Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+-activated K+ current
Abstract
The CA3 area of the mature hippocampus is known for its ability to generate intermittent network activity both in physiological and in pathological conditions. We have recently shown that in the early postnatal period, the intrinsic bursting of interconnected CA3 pyramidal neurons generates network events, which were originally called giant depolarizing potentials (GDPs). The voltage-dependent burst activity of individual pyramidal neurons is promoted by the well-known depolarizing action of endogenous GABA on immature neurons. In the present work, we show that a persistent Na+ current, I-Nap, accounts for the slow regenerative depolarization that triggers the intrinsic bursts in the neonatal rat CA3 pyramidal neurons (postnatal day 3–6), while a slow Ca2+-activated K+ current, sI-K(Ca), is primarily responsible for the postburst slow afterhyperpolarization and consequent burst termination. In addition, we exploited pharmacological data obtained from intracellular recordings to study the mechanisms involved in network events recorded with field potential recordings. The data as a whole indicate that I-Nap and sI-K(Ca) are involved in the initiation and termination, respectively, of the pyramidal bursts and consequent network events underlying GDPs.
Introduction
The interconnected network of mature hippocampal CA3 pyramidal neurons is well-known for its ability to generate population activity in the form of sharp waves and interictal spikes in vivo (Buzsaki, 1986) as well as interictal-like events in vitro (Traub & Wong, 1982; Miles & Wong, 1983). CA3 pyramidal neurons generate voltage-dependent intrinsic bursts (Kandel & Spencer, 1961; Hablitz & Johnston, 1981) in the early postnatal rat hippocampus (Menendez de la Prida & Sanchez-Andres, 2000; Sipiläet al., 2005). During the early postnatal developmental period, GABAA receptor-mediated inputs depolarize CA3 pyramidal neurons and thus promote burst activity (Sipiläet al., 2005). Synchronization of individual bursting pyramidal neurons via synaptic contacts results in network events, seen in individual neurons as giant depolarizing potentials (GDPs), which constitute the main type of electrophysiological activity in the immature hippocampus in vitro (Ben Ari et al., 1989). This view is consistent with the well-known facts that the depolarizing action of GABAA-mediated transmission promotes GDP occurrence (Ben Ari et al., 1989; Garaschuk et al., 1998; Khalilov et al., 1999) and that ionotropic glutamatergic transmission is an absolute requirement for the generation of these events (Ben Ari et al., 1989; Hollrigel et al., 1998; Bolea et al., 1999; Lamsa et al., 2000; Khazipov et al., 2001). In neonatal rats, GDPs are thought to be the in vitro counterparts of in vivo sharp waves (Leinekugel et al., 2002), both occurring at a preferred frequency of ∼0.3 Hz (Leinekugel et al., 2002; Sipiläet al., 2005).
Intrinsic bursts in immature CA3 pyramidal neurons are initiated by a depolarization that becomes regenerative by activation of an, as yet unidentified, slow inward current. This slow regenerative depolarization commences at ∼−60 mV while the action potential threshold is at around −50 mV (Sipiläet al., 2005). The termination of the bursts is associated with a slow afterhyperpolarization (sAHP). While previous studies point to a Ca2+-driven burst generation in mature CA3 pyramidal neurons (Wong & Prince, 1981) and in juvenile CA1 pyramidal neurons (Chen et al., 2005; Metz et al., 2005) as well as to a Na+-driven burst generation in mature CA1 pyramidal neurons (Yue et al., 2005), the intrinsic currents that initiate and terminate the intrinsic bursts in immature CA3 pyramidal neurons have not been identified. In addition, because most of the available literature on GDPs is focused on GABAergic and glutamatergic transmission (e.g. Ben Ari et al., 1989; Strata et al., 1995; McLean et al., 1996; Garaschuk et al., 1998; Hollrigel et al., 1998; Bolea et al., 1999; Khalilov et al., 1999; Lamsa et al., 2000; Ben Ari, 2001; Khazipov et al., 2001; Sipiläet al., 2004), we focused on drugs that likely act on intrinsic pyramidal cell currents in order to study the pharmacological properties of GDPs.
Our data indicate that the slow regenerative depolarization leading to intrinsic bursts in immature CA3 pyramidal neurons is driven by a persistent Na+ current (I-Nap), while a slow Ca2+-activated K+ current, sI-K(Ca), plays a major role in burst termination and the following refractory period. In addition, the data as a whole are consistent with the idea that I-Nap and sI-K(Ca) have a key role in the initiation and termination of GDPs, respectively.
Materials and methods
Experimental procedures were approved by the local Ethics Committee for Animal Research at the University of Helsinki.
Electrophysiological recordings
Wistar rat pups (postnatal days 3–6, where day 0 refers to the day of birth) were decapitated, and the brains were dissected in cold (0–4 °C) oxygenated (95% O2 and 5% CO2) standard solution containing (in mm): 124 NaCl, 3.0 KCl, 2.0 CaCl2, 25 NaHCO3, 1.1 NaH2PO4, 1.3 MgSO4, and 10 d-glucose, pH 7.4 at 32 °C. Coronal brain slices (350 µm) were cut with a vibrating blade microtome (VT1000S; Leica, Nussloch, Germany) and allowed to recover at least for 1 h at 32 °C before use.
Individual slices were transferred into a submersion-type recording chamber perfused with the standard solution (32–33 °C). Axopatch 200A amplifier was used for whole-cell recordings. Patch pipettes had a resistance of 5–8 MΩ when filled with (in mm): 95 K-gluconate, 40 KCl, 5 NaCl, 2 MgCl2, and 10 HEPES, pH 7.2 with KOH. In some recordings, 2 mm EGTA was included in the pipette filling solution. The recorded voltage was corrected with a calculated −10 mV liquid junction potential (Barry, 1994). The intracellular recordings were from CA3 pyramidal neurons that were visually identified using infrared video microscopy (Stuart et al., 1993). All of the cells included in the analyses had a resting membrane potential below −55 mV. Extracellular field potential (fp) recordings were performed with conventional NaCl filled (150 mm) glass capillary electrodes (tip diameter, 5–10 µm) placed in the stratum pyramidale of area CA3. In some fp recordings, the thickness of slices was increased to 600 µm, but this did not have any essential influence on the present results.
Drugs and modified solutions
2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonoamide disodium salt (NBQX), dl-2-amino-5-phosphonovaleric acid (d,l-AP-5), bicuculline methobromide, tetrodotoxin citrate (TTX), riluzole hydrochloride and ZD 7288 were from Tocris Cookson (Bristol, UK). 5,5-Diphenyl-2,4-imidazolidinedione sodium salt (phenytoin) and picrotoxin were from Sigma. Carbamylcholine chloride (carbachol, CCh) was from Acros Organics (Geel, Belgium). For stock solutions, d,l-AP-5 (40 mm) was dissolved in equimolar NaOH, riluzole (100 mm) in DMSO, picrotoxin (50 mm) in ethanol and NBQX (10 mm), bicuculline (10 mm), TTX (1 mm), ZD 7288 (10–100 mm), phenytoin (100 mm) as well as CCh (2.5 mm) in H2O. The final concentration of NBQX and d,l-AP-5 was 10 µm and 40 µm, respectively. Picrotoxin was always applied at 100 µm. The final concentrations of the other drugs are given in the Results.
The nominally Ca2+-free solution was made by omitting CaCl2. Adding 1 mm EGTA and increasing Mg2+ to 4 mm produced no further significant effects on the measured parameters. In some experiments, the extracellular K+ concentration [K+]O was raised up to 9 mm by adding KCl. Co2+ and Ni2+ were added as CoCl2 and NiCl2, respectively.
Analysis
Data were low-pass filtered at 1.6 kHz and digitized at 5 kHz for analyses using the Clampfit (Molecular Devices, Foster City, CA) and Strathclyde Electrophysiology WinWCP and WinEDR (John Dempster, Glasgow, UK) programs, and software programmed under Labview (National Instruments, Austin, TX).
In field recordings, the analysis of extracellular events was performed as described before (Sipiläet al., 2005). Spontaneous network events ‘field GDPs’, fGDPs (see Results) were detected with an amplitude threshold set at a fixed level (∼25–100 µV) in each experiment, and the detected events were visually verified. Network event onset was defined as the time point of the half-maximal amplitude during the negative fp rise and was used as the zero time point in crosscorrelation histograms. The duration of network events was taken from the time points where the averaged fp signal exceeded 10% of its maximal amplitude during rise and decreased below 10% of maximum during decay. Individual spikes in fp recordings reflect the activity of single cells because they are not seen in simultaneous fp recordings with tip separations of ∼30–100 µm, and spikes with distinct amplitudes in a given recording reflect the activity of distinct units.
Because the burst frequency of immature CA3 pyramidal neurons depends on membrane voltage (Menendez de la Prida & Sanchez-Andres, 2000; Sipiläet al., 2005), the interspike interval (ISI) histograms and autocorrelation histograms of spike intervals shown in Fig. 2 were obtained from current-clamp recordings where the Vm was set at a voltage level that was sufficiently positive to trigger bursts at a low frequency (∼0.3 Hz). Bursting neurons showed, as expected, a bimodal ISI histogram where ISIs > 400 ms and < 400 ms were taken as interburst intervals and intraburst spike intervals, respectively (see Fig. 2F). The duration of an intrinsic burst was defined as the time between the first and the last spike within a given burst. The input resistance at peak sAHP was obtained from a linear fit to I–V plots with postburst peak sAHP values at various levels of constant current (see Fig. 1 in Sipiläet al., 2005). Bandpass filtering was used to highlight unit or population events in the illustrations.
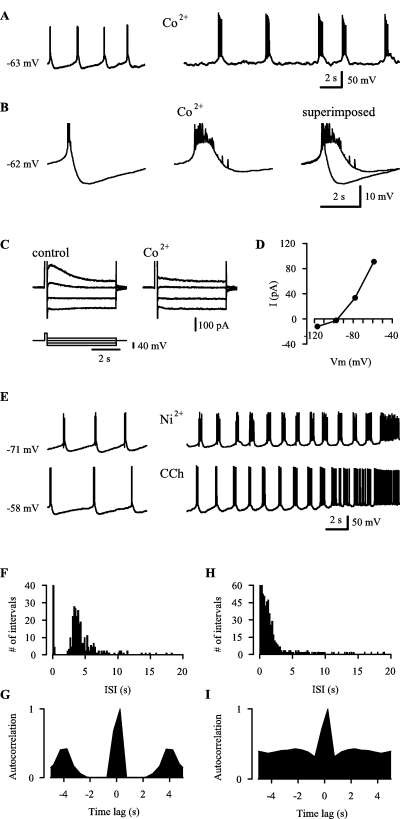
Properties of the postburst sAHP and its role in burst termination and refractoriness. (A) 200 µm Co2+ inhibits the postburst sAHP and leads to prolonged bursts. The initial membrane voltage is indicated at the onset of the traces in A, B and E. (B) Averaged traces aligned by first spikes of 13 and 17 bursts under control conditions and in Co2+, respectively, and superimposed traces. Note in A and B, that the slow regenerative depolarization preceding the spike bursts is not blocked by Co2+. (C) Voltage-clamp recordings showing currents at different voltages induced by a depolarizing step (200 ms) from −80 mV to −20 mV under control conditions and in Co2+. (D) An IV-plot obtained from the peak of the Co2+-sensitive current component in C. (E) 100 µm Ni2+ and 2.5 µm carbachol (CCh) inhibit the postburst sAHP and lead to prolonged bursts. (F and G) An ISI histogram (F) and an autocorrelation histogram of spike intervals (G) obtained from current-clamp recordings of the kind shown in A and E under control conditions. (H and I) Corresponding ISI (H) and autocorrelation (I) histograms obtained from pooled data in 0-Ca2+, Co2+, Ni2+ and CCh (see Results). Data in F–I were obtained from 12 experiments. The bars related to brief intervals reflecting intraburst spikes are truncated in the histograms in F and H. All recordings in NBQX, AP-5 and picrotoxin.
If not otherwise stated, data are presented as mean ± SD. Quantitative comparisons were based on Student's t-tests, and P-values < 0.05 were considered statistically significant.
Results
Intrinsic bursts of immature CA3 pyramidal neurons are driven by a persistent Na+ current
In the presence of NBQX, AP-5 and picrotoxin, 17 out of 26 neonatal CA3 pyramidal neurons were silent at rest (RMP 68.8 ± 5.1 mV) and nine out of 26 cells showed spontaneous bursting that was abolished under current-clamp by membrane hyperpolarization beyond ∼−60 mV (Menendez de la Prida & Sanchez-Andres, 2000; Sipiläet al., 2005). In all neurons, a slowly increasing positive current, starting from a level of membrane voltage where no spikes took place, first led to a depolarization with a linear I–V relationship (Rinput 1.3 ± 0.57 GΩ), but at around −60 mV a slow regenerative depolarization commenced, followed by a burst of action potentials, APs (Fig. 1, A1 and A2; AP threshold −45 ± 3.1 mV, AP amplitude 81 ± 13 mV). The slow regenerative depolarization as well as the spikes were blocked by the Na+ channel antagonist, TTX (0.5–1 µm; n = 6; Fig. 1). With bath application of another Na+ channel antagonist, riluzole (Urbani & Belluzzi, 2000), there was a concomitant positive shift (9.0 ± 2.0 mV; P = 0.003) in the threshold of the slow regenerative depolarization and action potentials, and the number of spikes within bursts was reduced from 3.0 ± 0.8 to 1.16 ± 0.2 within 10 min (n = 4, P = 0.013; Fig. 1B). This was also seen as a near-abolishment of ISIs of < 400 ms (i.e. those ISIs that reflect intraburst spike intervals; see Materials and methods; Fig. 1C). These results indicate that a Na+-dependent current underlies the slow regenerative depolarization leading to intrinsic bursts.
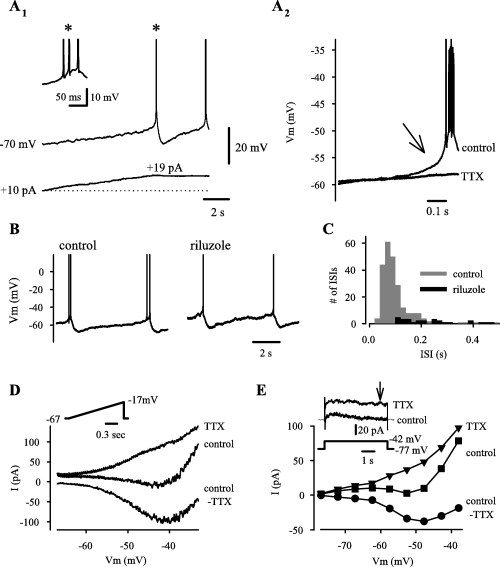
Intrinsic bursts in neonatal CA3 pyramidal neurons are driven by a persistent Na+ current (I-Nap). (A) In a neuron silent at rest, injection of a slowly increasing positive current triggers a slow regenerative depolarization, followed by a burst of spikes and an sAHP (A1). Current (bottom trace) was manually increased until the first burst was initiated. The first burst is shown on an expanded time scale with truncated spikes in the inset (asterisk). Averaged traces (A2) show that the bursts are preceded by a slow regenerative depolarization, which, in addition to the spikes, is blocked by 0.5 µm TTX (six individual bursts were aligned by the first spike for the averaged trace). (B) Bursts of action potentials are reduced to a single spike within 10 min of 10 µm riluzole application. (C) A histogram of interspike intervals (ISIs) obtained from data in control and in riluzole from four recordings. The number of ISIs < 0.4 s (i.e. intraburst-spike intervals) is markedly reduced in riluzole. (D) Voltage-clamp recordings showing currents induced by a depolarizing ramp (inset) in control, in 0.5 µm TTX, and their difference, control-TTX (I-Nap). The traces are averages of three low-pass filtered (30 Hz) recordings. (E) An IV-plot obtained from responses to depolarizing steps at the time point indicated by the arrow in the inset (upper two traces show current, lower trace shows voltage).
We further examined with voltage-clamp whether a persistent Na+-current, I-Nap (McBain & Dingledine, 1992; Azouz et al., 1996; Crill, 1996; Yue et al., 2005) could account for driving the intrinsic bursts. Slow (1.5–6 s) voltage ramps to −20 mV from a holding potential of −80 or −70 mV revealed a negative-slope inward current that was fully blocked by 0.5–1 µm TTX (Fig. 1D). Activation of this current commenced at around −60 mV and peaked at −41 ± 2.0 mV (74 ± 20 pA; n = 4), with a half-maximum value at −53 ± 2.8 mV. A persistent TTX-sensitive current with an I–V similar to that in Fig. 1D was also seen in three out of three cells examined with depolarizing steps lasting 5 s (Fig. 1E). Thus, the voltage-window of I–Nap activation is similar to that of the TTX-sensitive slow regenerative depolarization that precedes intrinsic bursts, which points to a causal relationship.
Properties of the slow AHP and its role in patterning intrinsic burst activity
Termination of the spike bursts of the immature CA3 neurons was associated with a slow afterhyperpolarization, sAHP (cf. Schwartzkroin & Stafstrom, 1980). The input resistance at peak sAHP (−69 ± 2.4 mV) was 55 ± 20% (n = 6; P = 0.03) of the value at a similar but nonoscillatory Vm that was more negative than the threshold of the slow regenerative depolarization (see Fig. 1A). This indicates a primary role of activation of an outward current rather than inactivation or deactivation of an inward current in the generation of the sAHP. The sAHP (measured from averaged traces aligned by the first spike in the burst, Fig. 2B) was reduced by at least 3 mV or completely blocked in 200 µm Co2+ (Fig. 2A). In parallel with the decrease in the sAHP, burst duration was increased in each recording to at least 180% of the control value (the upper limit of burst duration could not be given, because a complete block of the sAHP was associated with continuous spiking; data not shown, see also below). Co2+ had no significant effect on the slow regenerative depolarization during burst initiation (n = 4; Fig. 2A and B).
In voltage-clamp experiments where a 200-ms depolarizing step to −20 mV from a holding potential of −80 or −70 mV was applied to mimic the effects of a spike burst on voltage-gated Ca2+ channels (Sah, 1996), we observed a slowly rising and decaying outward current with a peak at ∼350 ms and duration of 2–4 s. Application of 200 µm Co2+ resulted in a near-complete block of the current (n = 4; Fig. 2C). The reversal potential of the Co2+-sensitive current was −96 ± 4 mV (Fig. 2D) which is close to the calculated equilibrium potential of K+ (−101 mV).
The above data indicate that a slow Ca2+-activated K+-current [sI-K(Ca)] accounts for the sAHP, and the consequent burst termination, in the neonatal CA3 pyramidal neurons (see Sah, 1996; Krause & Pedarzani, 2000; Vogalis et al., 2003). This conclusion was further supported by the findings that 50–1000 µm Ni2+ (n = 3; Fig. 2E), 2.5 µm carbachol (CCh; n = 3; Fig. 2E) or the 0-Ca2+ solution (data not shown) reduced the sAHP by 4 mV or more and prolonged the bursts, which resulted in continuous spiking in some recordings (see Fig. 2E). Furthermore, CCh reduced the sAHP following bursts elicited by depolarizing current steps (n = 3) and inhibited the slow outward current elicited by voltage steps (to −20 mV) in voltage-clamp experiments (n = 3). However, the sAHP was not inhibited by bicuculline arguing against a role for an apamin-sensitive medium AHP (cf. Stocker et al., 1999) (n = 4, not illustrated).
It is also interesting that, similar to Co2+, Ni2+ or the 0-Ca2+ solution did not inhibit the slow regenerative depolarization or the bursts themselves. Hence, our data do not reveal a salient role for voltage-activated Ca2+ currents in the initiation of intrinsic bursts in immature CA3 pyramidal neurons. In particular, low-threshold Ca2+-activated currents (mainly T-type Ca2+ currents; Thompson & Wong, 1991; Avery & Johnston, 1996) are not likely to be responsible for the slow regenerative depolarization that triggers the bursts.
Next, we tested the hypothesis that the sAHP shapes the rhythmicity of intrinsic bursting of the immature CA3 pyramids. The rhythmicity of bursts of spikes at a voltage-level inducing low burst frequencies (see Sipiläet al., 2005) was analysed using ISI histograms and autocorrelation histograms of spike intervals. As shown in Fig. 2F, under control conditions a peak of the ISI histogram was seen at 3.3 s, and a prominent peak in the autocorrelation histogram at ∼4 s (Fig. 2G), both corresponding to a regular burst frequency of ∼0.3 Hz. Furthermore, no ISIs of ∼0.4–2 s were observed indicating that individual neurons are in a functionally refractory state during this period following individual bursts. For analysis of the effect of sAHP on rhythmicity of bursting, we pooled the data obtained with Co2+, Ni2+, the 0-Ca2+ solution and CCh because all of these manipulations inhibited the sAHP as shown above. The peaks at ∼3.3 s and ∼4 s in the ISI and autocorrelation histograms, respectively, were abolished under those conditions that were found to inhibit the sAHP (Fig. 2H and I). Notably, a large number of brief ISIs (< 2 s) were seen under these conditions. Hence, these data indicate that the functional refractory period is set by the sI-K(Ca) in control.
Ih is not required for intrinsic bursting
A hyperpolarizing current step elicited a depolarizing sag that is typical for activation of the hyperpolarization-activated cation current, Ih (Pape, 1996) (Fig. 3A). Strikingly, while Cs+ (2–4 mm) blocked the depolarizing sag (Fig. 3A) in four out of four pyramidal cells, there was no detectable effect on intraburst spike interval, number of spikes per burst, or the sAHP (Fig. 3B). However, a slight, ∼10%, decrease in the duration of interburst intervals was seen under current-clamp. Because inhibition of Ih is, if anything, expected to increase interburst intervals, the observed decrease caused by Cs+ is most likely due to its well-known blocking action on K+ selective channels and consequent membrane depolarization (Hille, 2001).
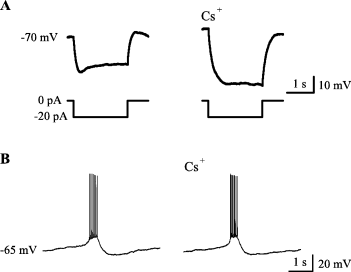
Ih is not crucially involved in the generation of intrinsic bursts in immature CA3 pyramidal neurons. (A) A current-clamp recording showing a depolarizing voltage sag, typical for Ih activation induced by negative current steps (lower traces), which is blocked by 2 mm Cs+. (B) 2 mm Cs+ has no obvious effect on intrinsic bursts.
Effects of inhibitors of I-Nap, sI-K(Ca) and Ca2+ currents on fGDPs
We have recently shown that the preferred frequency of GDP occurrence is similar to that of intrinsic bursts of immature CA3 pyramidal neurons (Sipiläet al., 2005). Furthermore, tonic depolarization not only increased, but also stabilized, GDP frequency in a manner that is consistent with the voltage-dependent properties of the intrinsic pyramidal bursts. Above, we identified the key currents that are involved in the initiation and termination of intrinsic CA3 pyramidal bursts. Our next aim was to examine further whether these intrinsic currents are crucial in the generation of GDPs. We want to emphasize that, because there are no specific antagonists available to block the key currents, I-Nap and sI-K(Ca) (see Stefani et al., 1997; Taddese & Bean, 2002; Vogalis et al., 2003), our inferences regarding network activity are based on the physiological and pharmacological data as a whole (see Discussion for detailed considerations).
The Na+-current blockers, riluzole (n = 4) and phenytoin (n = 4; Segal & Douglas, 1997), inhibited fGDPs in a dose-dependent manner (Fig. 4A and B). In addition, CA3 population bursts that are seen in the presence of ionotropic GABA receptor antagonists (see Ben Ari et al., 1989; Sipiläet al., 2005) were blocked by 10 µm riluzole (n = 4, not illustrated).
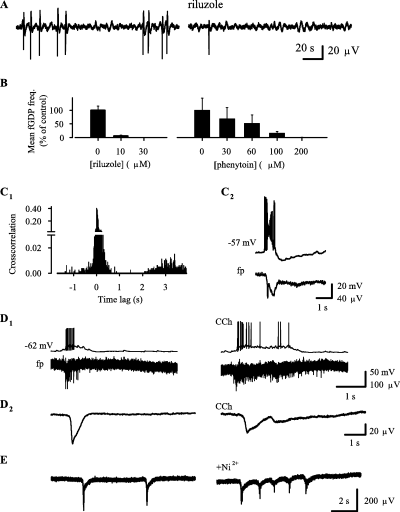
Effects of inhibitors of I-Nap and sI-K(Ca) on giant depolarizing potentials. (A) fp recording showing an inhibition of field GDPs (fGDPs) by riluzole (band-pass filter at 0.2–30 Hz). (B) Dose–response of riluzole and phenytoin on fGDP frequency (mean + SEM, n = 4). (C) A cross-correlation histogram of fp spikes vs. fGDPs (C1) show a silent period after fGDPs, which is similar to the duration of the sAHP following intracellular GDPs (upper trace in C2; lower trace shows a simultaneous fGDP with a low-pass filter at 20 Hz). (D1) Effect of 2.5 µm CCh on fGDPs as seen in simultaneous intracellular (upper trace) and fp (lower trace; no filter) recordings. (D2) Averaged traces, obtained from ten and seven individual fGDPs under control conditions and in 2.5 µm CCh, respectively, show the effect of CCh on the slow negative shift (low-pass filter at 30 Hz). (E) Effect of 100 µm Ni2+ on fGDPs.
fGDPs are associated with fp spikes as shown in primary recordings and cross-correlation histograms (Fig. 4C and D). Notably, cross-correlation histograms of fp spikes vs. fGDPs showed little spiking within a time window of ∼1–2 s following fGDPs, whereas the probability of spike occurrence showed a progressive increase up to ∼3.5 s (Fig. 4, C1). This is consistent with previous data where inter-fGDP interval histograms had a peak at ∼3.5 s but no intervals < 2 s (Sipiläet al., 2005). fGDPs were followed by an sAHP in six out of nine simultaneous intracellular and fp recordings (Fig. 4, C2). The functional refractory period of fGDPs is consistent with the duration of the sAHP.
Consistent with the inhibition of sAHP and prolongation of intrinsic CA3 pyramidal bursts (see Fig. 2), bath application of 2.5 µm CCh progressively increased fGDP duration, as calculated from the averaged slow fp shifts (see Materials and methods and Fig. 4, D2), to 250–1220% of the value in control (650 ± 150 ms) in four out of four recordings (Fig. 4D). Spikes and irregular slow fp shifts were often seen in the presence of CCh (Fig. 4, D1 and D2 right traces) during the ∼1–2 s interval following fGDPs that was silent under control conditions (Fig. 4, D1 and D2 left traces, see also Fig. 4, C1). The 0-Ca solution progressively increased fGDP duration in six out of six recordings to 200–1400% of the value seen under control conditions (not illustrated). Prolonged application of CCh and the 0-Ca solution often blocked fGDPs and only spike bursts (up to ∼4 s) could be seen (not illustrated).
In three out of four recordings, 100 µm Ni2+ prolonged the typical all-or-none waveform of fGDPs into ‘clusters’ of slow negative shifts that had a duration of 880–3270% compared to fGDPs under control conditions (Fig. 4E). This effect is most likely explained by a block of the sAHP in immature CA3 pyramidal neurons (see Fig. 2E) and the consequent disruption of the network refractory period. The finding that the network events occurred in the presence of Ni2+ in all four recordings suggests that T-type Ca2+ channels (Thompson & Wong, 1991; Avery & Johnston, 1996) are not crucial for fGDP initiation.
Ih is not required for fGDP generation
In a previous study, 0.3 mm Cs+ was reported to block intracellular GDPs (Strata et al., 1997) suggesting that Ih has an important role in GDP generation. In contrast to Strata et al. (1997), we saw an increase in fGDP frequency with 0.5–5 mm Cs+ in eight out of eight experiments. At a concentration of 2–4 mm, Cs+ increased fGDP frequency from 0.015 ± 0.01 Hz to 0.17 ± 0.06 Hz (n = 4, P = 0.014, [K+]O = 3 mm; Fig. 5A). This is an important observation, because if Ih were involved in pacemaking fGDP activity, inhibition of Ih would, of course, be expected to decrease fGDP frequency. Furthermore, in experiments where [K+]O was decreased to 1–2 mm, fGDPs were blocked (Sipiläet al., 2005). Strikingly, a subsequent addition of Cs+ (2–4 mm) re-induced the occurrence of fGDPs in six out of six recordings (Fig. 5B). Thus, the enhancement of fGDP frequency by Cs+ is most likely caused by inhibition of K+ selective channels (Hille, 2001). Another Ih blocker, ZD 7288 (Harris & Constanti, 1995), has been recently shown to inhibit bursts of synaptic currents that are associated with GDPs in intracellular recordings (Bender et al., 2005). We found that in four out of five fp recordings, bath application of 10 µm ZD 7288 increased fGDP frequency by a factor of 1.5–2 within 5–25 min (Fig. 5C). A higher concentration (50–100 µm) of ZD 7288 completely blocked fGDPs (n = 5; not illustrated). Because ZD 7288 is known to have unspecific effects at 10 µm and even lower concentrations (Chevaleyre & Castillo, 2002; Do & Bean, 2003; Chen, 2004; Nolan et al., 2004), it is unclear whether results with this drug indicate a modulatory role of Ih in the generation of fGDPs. While the postburst sAHP in immature CA3 pyramidal neurons (see above) is likely to activate Ih to some degree, the present experiments with Cs+ and 10 µm ZD 7288 clearly show that synchronous bursting of immature CA3 pyramidal neurons (fGDPs) occurs in the absence of Ih.
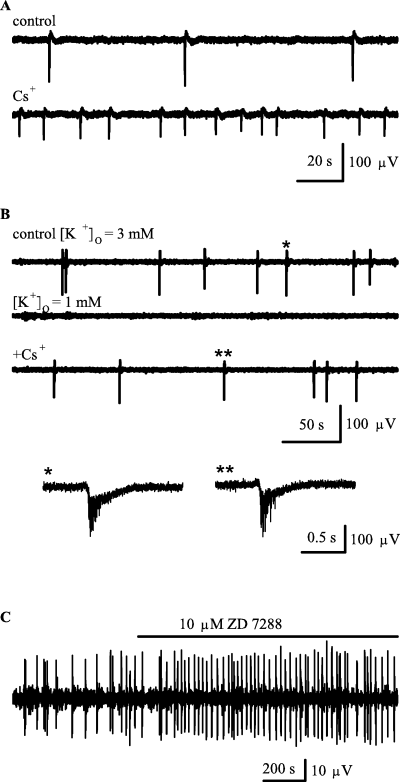
Ih is not required for fGDP generation. (A) fp recordings showing the effect of 2 mm Cs+ on fGDPs. (B) Lowering extracellular K+ concentration [K+]O to 1 mm blocks fGDPs, while a subsequent addition of 2 mm Cs+ induces a recovery of the network events. fp traces on the bottom show representative fGDPs (indicated by * and **; low-pass filter at 500 Hz) in control (left) and in the presence of 2 mm Cs+ and 1 mm[K+]O (right). (C) Field potential recording showing an enhancement of fGDP frequency by 10 µm ZD 7288.
Discussion
We have recently shown (Sipiläet al., 2005) that endogenous GABAergic depolarization promotes voltage-dependent intrinsic bursts in neonatal CA3 pyramidal neurons, which, in turn, pace network events seen as GDPs and fGDPs in intra- and extracellular recordings, respectively. Our present data show that the intrinsic bursts are driven by a slow regenerative depolarization, which is generated by I-Nap, while burst termination and the subsequent refractory period are largely attributable to sI-K(Ca). Consistent with the idea that pyramidal bursts generate fGDPs, these network events were blocked and prolonged by inhibitors of I-Nap and sI-K(Ca), respectively.
Roles of I-Nap and sI-K(Ca) at the unit level
An important feature of the intrinsic bursts of immature CA3 pyramidal neurons is that they are driven by a depolarization that becomes regenerative below action potential threshold (see Fig. 1). This type of a slow regenerative depolarization is not produced by Ih (Pape, 1996), or T-type Ca2+ currents (Thompson & Wong, 1991; Avery & Johnston, 1996), which would require a preceding hyperpolarization to remove channel inactivation (Perez-Reyes, 2003). Consistent with this view, the slow regenerative depolarization, and the bursts themselves, were not blocked by Co2+, Ni2+, the 0-Ca2+ solution or Cs+, while an inhibitory effect was seen with Na+-channel antagonists. Furthermore, the voltage-window of activation of both the slow regenerative depolarization and I-Nap were similar.
The observations that Co2+, Ni2+ and the 0-Ca2+ solution blocked the postburst sAHP, prolonged intrinsic bursts and disrupted the subsequent functional refractory period, clearly show that the sAHP is dependent on Ca2+ influx. This is in agreement with voltage-clamp data showing that a depolarizing pulse produced a slow transient outward current. Also, the sAHP was associated with a decrease in the input resistance, which argues for activation of sI-K(Ca) rather than deactivation of Ih. Furthermore, the sAHP was inhibited by CCh, which is known to inhibit sI-K(Ca) but to enhance Ih (Cobb & Davies, 2005). Thus, the data as a whole support a major role for sI-K(Ca) in burst termination in the immature CA3 pyramids. It should be noted that the Ca2+ influx required for triggering the sAHP may be mediated by low- as well as high-voltage-activated Ca2+ channels (Avery & Johnston, 1996) but this was not studied further. This Ca2+ influx is also expected to depolarize the neuron, but our results do not reveal a salient role for this type of a depolarization during the intrinsic pyramidal bursts. It should be noted also that the sAHP was not inhibited by bicuculline, which blocks the apamin-sensitive medium-duration AHP in hippocampal pyramidal neurons (Stocker et al., 1999). The slow AHP is not sensitive to apamin in pyramidal neurons (Stocker et al., 1999).
In summary, our data point to the following mechanism of burst generation in immature CA3 pyramidal neurons. I-Nap depolarizes the cell membrane to spike threshold, and the neuron will fire spikes in a burst until the sI-K(Ca) hyperpolarizes the cell and, consequently, terminates the spiking. After the decay of sI-K(Ca), I-Nap is again activated and a subsequent burst is generated. This sequence of events resulting in rhythmic cellular bursting occurs only if the membrane potential is positive to the activation threshold of I-Nap (∼−60 mV, see Fig. 1A). I-Nap can be activated by any type of depolarization, including elevation of [K+]O, current injection and synaptic GABAergic as well as glutamatergic responses. However, an endogenous GABAA receptor-mediated current tonically depolarizes the immature CA3 pyramidal neurons (Ben Ari et al., 1989) and, hence, has a significant role in promoting the recurrent burst activity (see Sipiläet al., 2005).
Functional roles of pyramidal intrinsic currents in the generation of fGDPs
Previous data (Sipiläet al., 2005) suggest that the intrinsic bursting behaviour of immature CA3 pyramidal neurons shapes the temporal pattern of fGDP activity. In this study, we have examined the underlying intrinsic currents in this process. fGDP initiation is characterized by a build-up period (∼0.3 s) of neuronal activity occurring simultaneously in pyramidal cells and interneurons (Menendez de la Prida & Sanchez-Andres, 2000; Sipiläet al., 2005). fGDPs are followed by a refractory period of ∼2–3.5 s (Fig. 4C) and fGDPs have the highest probability of occurrence at intervals ∼2.5–5 s with a peak at ∼3.5 s (∼0.3 Hz) although intervals up to many minutes are often seen under standard experimental conditions (Sipiläet al., 2005).
Consistent with the intracellular recordings, antagonists of I-Nap and sI-K(Ca) inhibited or prolonged fGDPs, respectively. Because prolonged fGDP ‘clusters’ were seen in the presence of Ni2+, T-type Ca2+ currents are not likely to be critically involved in fGDP initiation. The most prominent effect of the Ih blocker, Cs+, was an increase in fGDP frequency, which indicates that Ih is not critically involved in fGDP generation either. The increase in fGDP frequency is probably caused by inhibition of K+ selective channels (see Results). In our recordings, synchronous bursting of CA3 pyramidal cells (fGDPs) was not inhibited by ZD 7288 at a concentration that is known to significantly reduce Ih (Harris & Constanti, 1995). Notably, the enhancement of fGDP frequency by a maintained depolarization that takes place upon elevation of [K+]O or application of GABAA receptor agonists (Khalilov et al., 1999; Lamsa et al., 2000; Sipiläet al., 2005) is not to be expected from the typical behaviour of Ih or T-type Ca2+ currents (see above) but is consistent with the properties of I-Nap. In summary, the present data as a whole indicate that Ih and T-type Ca2+ currents are not required for fGDP generation although it is possible that they modulate fGDP activity in an as yet unidentified way. In addition, given that pyramidal bursting triggers fGDPs (Sipiläet al., 2005), I-Nap is likely to be involved in the generation of these network events. This conclusion is consistent with the present observations that antagonists of I-Nap blocked fGDPs, although it is clear that unspecific drug actions cannot be excluded (Stefani et al., 1997). It is worth noting here that riluzole does not have a general inhibitory effect on spontaneous network events. For instance, this drug did not block population activity generated by respiratory neurons even at a concentration that was ten times higher (Del Negro et al., 2002) than the one used presently.
Despite the lack of specific antagonists, a key role of sI-K(Ca) in fGDP termination and refractory period is supported by the following findings. First, the functional refractory period following fGDPs (Fig. 4, C1) is consistent with the duration of the sAHP (2, 4, C2; see also Sipiläet al., 2005). Second, inhibition of Ca2+ currents led to an increase in the duration and disrupted the functional refractory period of fGDPs. Third, CCh increased the duration and disrupted the functional refractory period following fGDPs. The effect of CCh cannot be explained solely by an effect on GABAergic transmission (Behrends & ten Bruggencate, 1993; Avignone & Cherubini, 1999), because this drug prolonged intrinsic CA3 pyramidal bursts in the presence of GABAA receptor antagonists (see Fig. 2E). A depressant effect on glutamate release (Vogt & Regehr, 2001) cannot explain the observed effect. In addition, a critical role for an M-current is unlikely, because this current is inhibited by higher concentrations of CCh (Madison et al., 1987).
As pointed out in the Introduction, most of the available pharmacological data on GDP activity is related to GABAergic and glutamatergic transmission. Because there are no entirely selective blockers of I-Nap or the sI-K(Ca), we used several pharmacological as well as other experimental manipulations to study their role in fGDP generation. As a whole, the present and previous data (Sipiläet al., 2005) indicate that the intrinsic bursting of pyramidal neurons plays a key role in the temporal patterning of fGDP activity. In particular, I-Nap drives fGDPs by generating spontaneous intrinsic bursts and by amplifying the excitatory postsynaptic responses in immature CA3 pyramidal neurons during the initiation of the network events. On the other hand, sI-K(Ca) has a direct role in the termination and refractory period of intrinsic as well as fGDP-associated pyramidal bursts. Notably, this inhibitory effect is qualitatively different from that of GABAB receptors, which influence the duration of fGDPs by suppressing presynaptically GABAergic and glutamatergic transmission (McLean et al., 1996), and that of GABA transporter 1, which shortens the decay of the slow GABAergic transients associated with fGDPs (Sipiläet al., 2004). Recent work in vivo (Leinekugel et al., 2002) has suggested that fGDPs are the in vitro counterpart of hippocampal sharp waves (Buzsaki, 1986). Hence, the presently characterized mechanisms are most likely involved in the patterning of the early sharp waves in the developing hippocampus in vivo (Sipiläet al., 2006).
Abbreviations
-
- CCh
-
- carbachol
-
- fGDP
-
- field giant depolarizing potential
-
- GDP
-
- giant depolarizing potential
-
- Ih
-
- hyperpolarization-activated cation current
-
- I-Nap
-
- persistent Na+ current
-
- ISI
-
- interspike interval
-
- sAHP
-
- slow afterhyperpolarization
-
- sI-K(Ca)
-
- slow calcium-activated potassium current
-
- TTX
-
- tetrodotoxin
Acknowledgements
This work was supported by the Academy of Finland and by the Sigrid Juselius Foundation.




