergic neurons. Under our experimental conditions there are sufficient amounts of 5-HT present in the media to enable receptor activation, as levels of 5-HT described in our work are similar to those described to be able to, for example, desensitise 5-HT1A or 5-HT1B receptors in the cortex (Lifschytz et al., 2004).
Up to now, the possible localization of 5-HT6 receptors on cholinergic neurons has been only indirectly shown, by means of cholinergic-mediated behaviours or ACh release. To further study the possible localization of 5-HT6 receptors on cholinergic neurons, the mRNA and protein expression of this receptor was measured after producing a cortical cholinergic deafferentation, as described in a previous work from our group (Gil-Bea et al., 2004). NBM lesions were chosen instead of a more extensive i.c.v. lesion in order to have areas, such as the striatum, not affected by the lesion, and therefore acting as negative controls. Cholinergic lesions failed to alter the expression of both the mRNA and protein of these receptors in the deafferentated frontal cortex, supporting the idea that 5-HT6 receptors are not located in cholinergic neurons. In addition, in the two brain areas studied, [3H]ACh release induced by SB-357134 was TTX-sensitive, suggesting that this effect was dependent on excitatory input from sites distal to the cholinergic terminal. Moreover, it has already been described in lesioning studies that 5-HT6 receptors are not located on serotonergic neurons (Gérard et al., 1996). Taken together, these data suggest the possible mediation of other neurotransmitter systems, such as the glutamatergic, dopaminergic or GABAergic, in the effects of 5-HT6 receptors on ACh release.
Due to the diversity of neurotransmitters measured, a non-specific depolarization, using high K+ concentration, was used to evoke neurotransmitter release. In our hands, and in accordance with previously reported data on systemic administration of 5-HT6 receptor antagonists (Dawson et al., 2001; Lacroix et al., 2004), SB-357134 was able to significantly influence the release of glutamate and DA. However, glutamate release by SB-357134 in the striatum does not seem to participate in the modulation of cholinergic neurotransmission by 5-HT6 receptors, as administration of the glutamate release inhibitor riluzole (Wang et al., 2004) only affected the increases of K+-evoked release of ACh in the cortex induced by SB-357134. As the putative interactions between ACh and glutamate have been widely studied in the cortex (Ghersi et al., 2003), we tested the hypothesis that glutamate released after 5-HT6 receptor blockade would stimulate cholinergic terminals by means of glutamatergic receptors. The blockade of the increases induced by SB-357134 of K+-evoked release of ACh by the AMPA receptor antagonist NBQX, together with the co-localization of AMPA receptors on cholinergic terminals (Ghersi et al., 2003) makes possible to suggest the mediation of AMPA receptors on the effects of 5-HT6 receptor antagonists on ACh release. It has also been found a significant enhancement of [3H]ACh release after concomitant administration of MK801 with SB-357134 in the cortex. It could be suggested that NMDA receptors, located on cortical serotonergic terminals, stimulate 5-HT release (Fink et al., 1995), which, in turn, would inhibit cholinergic activity through an, at the moment, unknown mechanism.
Almost 20% of 5-HT6-like immunoreactive neurons are GABAergic (Woolley et al., 2004) and, therefore, we also checked the possibility of a mediation of the GABAergic system in 5-HT6 receptor-mediated functions. Blockade of 5-HT6 receptors seems to increase the K+-evoked GABA efflux in the striatum. However, we did not see any effects of the GABAA receptor antagonist bicuculline on 5-HT6 receptor antagonist-evoked [3H]ACh release. This suggests that unlike other 5-HT receptors, such as the 5-HT3 receptor (Ramírez et al., 1996), the GABAergic system is not directly involved in 5-HT6 receptor regulation of cholinergic transmission.
In the cortex, and as previously reported with a different 5-HT6 receptor antagonist (Lacroix et al., 2004), SB-357134 produced a small increase in DA release, but only at the highest concentration used. In the striatum, even though high levels of expression of 5-HT6 receptors are found (reviewed by Woolley et al., 2004), it has been questioned whether 5-HT6 receptors affect dopaminergic mechanisms, as 5-HT6 receptor antagonists fail to alter basal DA release (Dawson et al., 2001), DA-induced contralateral rotation is not affected by 5-HT6 receptor blockade in the 6-hydroxydopamine-lesioned rat model (Bourson et al., 1995), and 5-HT6 receptor antagonists do not reverse DA-induced impairments in the novel object recognition test (Woolley et al., 2003). In our hands, a significant reduction in K+-evoked DA release by SB-357134 was demonstrated in the striatum. These observations are different to recent in vivo microdialysis studies showing that SB-271046 potentiates amphetamine-induced DA release in the striatum (Dawson et al., 2003). It has to be taken into account that in in vitro isolated slice preparations, key input circuitry may be lost. On the other hand, 5-HT6 receptors do not appear to be located on dopaminergic neurons (Roberts et al., 2002). Taken together, these data suggest that glutamate release induced by SB-357134 modulates DA release through AMPA receptors (Wu et al., 2002) and, subsequently, the release of DA would exert a facilitatory effect on ACh release through D1 receptors (Ramírez et al., 1997). However, interpretation of the haloperidol results is more challenging, but based on the relationship between D2 receptors and the cholinergic system (Day & Fibiger, 1993) and our present results, it cannot be ruled out that the 5-HT6 receptor modulation of [3H]ACh release could also be mediated through D2 postsynaptic receptors. Also, note the differences found between the cortex and striatum. Possible explanations include the different glutamatergic and dopaminergic innervation of these areas, in addition to the distribution of 5-HT6 receptors, with the highest levels found within the striatum (Monsma et al., 1993; Ward et al., 1995). These differences in the neurochemical outcome after 5-HT6 receptor blockade depending on the brain area considered should be taken into account at the time of analysing the functional effects of systemic administration of 5-HT6 receptor antagonists.
Taken together, the results from the present study clearly demonstrate that multiple neurotransmitter systems participate in the neurochemical actions following 5-HT6 receptor blockade. Given that a number of pharmaceutical companies are developing 5-HT6 receptor antagonists for clinical use, most likely for the cognitive dysfunction associated with disorders such as Alzheimer's disease or schizophrenia, and that this patient population will inevitably be taking AChE inhibitors, NMDA receptor antagonists (such as memantine) or a number of drugs that interact with the dopaminergic system, the results of the present study highlight the importance of further preclinical in vitro experiments and in vivo neurochemical and functional studies using combinations of 5-HT6 receptor antagonists with cholinergic, glutamatergic or dopaminergic compounds to further understand the potential interactions between these drugs.
Acknowledgements
This work has been supported by a grant from Gobierno de Navarra (Spain). B. Marcos and F. J. Gil-Bea have a scholarship from Gobierno de Navarra (Spain). M. Garcia-Alloza has a scholarship from the Secretaria de Estado de Educacion y Universidades, co-financed by the Fondo Social Europeo (EX2004-0250).
Abbreviations
-
- 5-HT
-
- serotonin
-
- ACh
-
- acetylcholine
-
- AChE
-
- acetylcholinesterase
-
- ChAT
-
- choline acetyltransferase
-
- DA
-
- dopamine
-
- GABA
-
- γ-aminobutyric acid
-
- HPLC
-
- high-performance liquid chromatography
-
- KRB
-
- Krebs–Ringer bicarbonate buffer
-
- NBM
-
- nucleus basalis magnocellularis
-
- NMDA
-
- N-methyl-d-aspartate
-
- TTX
-
- tetrodotoxin