Induction of parkin expression in the presence of oxidative stress
Abstract
Parkinson's disease (PD) is one of the most common neurodegenerative disorders. Gene mutations have been found in rare familial forms of PD, with mutations in parkin being the most common cause. Oxidative stresses have also been implicated as an important contributing factor in the pathogenesis of PD. Currently, there is accumulating evidence that parkin may play a role in maintaining mitochondrial function and preventing oxidative stress. We demonstrated here that parkin is up-regulated when SH-SY5Y dopaminergic neuroblastoma cells are exposed to the oxidant dopamine.The up-regulation of parkin appeared to be due to transcriptional activation as luciferase assays confirmed that specific parkin promoter constructs could confer enhanced transcriptional activation in response to dopamine. Moreover, this effect was also seen when SH-SY5Y cells were subjected to another oxidative stress, 1-methyl-4-phenylpyridinium. In parallel with these studies, we also observed similar transcriptional activation of the parkin coregulated gene by oxidative stress. This is the first demonstration that parkin expression is up-regulated by oxidative stresses and may suggest that this might be a general neuroprotective response of parkin to oxidative stresses.
Introduction
Parkinson's disease (PD) is the second most common neurodegenerative disorder after Alzheimer's disease (Huang et al., 2004). It is characterized clinically by tremor, rigidity, bradykinesia (slowness of movement) and postural instability. The pathological hallmarks of the disease include the accumulation of cytoplasmic aggregates known as Lewy bodies in relatively specific brain regions, such as the substantia nigra and locus ceruleus, as well as loss of dopaminergic neurones. The cause of PD is still unknown but many factors have been shown to contribute to the development of the disease including ageing, environmental factors and genetic factors. Six genes and three other genetic loci are associated with familial PD, with mutations in the parkin gene being the most common cause of early onset cases (Kitada et al., 1998; Abbas et al., 1999; Lucking et al., 2000).
Parkin is a 465-amino-acid protein containing two RING finger domains separated by an in-between-ring domain at the carboxyl terminal and a ubiquitin-like domain at the amino terminal (Sakata et al., 2003; Shimura et al., 2000). It has been identified as an E3 ubiquitin-protein ligase and plays a role in the ubiquitin proteasome system by recruiting E2 components and facilitating the transfer of ubiquitin to damaged or misfolded target substrates. These polyubiquitylated substrates are then targeted to the 20S proteasome complex where they are degraded (Imai et al., 2000, 2001; Shimura et al., 2001; Upadhya & Hegde, 2003).
The majority of parkin mutations are found to either impair its binding to putative substrates or render its ligase activity defective, thus resulting in a decrease in its activity. This loss-of-function mechanism leads to neurodegeneration and PD (Imai & Takahashi, 2004). Recent studies of parkin knock-out models have suggested that parkin loss-of-function may lead to mitochondrial dysfunction and oxidative stress (Greene et al., 2003; Palacino et al., 2004).
Oxidative stress has long been implicated in the pathogenesis of PD. Dopamine may be the major contributor as it can be generated endogenously by the substantia nigral neurones (Barzilai et al., 2001; Betarbet et al., 2002). An increase in dopamine may lead to mitochondrial dysfunction and impaired proteolysis via its reactive metabolites, dihydroxyphenylacetic acid and dopamine-quinones, or directly due to its oxidative nature (Berman et al., 1996; Berman & Hastings, 1997; Kuhn & Arthur, 1998; Stokes et al., 1999; Khan et al., 2001). The neurotoxin 1-methyl-4-phenylpyridinium (MPP+), a reactive metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, is a mitochondrial complex I inhibitor and is selectively taken up into dopaminergic neurones via the dopamine transporter leading to severe oxidative damage and neuronal degeneration resulting in Parkinsonism in rodents, primates and humans (Javitch et al., 1985; Javitch & Snyder, 1984; Ramsay et al., 1986; Mizuno et al., 1987).
There is accumulating evidence that parkin may play a role in maintaining mitochondrial function and preventing oxidative stress (Shen & Cookson, 2004). Parkin knock-out mice develop mitochondrial deficits (Palacino et al., 2004) and parkin knock-down in cell lines renders cells more vulnerable to oxidative stress (MacCormac et al., 2004). The ubiquitin ligase activity of parkin is also modified by nitric oxide-mediated oxidative stress. It was recently reported (Chung et al., 2004; Yao et al., 2004) that the reactive metabolite of dopamine, dopamine quinone, may decrease the solubility of endogenous parkin by covalently binding to cysteine residues of parkin. This results in the loss of activity of parkin (LaVoie et al., 2005). Furthermore, LaVoie et al. (2005) found increased insoluble parkin in the caudate nucleus of patients with PD (LaVoie et al., 2005). We have also previously shown that endogenous parkin localizes to aggregates following exposure to dopamine in neuroblastoma cells (Muqit et al., 2004).
Oxidative stress has been found to affect the expression of a variety of proteins implicated in PD, e.g. α-synuclein (Xu et al., 2005). However, no studies to date have examined the effect of oxidative stress on the regulation of parkin at the level of transcriptional expression. We wished to determine whether there is any up-regulation of parkin in the face of oxidative stress that might represent a compensatory mechanism following a reduction in parkin solubility. As such, in this study, we sought to examine the effects of two well-characterized oxidants, dopamine and MPP+, on the regulation of parkin expression.
Materials and methods
Cell culture
Human dopaminergic neuroblastoma SH-SY5Y cells were maintained in 85-cm2 flasks in Ham's F12 and minimum essential medium with Earles salts (1 : 1) (Gibco BRL, Paisley, UK) supplemented with 15% fetal calf serum (PAA Laboratories, Austria), 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mm l-glutamine and 1 × non-essential amino acids (all from Gibco BRL), and incubated in a humidified 5% CO2 atmosphere at 37 °C.
Real-time polymerase chain reaction
For real-time polymerase chain reaction (PCR), SH-SY5Y cells (passage 21–27) were plated onto six-well plates at 2 × 105 cells/well. Dopamine hydrochloride (Sigma) and MPP+ iodide (Sigma) were added to each well at a variety of concentrations. The cells were subsequently harvested using TRIZOL® reagent (Invitrogen) and retrieval of RNA was carried out according to the manufacturer's protocol. A DNase step using DNase I (Amersham-Pharmacia) was also subsequently carried out. cDNA was then made using Superscript II reverse transcriptase (Invitrogen), according to the manufacturer's protocol, primed by random hexamer primer mix. Each experiment was repeated three to five times.
Real-time PCR was performed on the DNA Engine Opticon system (MJ Research) using SYBR I Green technology (Platinum® SYBR® Green qPCR Supermix-UDG, Invitrogen), according to the manufacturer's instructions, with an initial denaturation step at 95 °C for 15 min (activating the Platinium®Taq DNA polymerase) and subsequently three-step cycling of 95 °C for 30 s, 58–67 °C (depending on the primer pair) for 30 s and 72 °C for 30 s. Fluorescence plate measurements were then taken after each cycle at 72 °C. A final melt curve was then carried out after the programme, from 65 to 95 °C (in 0.3-°C steps). Each cDNA sample was amplified in triplicate reactions simultaneously and a standard calibration curve for each target primer set was also produced using six appropriate serial dilutions of a cDNA template sample. To control for cross-contamination from other sources, blank reactions in which no cDNA was added were included in each PCR run. The opticon software on the DNA Engine Opticon system was used to determine the threshold amplification cycle, which represents the first discernible PCR cycle at which the product fluorescence increases to just above background noise, for each reaction [this process was described in detail previously in Diss et al. (2001)]. Housekeeping gene control primers, β2-microglobulin, β-actin and NADH cytochrome b5-reductase were subsequently used for the normalization of the target's expression relative to the housekeeper gene's expression with similar results obtained for each. The sequences and annealing temperatures of the normalizing gene primer pairs used are shown in Table 1. All primers spanned exon–intron boundaries to prevent amplification from genomic DNA.
| Primer | Primer sequence | Annealing temperature (°C) |
|---|---|---|
| Parkin, forward | 5′-CCAGTGACCATGATAGTGTT-3′ | 62 |
| Parkin, reverse | 5′-TGATGTTCCGACTATTTGTTG-3′ | |
| PACRG, forward | 5′-GGTTGTGTCAGCTGAGATGG-3′ | 62 |
| PACRG, reverse | 5′-AGGTTGCAACGAGCCATGAT-3′ | |
| β2-microglobulin, forward | 5′-TGCTGTCTCCATGTTTGATGTATCT-3′ | 64 |
| β2-microglobulin, reverse | 5′-TCTCTGCTCCCCACCTCTAAGT-3′ | |
| β-actin, forward | 5′-AGCCTCGCCTTTGCCGA-3′ | 67 |
| β-actin, reverse | 5′-CTGGTGCCTGGGGCG-3′ | |
| NADH cytochrome b5-reductase, forward | 5′-TATACACCCATCTCCAGCGA-3′ | 60 |
| NADH cytochrome b5-reductase, reverse | 5′-CATCTCCTCATTCACGAAGC-3′ |
- PACRG, parkin coregulated gene.
Data analysis
The data obtained from real-time PCR were analysed by the 2–ΔΔCt method (Livak & Schmittgen, 2001). We confirmed similar PCR efficiencies for parkin and housekeeping genes by undertaking reactions on serial dilutions of cDNA; plotting curves and calculating the slope of curves (data not shown). The variation in activation and expression levels in the experiments for each construct, determined by luciferase assays and real-time PCR, was assessed by one-way anova with a Bonferroni post-hoc test.
Western blot
SH-SY5Y cells (passage 21–27) were plated onto 10-cm plates at 18 × 105 cells/well. Dopamine hydrochloride (250 µm; Sigma) or MPP+ iodide (10 mm; Sigma) dissolved in fresh media was added to each plate. Each experiment was performed three times. The cells were subsequently lysed and harvested using radioimmuno-precipitation assay buffer (RIPA) buffer or Laemmli buffer to extract soluble or total protein, respectively. Lysates (∼30 µg for soluble protein; ∼20 µg for total protein) were run on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gels and transferred onto nitrocellulose membranes. The membranes were then blocked with 5% (w/v) milk powder in 0.1% Tris-buffered saline-Tween and probed overnight at 4 °C using anti-parkin antibody (Cell Signalling) or anti-β-actin antibody (Santa Cruz Biotechnology) in 5% bovine serum albumin in 0.1% Tris-buffered saline-Tween. Membranes were washed and probed using appropriate horseradish peroxidase-conjugated secondary antibodies (Dako Cytomation) and developed using enhanced chemiluminescence (Amersham Biosciences).
Constructs
The plasmids subsequently used were 4500, 363, 282, 140, Control (all gifts courtesy of Dr Andrew West, UCLA, Los Angeles, CA, USA) and PGL3 basic. 4500 was PGL3 basic containing the full promoter (4.5 kb) for parkin; 363 contained only the core promoter; 282 contained the antisense sequence; 140 contained only 40 bp of the promoter sequence and was deemed to be transcriptionally inactive and was thus used as a control similar to the empty PGL3 basic plasmid; and Control contained an SV40 promoter. SV40 Renilla plasmids were used in dual luciferase assays as a control against background signal.
Luciferase assays
For the luciferase assays, SH-SY5Y cells (passage 21–27) were plated onto 24-well plates at 4 × 104 cells/well and incubated overnight. Cells were cotransfected, in triplicate, with pRL-SV40 plasmid and one of the constructs at a ratio of 1 : 10, respectively. All transfections were performed using 2 µL Lipofectamine 2000 per well in OPTIMEM for 4 h followed by replacement of the full culture media. At 24 h post-transfection, dopamine hydrochloride and MPP+ iodide were dissolved in full culture media and a variety of concentrations of each stressor was added to 90% confluent wells at 0.5 mL/well. Cells were harvested at 24 h following stress with dopamine and at 6 h following stress with MPP+ using passive lysis buffer (Promega) and subsequently assayed for firefly and Renilla luciferase levels using the Dual-Luciferase® Reporter Assay System (Promega) according to the manufacturer's protocol. Each experiment was performed in triplicate and repeated three times for both stressors.
Results
Quantification of parkin mRNA
To determine if dopamine might have an effect on the transcription of parkin, real-time PCR was used to measure the amount of parkin mRNA in SH-SY5Y cells incubated with 0, 62.5, 125 and 250 µm dopamine for 24 h. Similar results were obtained when normalizing parkin mRNA values with three different control housekeeping genes, including β2-microglobulin, β-actin and NADH cytochrome b5-reductase (data not shown). Data normalized for β2-microglobulin are presented here. Once normalized, real-time PCR results demonstrated that dopamine caused a dose-dependent increase in endogenous parkin mRNA (Fig. 1). However, this did not indicate if the increase in parkin mRNA was due to transcriptional or post-transcriptional regulation.
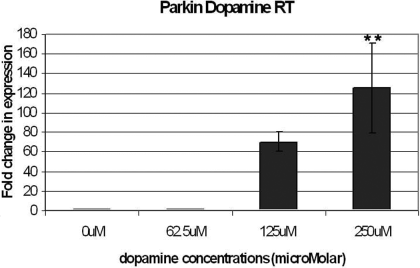
Cells were incubated for 24 h in 0, 62.5, 125 and 250 µm dopamine in full culture media, before mRNA was retrieved. The results of the real-time polymerase chain reaction were normalized onto results obtained using the housekeeping gene primer β2-microglobulin. The error bars indicate the SEM of three experiments performed in triplicate. Differences were analysed by anova and Bonferroni post-hoc test. The amount of parkin mRNA in cells stressed with 250 µm dopamine was significantly higher than in unstressed cells and cells stressed with only 62.5 µm dopamine (**P < 0.01).
To determine if this was specific to dopamine or a general response to oxidative stress, another oxidative stressor, MPP+, was used to carry out a similar quantitative analysis. Cells were incubated with 0, 1, 5 and 10 mm MPP+ for 6 h. The results from real-time PCR were similarly normalized and a similar dose-dependent increase in mRNA was observed (Fig. 2).
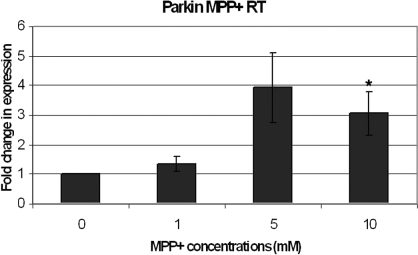
Cells were incubated for 6 h in 0, 1, 5 and 10 mm 1-methyl-4-phenylpyridinium (MPP+) in full culture media before mRNA retrieval. Similarly, the results of the real-time polymerase chain reaction were normalized onto results obtained using the housekeeping gene primer β2-microglobulin. The error bars indicate the SEM of five experiments. Differences were analysed by anova and Bonferroni post-hoc test. *P < 0.05, dose-dependent increase in parkin mRNA for cells stressed for 6 h.
Protein analysis
We then performed western blot analysis to determine if oxidative stress also altered parkin expression at the protein level. In the light of recent work showing altered parkin solubility by oxidative stress (LaVoie et al., 2005; Wang et al., 2005), we analysed parkin expression in both total protein (soluble and insoluble) and soluble extracts. Dopamine (250 µm) and MPP+ iodide (10 mm) were used to stress the cells. We found a significant increase in total parkin protein expression following dopamine stress compared with control that was consistent with our mRNA results. However, there was a significant decrease in soluble parkin protein expression, suggesting that increased parkin was sequestered into the insoluble fraction (Fig. 3A and B). In MPP+ iodide-stressed cells, we also observed an increase in total parkin protein expression compared with control but in contrast to dopamine there was no change in soluble parkin protein (Fig. 4A and B). Western blots for soluble and total protein analysis were performed separately with differences in protein concentration and film exposure times.
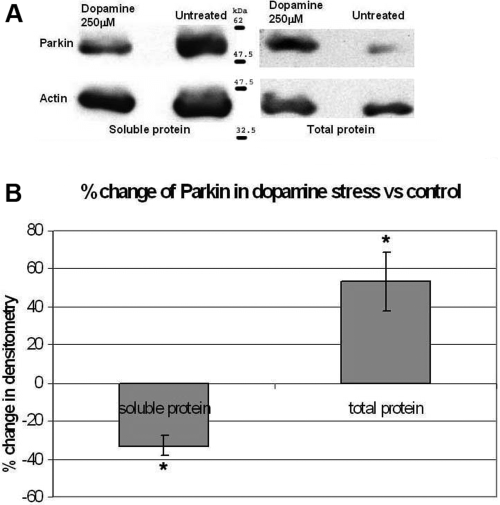
(A and B) Cells were incubated for 24 h in 0 and 250 µm dopamine in full culture media before lysing in either RIPA buffer or Laemmli buffer to extract soluble and total protein, respectively. The exposed bands were analysed using a GS-800 densitometer (Bio-Rad) and the results were normalized with β-actin protein and shown as a percentage change compared with untreated cells. Differences were analysed by paired t-test. Dopamine-stressed soluble parkin showed a significant decrease whereas total parkin showed a significant increase compared with untreated samples (*P < 0.05).
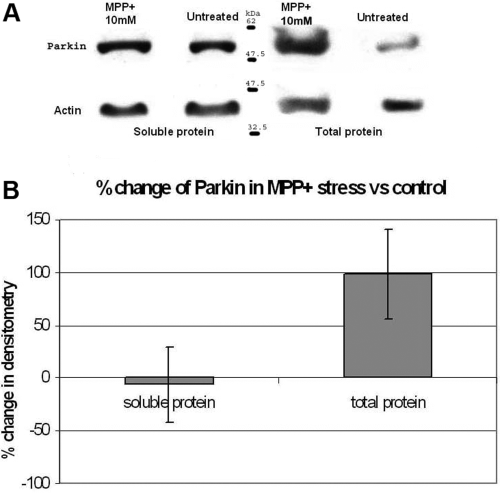
(A and B) Cells were incubated for 6 h in 0 and 10 mm 1-methyl-4-phenylpyridinium (MPP+) in full culture media before lysing in either RIPA buffer or Laemmli buffer to extract soluble and total protein, respectively. The exposed bands were analysed using a GS-800 densitometer (Bio-Rad) and the results were normalized onto β-actin protein and shown as a percentage change compared with untreated cells. There was a strong trend for an increase in total parkin protein expression compared with untreated control.
Parkin promoter activity
Dual luciferase assays were used to find out if the increase in parkin mRNA was due to transcriptional or post-transcriptional regulation. In dual luciferase assays, a test promoter is inserted upstream of the firefly luciferase reporter gene in a promoter-less plasmid (usually PGL3 basic). The inserted promoter activity will then drive the expression of luciferase. To account for transfection efficiency, another plasmid containing the Renilla (sea pansy) luciferase is cotransfected, both the luciferases are measured separately and the final value is corrected for the Renilla luciferase. A variety of parkin promoter regions cloned upstream of the luciferase gene (Fig. 5) were used to measure parkin promoter transactivation. These constructs were transfected into SH-SY5Y cells and luciferase activity assayed for basal levels and in response to varying concentrations of dopamine.
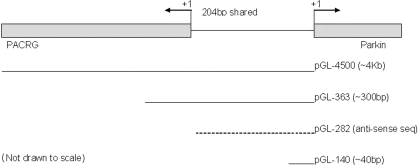
Constructs used for dual luciferase assays. Each of the inserts was cloned in PGL3 basic plasmid upstream of the firefly luciferase reporter gene. In all of the transfections, they were cotransfected with pRL-SV40, which produces Renilla luciferase and thus corrects for transfection efficiency when measuring for the overall luciferase activity. 4500 encodes for approximately 4 kb upstream of parkin exon 1 and thus contains the core parkin promoter and possible additional enhancer/repressor sites; 363 encodes for about 300 bp upstream of the parkin transcriptional start site and contains the core parkin promoter; 282 contains the antisense of the parkin promoter and codes for the parkin coregulated gene (PACRG) core promoter; 140 contains only about 40 bp upstream of parkin and PGL3 basic indicates the empty vector.
Under basal conditions, constructs 363 and 282 displayed significantly higher activity compared with 4500 (Fig. 6). There was no significant difference in activity between 4500 compared with the control construct 140. This indicated the presence of essential promoter sequences (core promoter) and possible enhancer domains of the parkin promoter (363) and also possible repressor domains in the sequences upstream of the promoter, present in 4500. The activity of 363 was also observed to be significantly higher than 282 which consists of the core promoter of the coregulated antisense gene, parkin coregulated gene (PACRG).
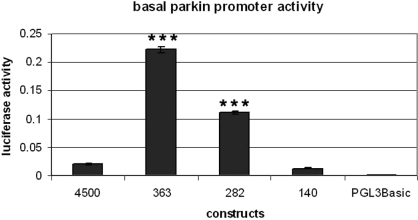
Cells were transfected with the different constructs and incubated in full culture media for 24 h. Data were analysed by anova with post-hoc Bonferroni correction. Constructs 363 and 282 expressed significantly higher luciferase activity compared with the other constructs (***P < 0.001) and 363, in turn, also expressed significantly higher luciferase than 282.
Administration of dopamine resulted in a significant increase in luciferase activity for the full-length parkin promoter construct 4500 at 125 µm and a dose-dependent increase for the 363 promoter (Fig. 7). This suggested that the increase in parkin mRNA observed in earlier experiments was due to transcriptional activation.
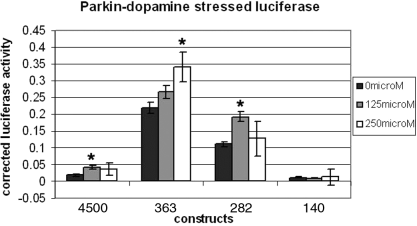
Cells were similarly transfected as those in Fig. 4 and incubated for 24 h in 0, 125 and 250 µm dopamine in full culture media. Constructs 4500 and 282 in 125 µm dopamine were significantly (*P < 0.05) different from untreated cells. The luciferase activity in cells transfected with construct 363, in 250 µm dopamine, was also significantly higher than untreated cells (*P < 0.05). Luciferase activity for 140 was not significantly altered in the different dosages of dopamine. The luciferase values were corrected for PGL3 basic vector activity. The error bars indicate the SEM of three experiments, each performed in triplicate.
Similar dual luciferase assays were carried out for the MPP+ stressor to determine if MPP+-induced regulation was also via transcriptional activation. MPP+ treatment also resulted in a similar activation of parkin transcription (Fig. 8). The increase seen, however, was much higher in all of the constructs tested when 10 mm MPP+ was used. Only construct 363 showed significant increases when stressed with 5 mm MPP+. The higher level of transactivation observed with 10 mm MPP+ compared with dopamine might suggest that the different transcriptional cofactors are required for each stress.
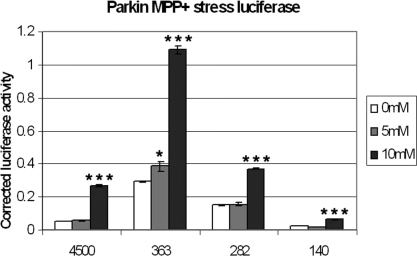
Transfected cells were incubated for 6 h in 0, 5 and 10 mm 1-methyl-4-phenylpyridinium (MPP+) in full culture media. ***P < 0.001, significant increase in luciferase activity for all of the constructs tested in 10 mm MPP+, and a less significant increase in activity of construct 363 in 5 mm MPP+ (*P < 0.05). The luciferase values were corrected for PGL3 basic vector activity. Error bars: SEM of three experiments, each performed in triplicate.
For both stresses, the largest construct, containing the core promoter and some possible enhancer and repressor sites (p4500), did not seem to be up-regulated as much as the 363 construct. This raises the possibility of repressor sites on the sequences upstream of the core promoter.
We also observed significant activation with the 282 construct when stressed with both dopamine and MPP+. 282 consists of the core promoter of the coregulated antisense gene PACRG, which suggests that activation of the PACRG promoter may also be stress responsive. There is currently some evidence that PACRG may play a role during cell stress (Imai et al., 2003; West et al., 2003).
Quantification of parkin coregulated gene mRNA
In view of the luciferase results for construct 282, which consisted of the core promoter of PACRG, we determined whether PACRG mRNA expression was increased in response to dopamine and MPP+ using PACRG primers (Table 1). There was a dose-responsive increase for both dopamine- and MPP+ iodide-stressed samples consistent with the luciferase data (9, 10).
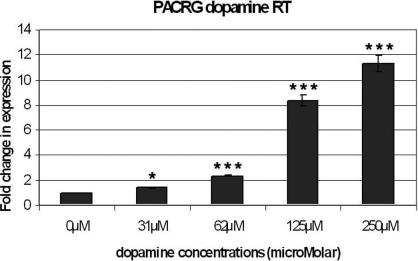
Cells were incubated for 24 h in 0, 31.25, 62.5, 125 and 250 µm dopamine in full culture media before mRNA was retrieved. The results of the real-time polymerase chain reaction were normalized onto results obtained using the housekeeping gene primer β2-microglobulin. Error bars: SEM of three experiments. There was a significant dose-dependent increase in parkin coregulated gene mRNA (*P < 0.05 for 31.25 µm, ***P < 0.001 for 62.5, 125 and 250 µm, anova and Bonferroni post-hoc test).
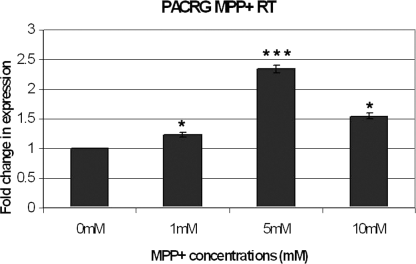
Cells were incubated for 6 h in 0, 1, 5 and 10 mm 1-methyl-4-phenylpyridinium (MPP+) in full media culture media before mRNA retrieval. Similarly, the results of the real-time polymerase chain reaction were normalized onto results obtained using the housekeeping gene primer β2-microglobulin. The error bars indicate the SEM of three experiments. Differences were analysed by anova and Bonferroni post-hoc test. All of the MPP+-stressed cells showed significant increases in parkin coregulated gene mRNA with those stressed with 5 mm MPP+ showing the highest increase (*P < 0.05 for 1 and 10 mm, ***P < 0.001 for 5 mm).
Discussion
Parkin is generally thought to be neuroprotective due to its role in the ubiquitin proteasome system, facilitating the transfer of ubiquitin to damaged or misfolded target substrates leading ultimately to their degradation. Mutations of the parkin gene have been associated with a large proportion of familial PD, especially early onset PD. As the age of onset of PD is usually greater in sporadic cases, the role of ageing and environmental stress in these cases becomes more important. There is also accumulating evidence for interplay between oxidative stress and parkin, and recently parkin has been shown to become more insoluble and inactivated by dopamine (LaVoie et al., 2005). One previous study has also shown that over-expression of parkin in cultured cells may confer protection against dopaminergic stress (Jiang et al., 2004) although we did not observe this in our system (Muqit et al., 2004). However, we have shown that parkin knock-down in cell lines renders them more susceptible to dopaminergic stress (MacCormac et al., 2004). However, no studies to date have focused on the regulation of endogenous parkin at the transcriptional level in the presence of oxidative stress.
In our studies, we have used both dopamine and MPP+ to determine if they might result in any alterations in the transcription of parkin. These stressors were used as dopamine has been shown to inactivate parkin, and MPP+/1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine is another potent neurotoxin that generates free radical-induced oxidative stress in vivo and in vitro (Javitch & Snyder, 1984; Javitch et al., 1985; Ramsay et al., 1986; Mizuno et al., 1987). Both of these stresses have been reported previously to increase the transcription levels of another PD-related gene, α-synuclein (Gomez-Santos et al., 2003; Xu et al., 2005). In the case of dopamine-stressed cells, it is believed that stress-response kinases such as SAPK/JNK and p38, as well as factors involved in autophagy, are activated which subsequently activate the expression of α-synuclein. For MPP+ stress, however, the mechanism of up-regulation of parkin is not clear.
We show here that dopamine treatment results in up-regulation of parkin at both the mRNA and protein level. Moreover, this appears to be due to transcriptional activation as luciferase assays confirmed that specific parkin promoter constructs could confer transcriptional activation. It is surprising that we did not see induction of luciferase for the 4500 construct (full-length promoter) in the light of our mRNA findings. However, this suggests that there may be additional enhancer sequences in the endogenous parkin promoter of SH-SY5Y cells. The parkin protein cellular distribution also appeared to be altered during dopamine treatment. In spite of an overall increase in total parkin protein, there was a decrease in soluble parkin protein. This indicated a shift in the solubility of parkin and is in line with previous reports of increased insolubility of parkin in the presence of dopamine (LaVoie et al., 2005; Wang et al., 2005). Whether the solubility shift in this case was due to the molecular alterations caused by dopamine or to the increase in parkin is not known.
Up-regulation of parkin also appears to be a general response to oxidative stress as MPP+ also caused up-regulation in parkin mRNA and activation of parkin promoter constructs. In addition, we also showed that possible repressor domains might be present in the promoter region upstream of the core parkin promoter, as the activation of the full promoter is less than that of the core promoter. It remains unknown whether our findings in undifferentiated SH-SY5Y cells occur in human brain in vivo. Further studies will therefore be required to be performed in differentiated SH-SY5Y cells, primary neurones as well as human post-mortem brain in vivo to confirm our findings but these approaches are beyond the scope of the current study.
Our results also show that PACRG was up-regulated in both dopamine- and MPP+-stressed cells. PACRG is a recently identified gene whose function is not yet clearly known. It is 0.6 Mb long and transcriptionally starts at 204 bp upstream and in antisense to parkin (West et al., 2003). There is some evidence that PACRG may play a role in protection against stress-induced cell death (Imai et al., 2003; West et al., 2003). However, the significance of PACRG in PD is unclear. Notably, the PACRG/parkin knock-out mouse mutant Quaking does not exhibit nigral degeneration (Lockhart et al., 2004; Lorenzetti et al., 2004).
It remains unclear how oxidative stress causes transcriptional activation of the parkin gene. It is likely that certain transcriptional factors that are stress related are up-regulated and activate specific parkin promoter domains. Therefore, we analysed the parkin core promoter sequence for putative stress-related transcription factor sites. Using matinspector (version 7.4) (Cross et al., 1994; Cartharius et al., 2005) several potential transcription factor sites of oxidative-stress-induced transcription factors were found in the parkin core promoter. These transcription factors include stimulating protein 1 and AP-1/CREB. They are known to be oxidative stress inducible and are increased or activated upon such stresses to enhance the transcription of prosurvival genes, which subsequently prevent cell death in response to the stress, DNA damage or both (Ryu et al., 2003; Shi et al., 2004). Nrf1 is another bZIP family transcription factor, whose potential binding site was predicted to be present within the parkin promoter. This transcription factor has also been shown to respond to oxidative stresses (Kwong et al., 1999). It would be of interest to determine if they play any role in the regulation of parkin.
In conclusion, we have shown that, when subjected to oxidative stresses such as dopamine and MPP+, the transcription of endogenous parkin increases. This may be a compensatory up-regulation in response to decreased parkin activity and decreased parkin solubility. The next step will be to investigate stress-specific transcription factors that may regulate parkin and determine whether these are altered in PD.
Acknowledgements
We thank Dr A. West for the generous gift of the parkin promoter constructs. This work was supported by the Parkinson's Disease Society (UK).
Abbreviations
-
- MPP+
-
- 1-methyl-4-phenylpyridinium
-
- PACRG
-
- parkin coregulated gene
-
- PCR
-
- polymerase chain reaction
-
- PD
-
- Parkinson's disease
-
- RIPA
-
- radioimmuno-precipitation assay buffer