Both α2 and α3 GABAA receptor subtypes mediate the anxiolytic properties of benzodiazepine site ligands in the conditioned emotional response paradigm
Abstract
Mice with point-mutated α2 GABAA receptor subunits (rendering them diazepam insensitive) are resistant to the anxiolytic-like effects of benzodiazepines (BZs) in unconditioned models of anxiety. We investigated the role of the α2 GABAA subtype in a model of conditioned anxiety. α2(H101R) and wildtype mice were trained in a conditioned emotional response (CER) task, in which lever-pressing for food on a variable interval (VI) schedule was suppressed during the presentation of a conditioned stimulus (CS+) that predicted footshock. The ability of diazepam, ethanol and pentobarbital to reduce suppression during the CS+ was interpreted as an anxiolytic response. Diazepam (0, 0.5, 1, 2, 4 and 8 mg/kg) induced a dose-dependent anxiolytic-like effect in wildtype mice. At high doses, diazepam (2, 4 and 8 mg/kg) was sedative in α2(H101R) mice. Analysis of the anxiolytic properties of nonsedative diazepam doses (0.5 and 1 mg/kg), showed that α2(H101R) mice were resistant to the anxiolytic effects of diazepam. Equivalent anxiolytic properties of pentobarbital (20 mg/kg) and ethanol (1 and 2 g/kg) were seen in both genotypes. These findings confirm the critical importance of the α2 GABAA subtype in mediating BZ anxiolysis. However, as a compound, L-838417, with agonist properties at α2, α3 and α5-containing receptors, gave rise to anxiolytic-like activity in α2(H101R) mice in the CER test, α3-containing GABA receptors are also likely to contribute to anxiolysis. Observations that α2(H101R) mice were more active, and displayed a greater suppression of lever pressing in response to fear-conditioned stimuli than wildtype mice, suggests that the α2(H101R) mutation may not be behaviourally silent.
Introduction
Benzodiazepines (BZs) have been used to treat anxiety disorders for over 40 years. BZs achieve their effects by binding to a specific recognition site on GABAA receptors, pentameric protein structures, assembled from two α1, α2, α3 or α5 subunits or two different α subunit forms (Benke et al., 2004), in combination with a β variant and the γ2 subunit (Pritchett et al., 1989; Benke et al., 1991). The BZ binding site occurs at the interface of the α and γ2 subunits and agonists at this site enhance the effects of GABA by increasing the frequency of GABA-induced channel opening events and thereby increase chloride flux (McDonald & Ra, 1979; Bormann & Kettenmann, 1988). The binding site for BZs requires a histidine residue at the drug-binding domain of the α subunit (Sigel & Buhr, 1997), which explains why GABAA receptors containing α4 and α6, which possess an arginine residue at the corresponding site (Wieland et al., 1992), are insensitive to BZ site agonists and are also known as diazepam-insensitive GABAA receptors. Introduction of a point-mutation at the BZ-binding domain of respective BZ-sensitive α subunits, which changes histidine to arginine, renders them insensitive to BZs (Benson et al., 1998).
Mice, bearing the histidine-to-arginine mutation in the BZ-sensitive α subunits have been used to investigate the distinct roles of the GABAA receptor subtypes in mediating BZ-induced effects (Mohler et al., 2001; Rudolph et al., 2001). It has been suggested that the distribution (Rudolph et al., 2001), level of expression, GABA transmission (Low et al., 2000), and receptor affinity and kinetics (Marowsky et al., 2004) at the mutated receptor remain unaffected (Wieland et al., 1992) so that the loss of a particular behavioural response to BZs in knock-in compared to wildtype mice can be attributed specifically to loss of BZ action at the respective mutated receptor subtype.
Using this approach, it has been reported that a histidine to arginine point mutation rendering α2-containing GABAA receptors insensitive to BZs result in loss of anxiolytic effects of diazepam, while the corresponding point mutations in other α subunits do not (Rudolph et al., 1999; Low et al., 2000; Crestani & Rudolph, 2001), strongly suggesting that diazepam's anxiolytic effects are mediated by, and only by, their action at α2-containing GABAA receptors. However, despite the observation that mice with α3-containing receptors made insensitive to BZs continue to show an anxiolytic-like action of diazepam (Low et al., 2000), pharmacological experiments have suggested that an anxiolytic effect can also be obtained by a drug acting with selective efficacy at GABA receptors containing α3 subunits (McKernan et al., 2000; Atack et al., 2005; Dias et al., 2005). Together, these findings may be reconciled by suggesting that anxiolyis may be achieved by an action at either α2- or α3-containing receptors (Atack et al., 2006), raising the question why diazepam loses its anxiolytic effects in mice with the point-mutated α2 subunit.
Evidence supporting the role of the α2 GABAA receptor subtype in mediating BZ anxiolysis, is based to date on results from unconditioned models of anxiety, the elevated plus maze and light-dark choice. To examine further the role of the α2-containing GABAA subtype in BZ-mediated anxiolysis, we investigated the anxiety-reducing effects of diazepam and two drugs, pentobarbital and ethanol, acting at GABAA sites other than the BZ site, in α2(H101R) mice in a model of conditioned anxiety. Secondly, we tested the ability of a compound, L-838417 (7-(1,1-dimethylethyl)-6-(2-methyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2,5-difluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine), that possesses partial agonist actions at α2, α3 and α5 subunit-containing GABAA receptors, but is an antagonist at (i.e. has no effect upon) those containing α1 subunits (McKernan et al., 2000) to induce an anxiolytic-like effect both in wildtype mice, and those in which the α2 subunit had been made insensitive to diazepam. We predicted that, if α3-containing receptors are involved in mediating the anxiolytic properties of BZ site ligands, then L-838417 should retain anxiolytic activity even in the mice with insensitive α2-containing GABAA receptors.
Materials and methods
Animals
Wildtype and α2(H101R) male and female mice were bred in the Department of Psychology at the University of Sussex from parents obtained from Merck, Sharp and Dohme (Dias et al., 2005). The GABAA receptor α2H101R knock-in mice were generated using a method similar to that for α1H101R mice (McKernan et al., 2000). In brief, an α2 subunit-specific cDNA probe was used to screen a bacterial artificial chromosome (BAC) library containing genomic mouse DNA (Research Genetics, Huntsville, AL). The targeting vector was generated from overlapping BAC-derived subclones in pBlue-script covering 12 kb of genomic DNA containing exons 4 and 5 of the α2 gene. The His101 to Arg101 codon change was introduced by site-directed mutagenesis and was labelled with a novel BspE1 restriction site. The targeting vector containing an 8.1 kb NdeI-SacI long arm including the H101R mutation, the 1kb NdeI short arm, the neomycin resistance gene, and the HSV-TK gene was introduced into AB2.2 embryonic stem (ES) cells in several independent experiments. Targeting frequency, which was confirmed by PCR and Southern blot analysis, was 1 : 100, but only 1 : 250 ES cell clones retained the mutation. Only one of several highly chimeric males gave rise to germ-line transmission. Chimeric mice were bred with deleter mice (Schwenk et al., 1995) to eliminate the neomycin resistance gene in the genome. Homozygous and wild-type littermate controls were established after additional breeding.
Animals used in the present experiment were offspring of homozygous mutant or wildtype pairings in a mixed 50% C57BL6J – 50% 129 SvEv genetic background. At the start of the experiment the mice were 8-weeks old and the males and females weighed between 20 and 27 g and 18–23 g, respectively. The mice were housed in groups of two or three under a 12-h light : 12-h dark cycle (lights on at 07:00 h) at a temperature of 19–21 °C and humidity of 50 ± 10%. All mice were food restricted to maintain weights at approximately 85% of the free-feeding weight. All animals had ad libitum access to water. All experiments were carried out under the UK Animal (Scientific Procedures) Act 1986, following ethical review from the appropriate committee at the University of Sussex.
Drugs
Pentobarbital hydrochloride, purchased from Sigma–Aldrich, Poole, UK, was dissolved in sterile 0.9% saline. Diazepam (Hoffman LaRoche, Basel, Switzerland) was suspended in a saline solution containing 0.2% Tween 80. Ethanol (95%) was diluted with distilled water. L-838417 (McKernan et al., 2000) was obtained from Merck, Sharp & Dohme, Terlings Park, UK and suspended in a saline solution containing 0.5% methyl cellulose. All drugs were administered intraperitoneally (i.p.) at a volume of 10 mL/kg.
Apparatus
For the conditioned emotional response (CER) experiment, operant chambers (Medical Associates, Vermont, USA), containing two levers, were used. Cue lights were situated above each lever and the food magazine was located between the levers with a speaker positioned above it. The floor consisted of steel rods connected to a shock generator (Medical Associates, Vermont, USA). The operant boxes were connected to a computer, which ran Medical Associates software and collected data for subsequent analysis.
Conditioned emotional response
Food-shaping
Initially, all animals were food restricted for 1 week (weight did not fall below 85% of free-feeding weight). In a counterbalanced design the mice were trained to lever press for food reward on the active lever (right or left) during a single 16-h overnight session. The session consisted of six phases. During phase 1–3 mice were trained to press on a fixed ratio (FR) schedule of reinforcement (Phase 1, FR1 until ten reinforcers; Phase 2, FR2 until ten reinforcers and Phase 3, FR4 until ten reinforcers), and during phase 4–6 they were trained to lever press on a variable interval (VI) schedule of reinforcement (Phase 4, VI 10-s until 20 reinforcers; Phase 5, VI 30-s until 20 reinforcers and Phase 6, VI 60-s until the session had finished. In the next stage, animals were trained to lever press on the active lever for food reinforcement in 30-min sessions on a VI 60-s schedule, during which a lever press performed after an elapsed time, averaging 60 s, from the previous reinforcement was rewarded, until the preset criteria had been met (more than 50 lever presses, and delivery of > 10 reinforcers per session). This stage was completed by all animals within the first two sessions. Subsequently, mice were habituated in two sessions to the light (house lights were extinguished and stimulus lights above each lever flashed at 1 Hz for 30 s) and tone (2.9 KHz for 30 s) cues, in 30-min VI 60-s sessions, in which five random presentations of a tone or light, in tone and light habituation sessions, respectively, were presented.
CER training
During CER training sessions, food was available on a VI 60-s schedule. Each 35-min session included ten cue presentation trials; five CS+ and five CS–, presented in a randomised order. Half of the animals received CS+ tone and CS– light and the other half received CS+ light and CS– tone, cue pairings. During each session, following a variable time period (mean value, 120 s; range 30–210 s), a response on the active lever activated either the CS+ or the CS– for 30 s. The CS+ was immediately followed by 0.5 s unavoidable footshock whereas the CS– had no programmed consequence. There had to be at least a 5-min interval between each CS+ presentation, or the trial defaulted to the CS–.
The rate of lever pressing was recorded during the 30-s period immediately preceding the CS, the 30-s period during the CS and for 60 s after the end of the CS presentation. The suppression ratios were calculated using the formula A/(A + B), where A is the responding rate during the CS and B is the responding rate in the period preceding the CS. The average CS+ and CS– suppression ratios were calculated for each session. The degree of suppression to the CS+ was used as a measure of anxiety. A suppression ratio of 0.5 indicated a lack of suppression of lever pressing to the CS, a suppression ratio < 0.5 indicated suppression of lever pressing to the CS and complete suppression of lever pressing was represented by a suppression ratio of 0. The average number of lever presses per minute was calculated from the number of lever presses made during the 60-s period after the end of the CS presentation and used as a measure of drug-induced stimulation or sedation.
CER training began with a shock intensity of 0.25 mA for 4 days. The shock level was then gradually increased: 0.30 mA for 3 days, 0.35 mA for 3 days and 0.40 for 12 days. Due to a greater degree of suppression of lever pressing to the CS+ in α2(H101R) compared to wildtype mice at all of the shock levels, shock levels were adjusted for each animal, to achieve a CS+ suppression ratio of approximately 0.2 in all mice. Training at the assigned shock level continued for the following 8 days. At this stage, only mice in which lever pressing had been sufficiently suppressed by the CS+ (i.e. CS+ suppression ratio < 0.3) and lever pressing was consistent (lever pressing/min > 5), entered the test phase. This included eight wildtypes out of 16 (five males and three females) and nine α2(H101R) mutant mice out of 16 (six males and three females). At the time of entry into the drug-testing phase, there were no longer differences in CS+ suppression ratios between genotypes (mean CS+ suppression ratios for both genotypes was approximately 0.2). The shock levels assigned to each animal remained unchanged throughout all subsequent training and test sessions. There was at least one training day between each drug test day.
CER test sessions
In the diazepam test session, wildtype and α2(H101R) mice were treated with diazepam (0, 0.5, 1 and 2 mg/kg) 30 min prior to testing. A latin-square design was used, followed by escalating doses 4 mg/kg and 8 mg/kg. To ascertain whether a drug acting at a site other than the BZ binding site was anxiolytic in the α2(H101R) mice, all mice were administered pentobarbital (0 vs. 20 mg/kg) 20 min before testing, two days after receiving the final diazepam dose. Following a further 5 days of training the mice were treated with vehicle, or 1 or 2 g/kg ethanol 10 min before testing in latin-square design. In the ethanol test phase of the experiment there were six wildtypes (five males and one female) due to the death of one animal and incomplete data for another.
To investigate the ability of a BZ receptor agonist with functional selectivity for α2, α3 and α5-containing GABAA receptors to elicit anxiolytic-like activity, a further experiment was carried out. At this stage of the experiment, following 2 weeks of retraining on the CER procedure, four wildtype and one α2(H101R) mice no longer suppressed lever pressing to the CS+, and were excluded from further testing. Four wildtype and eight α2(H101R) mice were treated with vehicle or 10 or 30 mg/kg L-838417, 30 min prior to testing, using a latin-square design.
Shock sensitivity
To identify whether there were any differences in sensitivity to footshock levels across genotypes, flinch thresholds were measured. Footshock levels started at 0.1 mA (for 0.5-s) and were increased by 0.05 mA, until a flinch was observed. Footshock did not exceed 0.5 mA.
Statistics
Where sphericity assumptions were violated, the Greenhouse-Geisser correction was applied. The rate of lever pressing under different schedules of reinforcement during the 16-h overnight food-shaping session, and the influence of the light and tone cues, across male and female, wildtype and α2(H101R) mice prior to CER training, were compared. A three-way mixed design anova, within subjects variable, lever (active vs. inactive) and between subjects variables, genotype (wildtype vs. α2(H101R)) and sex (male vs. female), was used to compare active and inactive lever responses on VI 10 s, VI 30 s and VI 60 s schedules of reinforcement. Activity levels were measured as the total number of lever responses during the 16 h overnight session, using a three-way mixed design anova, within subjects variable, lever (active vs. inactive) and between subjects variables, genotype (wildtype vs. α2(H101R)) and sex (male vs. female). To identify whether the presentation of the light and tone cues alone suppressed food-maintained lever pressing on a VI 60-s schedule, the results were subjected to a three-way mixed design anova, within subjects variable, cue (light vs. tone) and between subjects variables, genotype [wildtype vs. α2(H101R)] and sex (male vs. female).
A three-way mixed-design anova was used to analyse CS– and CS+ suppression ratios at the different footshock levels across genotypes. The between-subject variable was genotype (wildtype vs. α2(H101R)), and the within-subject variables were shock level (0.25 vs. 0.3 vs. 0.35 vs. 0.4 mA) and CS (CS+ vs. CS–). The CS+ suppression ratio was subsequently compared between genotypes using a two-way mixed design anova, with the between-subject variable genotype (wildtype vs. α2(H101R)), and the within-subject variable, shock level (0.25 vs. 0.3 vs. 0.35 vs. 0.4 mA). Independent samples t-tests were used for posthoc analysis and to compare CS+ suppression ratios on the last training day.
A three-way mixed-design anova was used to compare drug effects across genotypes on lever pressing suppression during CS+ and CS–; independent measure, genotype [wildtype vs. α2(H101R)] by repeated measures, dose (0 vs. other doses) and CS (CS– vs. CS+). Where a main effect of CS type was found, a two-way mixed design anova, independent measure, genotype [wildtype vs. α2(H101R)] by repeated measure, dose (0 vs. other doses) was subsequently used to investigate the effect of genotype and different drug doses on the CS+ suppression ratio. Posthoc analysis was performed using simple contrasts. The rate of lever pressing at different drug doses was compared using a two-way mixed design anova; independent measure, genotype (wildtype vs. α2(H101R)) by repeated measure, dose (0 vs. other doses).
Results
Conditioned emotional response
Food-shaping
Data from 30 out of the 32 mice were analysed because one wildtype female and one α2(H101R) female failed to lever press. During the 16-h overnight food-shaping session, male mice made significantly more active and fewer inactive lever responses than females on the VI 10 s (significant lever by sex interaction, F1,26 = 5.54, P = 0.026) and VI 60 s (significant lever by sex interaction, F1,26 = 7.48, P = 0.011, significant main effect of sex, F1,26 = 7.08, P = 0.013) schedules of reinforcement. Both genotypes made a similar number of active responses on VI 10 s (nonsignificant lever by genotype interaction, F1,26 = 0.26, P = 0.613), VI 30 s (nonsignificant lever by genotype interaction, F1,26 = 0.02), and VI 60 s (nonsignificant lever by genotype interaction, F1,26 = 0.16, P = 0.693), schedules of reinforcement (data not shown). During this overnight session, a comparison of total inactive and active lever responses showed that, α2(H101R) males were more active than the other groups, shown by a greater rate of active and inactive lever pressing (significant genotype by sex interaction, F1,27 = 4.16, P = 0.051; Fig. 1A).
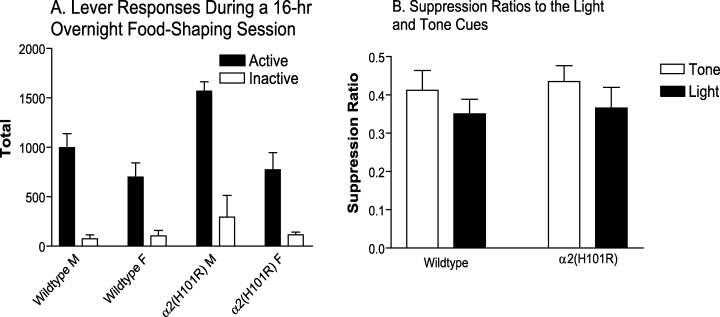
Food Shaping in 16 wildtype (eight male and eight female) and 16 α2(H101R) (eight male and eight female) mice. (A) In a single 16-h overnight food-shaping session, α2(H101R) male mice displayed a significantly higher rate of lever pressing than the other groups (P = 0.051). (B) In the first cue habituation sessions, lever pressing in both wildtype and α2(H101R) mice was mildly suppressed by initial presentation of the unconditioned light and tone cues. There were no significant differences between suppression ratios to the tone and light cues (P = 0.178) or between genotypes (P = 0.839).
Analysis of the data from habituation sessions showed that there were no significant effects of sex (no main effect of sex, F1,21 = 0.22, P = 0.642) so male and female data were combined. Lever pressing in both wildtype and α2(H101R) mice was suppressed by the unconditioned light and tone cues, shown by suppression ratios that were lower than 0.5 (Fig. 1B). However, there were no significant differences between suppression ratios to the tone and light cues (nonsignificant main effect of cue, F1,23 = 1.93, P = 0.178) or across genotypes (nonsignificant main effect of genotype, F1,23 = 0.04, P = 0.839; nonsignificant cue by genotype interaction, F1,23 = 0.02, P = 0.903; Fig. 1B).
CER training
Data from 30 mice were analysed. There was no main effect of sex (F1,26 = 0.70, P = 0.793), so male and female data were combined. Figure 2A indicates that presentation of the CS– had no effect on lever pressing rates in either genotype, while the CS+, which predicted mild footshock, suppressed lever pressing in both genotypes (main effect of CS, F1,28 = 36.18, P < 0.001; significant CS by shock interaction, F3,84 = 2.97, P = 0.037). For this reason, data from CS+ and CS– components were analysed separately.
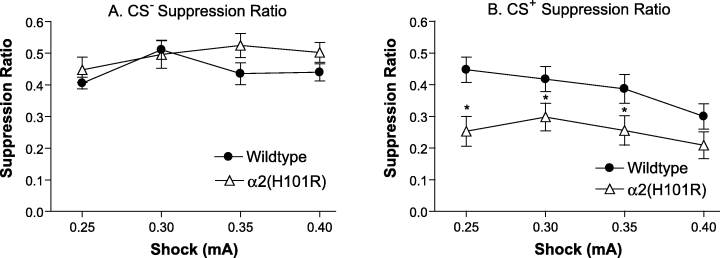
CER training. During each 35-min CER training session, wildtype (n = 16) and α2(H101R) (n = 16) mice were exposed, in a randomised order, to five CS+ presentations, which were immediately followed by a mild footshock and five CS– presentations, which had no consequence. Footshock levels were gradually increased, 0.25 mA (4 days), 0.30 mA (3 days) 0.35 mA (3 days) and 0.40 mA (12 days). The scores shown represent the CS+ and CS– suppression ratios (a measure of lever pressing suppression during the presentation of the CS), produced on the third day of testing at the respective footshock level. (A) Presentation of the CS– had no effect on lever pressing rates. (B) The degree of suppression to the CS+ increased with footshock intensity (P = 0.003). α2(H101R) mice showed a greater suppression of lever pressing than the wildtypes to the CS+ (P = 0.001). *P < 0.05 compared with wildtype.
Analysis of the CS+ data using a two-way mixed design anova, showed that α2(H101R) mice displayed a greater suppression of lever pressing to the CS+ than wildtype mice (Fig. 2B) (main effect of genotype, F1,28 = 7.58, P = 0.01), that was already evident at the lowest shock level employed (0.25 mA; t28 = 3.17, P = 0.004). This difference was also apparent at 0.30 mA (t28 = 2.03, P = 0.052) and 0.35 mA (t28 = 2.02, P = 0.053).
In order to test drug effects on comparable levels of suppression, footshock levels were adjusted for each animal. There were no significant differences in CS+ suppression ratios between genotypes on the last day of CER training (t15 = −1.16, P = 0.263), but consequently, the mean footshock level was significantly higher in wildtype mice (mean, 0.43 ± 0.02 mA) than α2(H101R) mice (mean, 0.38 ± 0.02 mA; t15 = 2.29, P = 0.036, SEM, 0.02).
CER test sessions
Diazepam
Figure 3A shows that lever pressing in both wildtype and α2(H101R) mice was suppressed by the CS+ and not the CS– (main effect of CS, F1,15 = 145.35, P < 0.001). We therefore carried out separate analyse of the data from CS+ and CS– phases using a two-way mixed design anova.

Effect of diazepam on conditioned suppression of lever pressing. Wildtype (n = 8) and α2(H101R) mice (n = 9) in which lever pressing had been sufficiently suppressed by the CS+ (i.e. CS+ suppression ratio < 0.3) and lever pressing was consistent (lever pressing/min > 5), were treated with diazepam (0, 0.5, 1 and 2 mg/kg) 30 min prior to testing in a latin-square design, followed by escalating doses 4 mg/kg and 8 mg/kg (A) Lever pressing in both wildtype and α2(H101R) mice was suppressed by the CS+ and not the CS– (P < 0.001). At the nonsedative doses of diazepam in α2(H101R) mice (0.5 and 1 mg/kg), diazepam produced a dose-dependent increase in the CS+ suppression ratio in wildtype but not α2(H101R) mice (P = 0.012). (B) At the higher doses, diazepam was sedative in the α2(H101R) mice, yet increased lever pressing in the wildtypes (P = 0.001). *P < 0.05, in A compared with wildtype, in B compared with vehicle.
Analysis of the CS– data showed that diazepam did not affect the CS– suppression ratio (no main effect of diazepam F5,70 = 1.41, P = 0.233). There were no differences in CS– suppression ratios between wildtype and α2(H101R) mice, (no main effect of genotype, F1,14 = 0.38, P = 0.548, nonsignificant diazepam by genotype interaction, F5,70 = 2.04, P = 0.102). Analysis of the CS+ data showed that diazepam dose-dependently increased the CS+ suppression ratio (main effect of diazepam F5,75 = 3.39, P = 0.014, e = 0.610). Simple contrasts (comparing each dose to 0 mg/kg) show that this was apparent at 0.5 mg/kg (F1,14 = 3.6, P = 0.079), 2 mg/kg (F1,14 = 11.00, P = 0.005), 4 mg/kg (F1,14 = 5.77, P = 0.031) and 8 mg/kg (F1,14 = 6.27, P = 0.025).
Although the diazepam by genotype interaction showed only a trend (F5,75 = 2.14, P = 0.085, e = 0.610), these data were influenced by the effect of high doses of diazepam in greatly reducing the rate of lever pressing in α2(H101R) mice, so that the suppression ration values at higher doses of diazepam in the mutant mice were calculated from very small numbers of lever presses in the CS phases. Thus the average number of lever presses/min after high doses of diazepam was reduced in α2(H101R) mice, yet increased in wildtype mice (diazepam dose by genotype interaction, F5,70 = 7.31, P = 0.001, e = 0.480]. One-way repeated measures anovaposthoc analysis revealed that 0.5 (P < 0.05), 2 (P = 0.01), 4 (P < 0.001) and 8 mg/kg (P < 0.01) of diazepam increased lever pressing in wildtype mice and 2 (P < 0.05), 4 (P = 0.01) and 8 mg/kg (P = 0.01) decreased lever pressing in α2(H101R) mice (Fig. 3B).
For this reason, we reanalysed the data, comparing the effect of diazepam on CS suppression ratios in wildtype and α2(H101R) mice at nonsedative doses, 0, 0.5 and 1 mg/kg (Fig. 3A). Again, lever pressing in both wildtype and α2(H101R) mice was suppressed by the CS+ and not the CS– (main effect of CS, F1,15 = 145.35, P < 0.001, nonsignificant CS by genotype interaction, F1,15 = 0.06, P = 0.806, no main effect of diazepam, F2,30 = 0.27, P = 0.760). Analysis of the CS+ data showed that diazepam dose-dependently increased the CS+ suppression ratio in wildtype but not α2(H101R) mice (diazepam by genotype interaction, F2,30 = 5.10, P = 0.012). Posthoc contrasts show that there was no difference in mean CS+ suppression ratio between the genotypes (t15 = −1.55, P = 0.14) during vehicle treatment, but the effect of diazepam differed between wildtype and mutant mice at 0.5 (P = 0.016) and 1 mg/kg (P = 0.005). There was no main effect of diazepam (F2,30 = 1.73, P = 0.195) and no main effect of genotype (F1,15 = 0.08, P = 0.78). Analysis of the CS– data showed that diazepam did not affect the CS– suppression ratio (no main effect of diazepam F2,30 = 1.82, P = 0.179]. There were no differences in CS– suppression ratios between wildtype and α2(H101R) mice, (no main effect of genotype, F1,15 = 0.00, P = 0.981, nonsignificant diazepam by genotype interaction, F2,30 = 0.04, P = 0.964).
In keeping with genotype differences in lever-pressing rates during food shaping, at 0 mg/kg diazepam, α2(H101R) mice made significantly more lever presses/min than wildtype mice (t9.33 = −2.20, P = 0.055), but in this case there was no sex difference.
Pentobarbital
Lever pressing in both wildtype and α2(H101R) mice was sufficiently suppressed by the CS+ and not the CS– (Main effect of CS, F1,15 = 9.87, P = 0.007). A two-way mixed design anova of the CS+ data showed that pentobarbital (20 mg/kg) significantly increased the CS+ suppression ratio (main effect of pentobarbital, F1,15 = 24.16, P < 0.001). This anxiolytic-like effect was apparent in both genotypes (genotype by pentobarbital interaction, F1,15 = 0.55, P = 0.472; Fig. 4A). Lever pressing was unaffected by pentobarbital (nonsignificant main effect of pentobarbital, F1,15 = 0.21, P = 0.650; Fig. 4B).
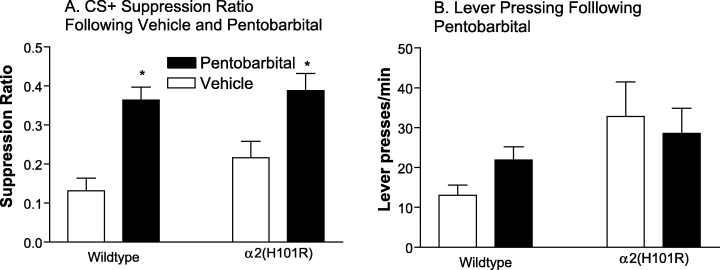
Effect of pentobarbital on conditioned suppression of lever pressing. Wildtype (n = 8) and α2(H101R) (n = 9) mice were administered pentobarbital (0 vs. 20 mg/kg) 20 min before testing. (A) Pentobarbital (20 mg/kg) significantly increased the CS+ suppression ratio (P < 0.001) in both genotypes (P = 0.472). (B) Lever pressing was unaffected by pentobarbital (P = 0.650). *P < 0.05, compared with vehicle.
Ethanol
Lever pressing in both wildtype and α2(H101R) mice was suppressed by the CS+ and not the CS– (Main effect of CS, F1,13 = 118.73, P < 0.001). A two-way mixed design anova of the CS+ data, showed that ethanol significantly increased the CS+ suppression ratio (main effect of ethanol, F2,26 = 3.83, P = 0.035). This anxiolytic-like effect was apparent in both genotypes (nonsignificant genotype by ethanol interaction, F2,26 = 0.12, P = 0.888; Fig. 5A). Posthoc contrasts show that the CS+ suppression ratio was significantly increased by 2 g/kg ethanol. However, lever pressing was reduced by 2 g/kg ethanol (P = 0.011; significant main effect of ethanol, F2,26 = 4.27, P = 0.025; Fig. 5B).
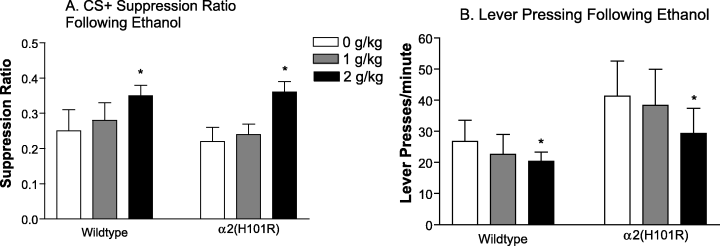
Effect of ethanol on conditioned suppression of lever pressing. Wildtype (n = 6) and α2(H101R) (n = 9) mice were administered 0, 1 and 2 g/kg ethanol 10 min before CER testing using a latin-square design. (A) Ethanol significantly increased the CS+ suppression ratio (P = 0.035) in both genotypes (P = 0.888). Posthoc contrasts show that the CS+ suppression ratio was significantly increased by 2 g/kg ethanol. (B) However, lever pressing was reduced by 2 g/kg ethanol (P = 0.011). *P < 0.05, compared with vehicle.
L-838417
Once again, lever pressing in both wildtype and α2(H101R) mice was suppressed by the CS+ and not the CS– (Main effect of CS, F1,20 = 133.91, P < 0.001), but in this experiment, the initial values of suppression ratio induced by the CS+ in the absence of drug was lower than in the previous experiment. Nevertheless, there was no significant difference between genotypes (no main effect of genotype, F1,10 = 0.01, P = 0.943). A two-way mixed design anova of the CS+ data, showed that L-838417 significantly increased the CS+ suppression ratio (main effect of L-838417, F2,20 = 6.24, P = 0.008). This anxiolytic-like effect was apparent in both genotypes (nonsignificant genotype by L−838417 interaction, F2,20 = 0.81, P = 0.459; Fig. 6A). Posthoc contrasts show that the CS+ suppression ratio was significantly increased by both doses tested (P < 0.05). There were no effects of L-838417 on lever pressing rates (no main effect of dose, F2,20 = 1.61, P = 0.226), no genotype differences (F1,10 = 0.55, P = 0.476), and no interaction effect (F2,20 = 0.22, P = 0.806; Fig. 6B).
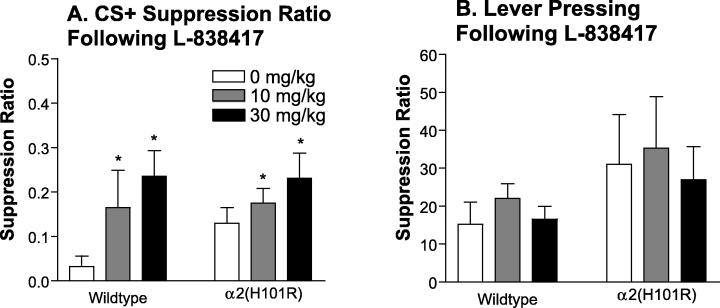
Effect of L-838417 on conditioned suppression of lever pressing. Wildtype (n = 4) and α2(H101R) (n = 8) mice were administered 0, 10 and 30 mg/kg L-38417 30 min before CER testing using a latin-square design. (A) L-838417 significantly increased the CS+ suppression ratio (P = 0.008) in both genotypes (P = 0.459). (B) Lever pressing was unaffected by L-838417 (P = 0.226), no genotype differences (P = 0.476), and no interaction effect (P = 0.806).
Shock sensitivity
Flinch thresholds were similar for both genotypes (wildtype mean, 0.38 ± 0.03 mA and α2(H101R) mean, 0.34 ± 0.02 mA).
Discussion
Point mutations, leading to substitution of arginine for histidine at position 101 of α2 subunits of GABAA receptors, prevent binding of conventional benzodiazepines to these receptors, and lead to the loss of the anxiolytic properties of BZs in mice bearing the mutation (Low et al., 2000). Previous experiments have concentrated on unconditioned tests of anxiolytic activity, the elevated-plus maze and light-dark tests (Low et al., 2000); the current data set indicates that such anxiolytic effects are also lost in an animal model employing conditioned fear. This loss of response to diazepam is not attributable to an inability to show an anxiolytic response, as two other drugs, a barbiturate, and ethanol, with anxiolytic activities achieved by interactions at other sites on the GABAA receptor complex, were not affected by the mutation.
During the CER training phase of the experiment, lever pressing for food reinforcement on a VI 60-s schedule of food reinforcement, was suppressed in both wildtype and α2(H101R) mice during the presentation of the CS+, which predicted mild footshock. The presentation of the CS–, which had no consequence, did not affect response rates. Interestingly, lever pressing was suppressed to a greater degree in α2(H101R) mice, shown by a significantly lower mean CS+ suppression ratio in α2(H101R) mice compared to wildtype mice, at 0.25 mA, 0.30 mA and 0.35 mA levels of footshock. To achieve comparable levels of CS+ suppression ratios in both genotypes before testing, footshock levels were individually titrated to obtain suppression ratios between 0.2 and 0.3 that did not differ between genotypes. For this reason, footshock levels were significantly higher in wildtype compared to α2(H101R) mice.
Doses of diazepam between 2 and 8 mg/kg produced a significant decrease in the average number of lever presses per minute in α2(H101R) but not wildtype mice, suggesting increased sensitivity to the sedative effects of diazepam in α2(H101R) in the familiar environment. For this reason, the effects of the lower, nonsedative diazepam doses, 0.5 mg/kg and 1 mg/kg, on the CS suppression ratios were investigated. Diazepam dose-dependently increased the CS+ suppression ratio in wildtype but not α2(H101R) mice. The reduction of conditioned suppression is interpreted as an anxiolytic effect (LeDoux, 2003). Thus, in contrast to wildtype mice, α2(H101R) mice were resistant to the anxiolytic properties of diazepam in this test. The anxiolytic-like effect of diazepam in wildtype mice is not likely to be due to an overall increase in rate of lever pressing, as the suppression ratio [(responding during the CS+ presentation)/(responding during the CS+ presentation) + (responding outside the CS+ presentation)] takes the increased rate of lever pressing under diazepam outside the CS+ period into account.
Wafford et al. (2004), have suggested that interpretation of the apparent anxiolytic-like effects in the plus-maze and light–dark box (Low et al., 2000) may have been influenced by the effects of the mutation in increasing the sedative action of benzodiazepines, as sedation may give rise to false positive effects in tests employing exploratory activity (Dawson & Tricklebank, 1995). An increased sedative effect of the BZ in the mutant was also seen in the present experiments as a decrease in the lever-pressing rate at higher diazepam doses. However, it must be noted that the α2(H101R) mice used by Wafford et al. (2004) and in the present study differed in their genetic background from those used by Low et al. (2000); the mice in the present report were from a mixed 50% C57Bl/6J – 50% 129 SvEv genetic background, whilst those used by Low et al. (2000) were from the F6 generation of repeated backcrossing to a 129/SvJ background. Thus, it cannot be excluded that the observed enhanced sensitivity to the sedative effects of BZs in α2(H101R) mice in the present study may be limited to the genetic background used. Nevertheless, on either background, the mutation blocked the anxiolytic-like effect of diazepam, strongly suggesting that α2-containing subunits of GABAA receptors contribute importantly to the ability of BZs to exert anxiolytic effects across a variety of models.
That the α2 subtype plays an important role in BZ anxiolysis is supported by its dominant localization in the amygdala, known to play a central role in anxiety and fear, and in mediating the anxiolytic effects of BZs (Scheel-Kruger & Petersen, 1982; Thomas et al., 1985; LeDoux, 2000). While all of the BZ receptor subtypes are expressed in the amygdala, α1, α2 and α3 at a moderate level and α5 to a minor degree (Pirker et al., 2000), it was originally postulated that either the α1 or α2 amygdala-localized subtypes mediate BZs' anxiolytic effects (Sibille et al., 2000). However, subsequent behavioural experiments have suggested that the α1 receptor subtype mediates the sedative but not the anxiolytic properties of BZs (Rudolph et al., 1999; McKernan et al., 2000; Crestani et al., 2002a). The disparate roles of the α1 and α2 subtypes, in mediating BZ effects has been explained by their differential localization in the amygdala. In the central amygdaloid nucleus (CEA), whilst the α2 subtype is predominant, expression of the α1 subtype is very low (Fritschy & Mohler, 1995; Kaufmann et al., 2003), or, in the mouse, even absent (Marowsky et al., 2004). Kaufmann et al. (2003) suggest that the α2 receptor subtype localized in the CEA mediates BZ anxiolytic effects by influencing activity in the direct projections from the CEA to the pedunculopontine tegmental nucleus, an area known to be involved in anxiety and depression (Podhorna & Franklin, 2000), whilst α1 receptors localized in the basolateral amygdala (BLA) (Fritschy & Mohler, 1995) may mediate the sedative effects of BZs.
Further support for the predominant role of the α2 subtype in mediating diazepam's inhibitory effects in the amygdala is provided by evidence that the inhibitory signal induced by diazepam was almost completely abolished in the CEA of mice with point-mutated α2 receptors, whilst it remained unaffected in mice with point mutated α1 and α3 receptors (Marowsky et al., 2004). The α2 subtype is also predominant in mediating the inhibitory signal in the BLA. The strong expression of the α2 subtype in the CEA (Kaufmann et al., 2003) is known to play an important role in mediating the anxiolytic properties of BZs (Kang-Park et al., 2004) and the predominant role of the α2 subtype in mediating the inhibitory signal of diazepam in the amygdala (Marowsky et al., 2004), are all strongly consistent with the behavioural observations that the α2 subtype plays a crucial role in BZ anxiolysis.
Nevertheless, the present data with the α2-subunit mutant do not rule out a role for other subtypes in mediating anxiolytic-like actions of benzodiazepines, and chlordiazepoxide remains effective in the α2(H101R) mutant in the stress-induced hyperthermia model (Dias et al., 2005), implicating receptor subunits other than α2 in BZ anxiolysis, with significant evidence from α3 subtype-selective compounds suggesting a role for the α3 subtype (Collins et al., 2002). More specifically, although α3(H126R) mice continued to display an anxiolytic response to diazepam in the elevated plus maze and light-dark choice test (Low et al., 2000), indicating that BZ modulation of subtypes other than the α3 subtype mediates the anxiolytic properties of BZs in these tests in the mouse, in the rat, an α3-selective inverse agonist was found to be anxiogenic in the plus maze (Collins, 2002). Correspondingly, an agonist selective for α3-containing GABAA receptors has been reported to possess anxiolytic properties in the rat elevated-plus maze and in the CER paradigm (Dias et al., 2005). From this evidence, it seems possible that both the α2 and α3 receptor subtypes might be involved in mediating perhaps different aspects of anxiolytic properties of BZs and would be consistent with the anxiolytic properties of a compound with partial agonist efficacy at these two subtypes (Atack et al., 2006).
This possibility was tested further in the current experiment using the drug, which has selective agonist efficacy at α2, α3, and α5-containing GABAA receptors, while not demonstrating agonism α1-containing receptors. L-838417 showed anxiolytic-like effects at 30 mg/kg in the conditioned emotional response test in both wildtype and α2 mutant mice. While its action at α2-containing receptors may account for L-838417′s action in wildtype mice, it is unlikely to do so in the mutant mouse, and it can thus be concluded that in the mutant, L-838417 must be exerting anxiolytic-like effects, at α3- or α5-containing GABA receptors. As there is no suggestion to date that α5 subunits are involved in BZ's anxiolytic properties (Collinson et al., 2002; Crestani et al., 2002b; Dawson et al., 2005), then these findings suggest strongly that it achieved an anxiolytic effect via its action at α3-containing receptors. A dose of 10 mg/kg L-38417 was anxiolytic in wildtype but not α2(H101R) mice, suggesting that high occupancy levels of α3-containing GABAA receptors induced by 30 mg/kg L-838417 (see Scott-Stevens et al., 2005) are necessary to induce an anxiolytic effect.
Why then, did diazepam not demonstrate an anxiolytic-like effect in these animals, as it too, possesses agonistic activity at α3-containing receptors? The most likely explanation is that at those doses necessary to achieve an anxiolytic effect by agonism at α3-containing receptors, diazepam was markedly sedative in the α2 mutant, obscuring the behavioural expression of anxiolysis. However, it must be noted that is not yet known whether L-838417, in common with other benzodiazepine-site agonists, loses its agonist effects at histidine to arginine point-mutated α2-containing GABAA subtype.
Why should α3-containing receptors be expected to play a role in BZ-mediated anxiolysis? The expression of α3 in both the CEA and BLA (Pirker et al., 2000) would be consistent with its involvement in anxiolysis in the CER model (present experiments) and in the plus maze (Dias et al., 2005). Additionally, α3-containing GABAA receptors are known to be expressed by monoaminergic neurons (Fritschy et al., 1992) and there has long been evidence that disruption of transmission in serotonin (Brody, 1970; Thiebot, 1986; Graeff, 1994) and noradrenergic pathways (Tsaltas et al., 1984; Cole & Robbins, 1987) interferes with acquisition and/or expression of a CER. An ability of BZs to modulate activity of these pathways through activation of α3-containing GABAA receptors would be consistent with their ability to mimic the effects of both serotonin and noradrenaline depletion (Gray & McNaughton, 2000).
Although it has generally been held that the substitution of a histidine for an arginine residue at position 101 of the α2 subunit (or its homologue in other α subtypes) is functionally silent (Low et al., 2000; McKernan et al., 2000), our observations lead us to question this. We found clear differences in the ability of the CS+, when it predicted a mild shock, to suppress operant responding, with the mutant substantially more sensitive than the wildtype. This observation might be consistent with several accounts. The mutation might increase shock sensitivity; it might facilitate learning of the shock-CS+ relationship; or it might lead to an enhanced state of anxiety. We are unable at present to distinguish these possibilities completely. Nevertheless, we saw no evidence that the mutation increased shock sensitivity in terms of flinch threshold. In addition to an apparent increased anxiety-like phenotype, α2(H101R) male (but not female) mice performed a significantly greater number of lever presses in an overnight food-shaping session, compared to the other groups, and α2(H101R) mice, both males and females, performed a greater number of lever presses in the vehicle condition of the diazepam test phase. We place little emphasis on the apparent male-female difference in the food shaping measure as in other experiments at the Sussex laboratory both males and female mutants show increased activity (H.V. Morris and D.N. Stephens, unpublished). These findings, suggest that α2(H101R) mice have higher baseline activity levels than wildtype mice. Again it is important to note that these genotype differences may be specific to the mixed 50% C57BL6J – 50% 129 SvEv genetic background, as these behavioural changes have not been tested in mutants with an alternative genetic background.
Although many studies indicate that the point mutation does not disrupt the distribution of (Rudolph et al., 2001), level of expression of (Wafford et al., 2004) or GABA transmission at (Low et al., 2000; Marowsky et al., 2004), the mutated GABAA receptor subtype, other studies suggest otherwise. It has been shown that the substitution of the histidine with an arginine residue at position 101 in α2 receptors, caused a rightward shift of the GABA dose–response curve when the mutant receptor was coexpressed with γ2 and β3 subunits in HEK 293 cells (Benson et al., 1998). In those experiments, the EC50 value in wildtype α2β3γ2 receptors (74 µm) was increased in the mutated α2(H101R)β3γ2 subtype (154 µm). This observation would be consistent with the enhanced levels of anxiety observed in α2(H101R) mice being due to the reduction of GABA's inhibitory action at mutated α2 receptor subtypes. Further evidence consistent with a nonsilent mutation is shown by the shift to lower GABA affinity in the α1, α3 and α5 mutated receptor subtypes in a heterologous expression system (Benson et al., 1998; Kelly et al., 2002) and the observation that levels of α5-containing GABAA receptors are reduced in α5(H105R) mice (Crestani et al., 2002b). Although Low et al. (2000) found no differences in the response to GABA of wildtype and mutant receptors, in electrophysiological experiments employing cultured hippocampal pyramidal cells, only a single, low concentration of GABA was tested. Nevertheless, both spontaneous miniature inhibitory postsynaptic currents (mIPSCs) and IPSCs evoked by electrically induced synaptic GABA release are normal in CEA slices of the α2 mutant mice (Marowsky et al., 2004), suggesting that at physiological GABA concentrations the H101R mutation has no effect on GABAergic transmission, at least at room temperature. For the present, then, there is no unequivocal explanation of the behavioural changes, including increased anxiety, in our mutant mice.
In conclusion, our findings strongly support the view that the α2 subtype is involved in mediating BZ anxiolysis, shown in the present experiments by a resistance to the anxiolytic properties of a nonsedative dose of diazepam in α2(H101R) mice in the CER paradigm. However, we also found good evidence that another subtype is also implicated in the anxiolytic action of BZs, as L-838417 maintained its anxiolytic properties in the α2(H101R) mice. Interestingly, we observed substantial behavioural differences between wildtype and α2(H101R) mice, including increased activity and enhanced levels of anxiety in α2(H101R) mice in the CER paradigm, which suggests that the point-mutation may not be phenotypically silent.
Acknowledgements
HVM was supported by a CASE studentship provided by the MRC. Thanks to Dr Thomas Rosahl for the generation of the α2(H101R) mice.
Abbreviations
-
- BLA
-
- basolateral nucleus of the amygdala
-
- BZ
-
- benzodiazepine
-
- CEA
-
- central nucleus of the amygdala
-
- CER
-
- conditioned emotional response
-
- CS
-
- conditioned stimulus
-
- VI
-
- variable interval




