Isolation and characterization of neural precursor cells from the Sox1–GFP reporter mouse
Abstract
We have made use of a reporter mouse line in which enhanced green fluorescence protein (GFP) is inserted into the Sox1 locus. We show that the GFP reporter is coexpressed with the Sox1 protein as well as with other known markers for neural stem and progenitor cells, and can be used to identify and isolate these cells by fluorescence-activated cell sorting (FACS) from the developing or adult brain and from neurosphere cultures. All neurosphere-forming cells with the capacity for multipotency and self-renewal reside in the Sox1–GFP-expressing population. Thus, the Sox1–GFP reporter system is highly useful for identification, isolation and characterization of neural stem and progenitor cells, as well as for the validation of alternative means for isolating neural stem and progenitor cells. Further, transplantation experiments show that Sox1–GFP cells isolated from the foetal brain give rise to neurons and glia in vivo, and that many of the neurons display phenotypic characteristics appropriate for the developing brain region from which the Sox1–GFP precursors were derived. On the other hand, Sox1–GFP cells isolated from the adult subventricular zone or expanded neurosphere cultures gave rise almost exclusively to glial cells following transplantation. Thus, not all Sox1–GFP cells possess the same capacity for neuronal differentiation in vivo.
Introduction
The biology of neural stem cells (NSCs) is of considerable interest, not only for understanding their roles in normal development of the nervous system but also for use in cell replacement therapy for neurodegenerative disorders (Bjorklund and Lindvall, 2000). Self-renewing and multipotent NSCs reside initially in the early neuroepithelium and later in the ventricular zone (VZ) and subventricular zone (SVZ) during embryogenesis, and they also persist in some areas of the adult brain (Temple, 2001). Several markers are currently used to identify stem cells in the nervous system. These markers include the intermediate filament protein Nestin (Lendahl et al., 1990), the RNA-binding protein Musashi-1 (Sakakibara et al., 1996; Sakakibara and Okano, 1997), and cell-surface markers such as Prominin/CD133 (Corbeil et al., 2000; Weigmann et al., 1997; Uchida et al., 2000), CD15 (Capela and Temple, 2002) and the P75 receptor (Stemple and Anderson, 1992). These markers have been used in attempts to isolate NSCs using fluorescence-activated cell sorting (FACS), either by means of antibodies raised against cell-surface epitopes or by introducing a reporter gene under the control of cell-specific promoters (Kawaguchi et al., 2001; Keyoung et al., 2001; D'Amour and Gage, 2003). However, all these markers have been shown to be selective rather than specific for NSCs.
To identify and isolate a transplantable population of cells highly enriched in NSCs, we made use of a reporter mouse line in which the gene coding for green fluorescent protein (GFP) is inserted into the Sox1 locus (Aubert et al., 2003). Sox1 is the earliest known marker of neural precursors in the mouse embryo (Pevny et al., 1998; Wood and Episkopou, 1999) and, except for expression in the lens, Sox1 is exclusively present in proliferating neural precursors from the neural plate stage and onwards. It is down-regulated when the cells start to differentiate into neurons and glia (Pevny et al., 1998). The GFP knock-in mice permit visualization of Sox1 expression by fluorescence microscopy both in fixed tissue and in live cells, and it also allows identification and purification of Sox1-expressing cells by FACS.
We have carefully analysed the in vivo expression pattern of Sox1–GFP and confirm that there is a good overlap between Sox1 protein and GFP expression in the embryonic forebrain VZ, where GFP also colocalizes with other markers of immature neural cells including Nestin, Musashi-1 and Sox-2. Although expressed widely throughout the developing nervous system, Sox1–GFP expression was clearly absent from the ventral midbrain, in the region that contains the precursors for the dopaminergic neurons. The Sox1–GFP cells isolated from the forebrain are expandable in neurosphere culture, and we show that all multipotent and self-renewing NSCs are contained in the Sox1–GFP-positive subpopulation within these spheres. Further, after grafting to the neonatal striatum, foetal Sox1–GFP-expressing cells are capable of generating astrocytes and oligodendrocytes as well as regionally specified neurons. Thus, in the brain regions where Sox1 is expressed, Sox1–GFP appears to be an excellent marker for the isolation of transplantable neural precursors and putative NSCs.
Materials and methods
Animals
Neonatal Sprague-Dawley rats were used as graft recipients in the transplantation experiments. Timed pregnant [day of vaginal plug = embryonic day 0.5 (E0.5)] wild-type (WT) or Sox1–GFP transgenic mice were generated by crossing WT NMRI females with WT or heterozygous (+/–) Sox1–GFP (Aubert et al., 2003) males. Following lethal exposure to CO2 the embryos were removed and the WT and Sox1–GFP+/– embryos were identified under a fluorescence microscope before being taken for dissection or immersion-fixed overnight in 4% paraformaldehyde (PFA) in phosphate-buffered saline (PBS; 0.1 m) at 8 °C. Fixed embryos were cryoprotected in 15% sucrose and cryosectioned at a thickness of 12 µm. All animal-related procedures were conducted in accordance with local ethical guidelines and approved animal care protocols.
Tissue dissection and preparation of single-cell suspensions
Whole telencephalon or subdissections of various brain regions [brainstem, cortex, lateral ganglionic eminence (LGE) and medial ganglionic eminence (MGE)] were dissected from E12.5–13.5 Sox1–GFP or WT mice and mechanically dissociated into single-cell suspensions in PBS without Ca2+ or Mg2+ (PBS–Ca2+/Mg2+ Gibco). Tissue from 20 Sox1–GFP+/– embryos was pooled for the transplantation experiments. In order to obtain GFP+ve cells from adults, three adult Sox1–GFP mice received lethal injections of pentobarbitone before the brains were removed and placed on ice for 5 min. A brain block (Kopf, Germany) was used to collect 1-mm coronal sections which were placed in L-15 dissection medium (Gibco) and the lateral aspects of the ependymal zone and SVZ were precisely removed under a Leica dissection microscope. The tissue pieces were incubated at 37 °C for 15 min in 10 mL of dissociation solution (HBSS–Ca2+/Mg2+ Gibco) supplemented with HEPES, 0.015 m (Gibco); d-glucose, 5.4 mg/mL, 1 × trypsin, DNase, 80 U/mL; hyaluronidase, 0.7 mg/mL; and kynerenic acid, 2 mg/mL (all from Sigma), followed by gentle trituration, and incubation in the same solution at 37 °C for another 10 min. Excess solution was removed and the tissue further mechanically dissociated to a single-cell suspension.
Bulk neurosphere cultures
Single cells were plated at a density of 5 × 105 cells/mL in a serum-free expansion medium composed of a 1 : 1 mixture of Dulbecco's modified Eagle's medium (DMEM) and F12 (both Gibco), supplemented with epidermal growth factor (20 ng/mL; human recombinant; R and D Systems, Oxon, UK) and basic fibroblastic growth factor (10 ng/mL; human recombinant; R and D Systems), a defined hormone and salt mixture (Reynolds and Weiss, 1992) including insulin, 20 µg/mL; transferrin, 100 µg/mL; progesterone, 20 nm; putresceine, 60 µm; sodium selenite, 30 nm (all from Sigma); and penicillin–streptomycin, 1% (Gibco). Cultures were maintained at 37 °C in a humid atmosphere with 95% O2 and 5% CO2. Fresh medium was added to the cultures every 2nd or 3rd day. After 7 days, neurospheres were either processed for FACS analysis or passaged by mechanical dissociation to a single-cell suspension and then re-suspended at a density of 105 cells/mL. For the transplantation experiments, cultures passaged 5 or 6 times were prepared for FACS on day 7 after the last passage.
Cell preparation for FACS
Cells were mechanically dissociated to obtain a single-cell suspension in a solution containing 2 mm EDTA (Sigma) and 0.5% bovine serum albumin (Sigma) in PBS–Ca2+/Mg2+. Cells were harvested by centrifugation (500 g, 5 min, 4 °C), filtered through a 70-µm cup filter (Falcon) and kept on ice until FACS analysis. Cell sorting was performed on a FACS Vantage TS flow cytometer (Becton Dickinson, San Jose, CA, USA) equipped with 488-nm argon and 633-nm He-Ne lasers. In all sorting procedures, an initial gating was performed in order to exclude cell debris and cell doublets based on forward- and side-scatter information, dead cells were excluded based on labelling with 7-aminoactionomycin-D (7-AAD; Sigma; 10 µL/mL) and the system was first calibrated with WT tissue in order to determine the background signal for GFP fluorescence. For in vitro experiments, three subpopulations were sorted according to GFP intensity: GFPNegative, GFPHigh and GFPLow populations. The GFPLow and GFPHigh populations were defined arbitrarily by gating approximately half-way between the lower and upper limits for the GFP signal. When sorting from neurospheres or acutely dissociated foetal or adult brain for the transplantation experiments, all GFP+ve cells were taken.
Clonal analysis and in vitro differentiation
Cells were plated at clonal density (10 cells/µL) with an automated cell deposition unit (Becton Dickinson) into a 96-well plate containing a mixture (1 : 4) of conditioned medium and expansion medium (see experimental design in Fig. 4D). After 7 days in vitro, the number of first-generation spheres was counted. Individual first-generation spheres were then transferred into new wells and the single isolated spheres were dissociated into a single-cell suspension to study the formation of second-generation spheres. This procedure was repeated to study third-generation sphere formation. For in vitro differentiation, 10-day-old third-generation spheres were plated onto poly l-lysine-coated chamber slides in the same medium, with the exception that growth factors were replaced with 1% foetal bovine serum. After 7 days in vitro cells were fixed for 15 min with 4% PFA at room temperature (RT), rinsed three times in PBS and processed for immunocytochemistry.
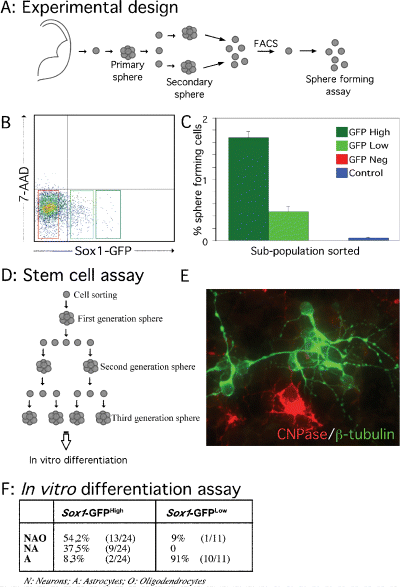
Stem cell properties of Sox1–GFP cells. (A) Experimental design: whole forebrains from E12.5 Sox1–GFP+/– mice were dissected and dissociated into a single-cell suspension to generate neurosphere cultures. After the first passage, cells from secondary spheres were analysed by FACS and three subpopulations were isolated based on the GFP intensity. (B) Dot plot from FACS of secondary spheres showing the gates used to define three subpopulations (Sox1–GFPNegative, Sox1–GFPLow and Sox1–GFPHigh). Dead cells were identified by labelling with 7-AAD (y-axis) and excluded from subsequent analysis. (C) Sphere-forming assay: the three subpopulations and a control (all 7-AAD–ve cells, but not sorted for GFP) were plated directly after FACS isolation into a 96-well plate at a clonal density (10 cells/µL). The bars in the histogram indicate the percentage of spheres generated after 7 days in vitro per total number of single sorted cells for each subpopulation (n = 3). (D) Experimental design of the clonal stem cell assay. To study self-renewal and multipotent properties of sorted cells, first-generation spheres were subcloned and dissociated into single cells for a second-generation sphere-forming assay. After 7 days in vitro, second-generation spheres were subcloned, dissociated and cultured for a futher 7 days to generate third-generation neurospheres. Third-generation spheres were transferred into a poly l-lysine-coated chamber and differentiated. (E) Immunocytochemical detection of (green) β-III tubulin and (red) CNPase, identifying neurons and oligodendrocytes, respectively, in third-generation spheres. (F) In vitro differentiation assay. Percentages of tripotent, bipotent and unipotent clones generated by third-generation GFPLow and GFPHigh cells.
Transplantation procedure
Sorted GFP+ve cells were resuspended in HBSS–Ca2+/Mg2+ at 1 × 105 cells/µL (foetal tissue), 1 × 104 cells/µL (neurospheres) or 0.5 × 104 cells/µL (adult tissue), with the low yield of neurosphere and adult sorted cells dictating the lower density of these cell suspensions. Under deep hypothermic anaesthesia, 1 µL of cell suspension was delivered over 2 min to the striatum of postnatal day 3 rats using a glass capillary attached to the tip of a 5 µL Hamilton syringe as previously described (Nikkhah et al., 2000). Injection co-ordinates (mm) were anterior, 0.7 and lateral, 1.9 to bregma, and 2.9 below the dural surface. A minimum of four animals were grafted with each cell preparation. Six weeks after transplantation animals received lethal doses of pentobarbitone and were transcardially perfused with 0.9% saline followed by 4% PFA. The brains were postfixed for 2 h, cryoprotected in 25% sucrose and sectioned on a freezing microtome (30-µm coronal sections).
Immunohistochemistry and immunocytochemistry
Slide-mounted or free floating sections, or plated cells, were preincubated in potassium-buffered PBS (KPBS) with 5% normal serum and 0.025% Triton-X (Amresco, Solon, Ohio, USA) for 1 h at RT, followed by incubation overnight at RT with primary antibodies diluted in the incubation solution. For peroxidase-based detection of the M2M6 antibody, the tissue was quenched of endogenous peroxidase activity for 15 min with 3% H2O2 in KPBS prior to incubation with primary antibody. The primary antibodies used were: mouse anti-adenomatous polyposis coli (APC; Calbiochem, 1 : 200), mouse anti-polysialylated neural cell adhesion molecule (PSA-NCAM) (Sigma, 1 : 500), mouse anti-Nestin (BD Biosciences, 1 : 500), rat anti-Musashi1 (kindly provided by Dr H. Okano, 1 : 1000), mouse anti-neuronal nuclei (NeuN; Chemicon, 1 : 100), chicken anti-GFP (Chemicon, 1 : 5000), rat anti-M2 and -M6 (courtesy of Dr C. Lagenaur, available from Developmental Studies Hybridoma Bank; both 1 : 50), rabbit anti-dopamine- and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32; Chemicon, 1 : 1000), rabbit anti-choline acetyltransferase (ChAT; Chemicon, 1 : 500), mouse antiparvalbumin (Sigma, 1 : 2000), rabbit anti-SOX1 and rabbit anti-SOX2 (both kindly provided by Dr R. Lovell-Badge, 1 : 1000), mouse anti-2′3′-cyclic nucleotide-3′-phosphodiesterase (CNPase) (1 : 100, Sigma), rabbit anti-glial fibrillary acidic protein (GFAP; 1 : 1000, DAKO), and rabbit antiβ-III-tubulin (1 : 1000, Covance, Berkeley, California, USA). After three rinses in kPBS, sections or plated cells were incubated with fluorescein isothionocyanate–Cy2- and/or Cy3-conjugated secondary antibodies (Jackson Immunoresearch, 1 : 200) for 2 h at RT for fluorescent labelling of the primary antibodies. For nuclei staining, plated cells were incubated for 5 min in 4,6-diamidino-2-phenylindole (DAPI; Sigma, 1 : 1000). For peroxidase-based labelling of the M2M6 antibody, a peroxidase-conjugated donkey antirat secondary antibody (Jackson Immunoresearch, 1 : 200) was used (2 h, RT), before the material was incubated with 3,3-diaminobenzidine (0.5 mg/mL, Sigma) and precipitation of the chromophore was catalysed by the addition of 1% H2O2. Material labelled with fluorescent markers was coverslipped using PVA-DABCO (Peterson, 1999), while the 3,3-diaminobenzidine-labelled material was dehydrated in alcohol and xylene before being coverslipped with DEPEX™ mounting media (BDH).
Quantification
Single-cell suspensions generated from E12.5 cortex, LGE and MGE were re-suspended at a density of 105 cells/mL in DMEM solution, and plated on poly l-lysine-coated chamber slides. Less than two hours after plating, cells were fixed for 15 min in ice-cold 4% PFA and immediately processed for immunostaining as described above. Total cell numbers in random visual fields were quantified by counting all DAPI-stained nuclei, and the proportion of cells expressing Sox1, Sox2, Nestin, Musashi1 or PSA-NCAM was also determined. Quantifications were performed in triplicate and a minimum of 1000 cells were counted. All results are reported as mean ± 1 SEM.
Imaging and schematic drawings
The macroscopic fluorescence image of an embryonic Sox1–GFP brain illustrated in Fig. 1 was acquired using a dissection microscope (Leica) equipped with a digital camera (ProgRes; Jenoptik, Germany). Confocal images (5-9) were acquired using a Leica DMRE laser-scanning microscope equipped with GreNe, HeNe and Argon lasers.
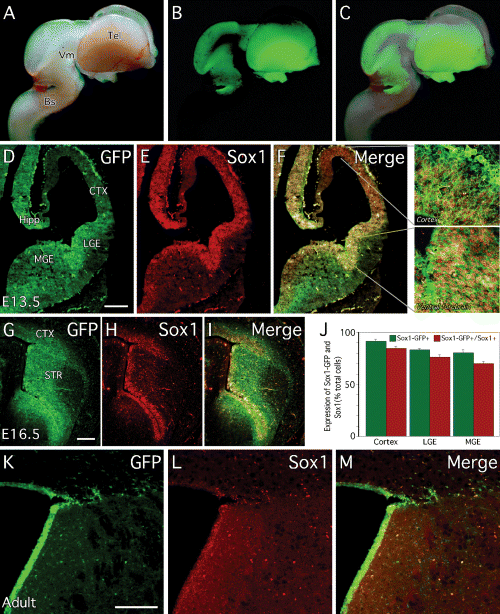
GFP expression in the developing and adult Sox1–GFP mouse brain. (A) Brightfield, (B) native GFP fluorescence and (C) a merge of these panels show the wide distribution of GFP throughout the E13.5 brain of Sox1–GFP+/– mice. At (D–F) E12.5 and (G–I) E16.5, immunohistochemistry for (D and G) GFP and (E and H) Sox1 shows (F and I, and high magnification panels from F) extensive overlap of these proteins. (J) Cell counting confirmed that the vast majority of cells from the developing (E12.5) telencephalon of Sox1–GFP mice express both GFP and Sox1. In the adult brain, (K) GFP and (L) Sox1 are (M, merged image) expressed in cells aligning the lateral ventricles. Bs, brainstem; CTX, cortex; Hipp, developing hippocampus; Str, striatum; Tel, telencephalon; Vm, ventral mesencephalon. Scale bars, 200 µm.
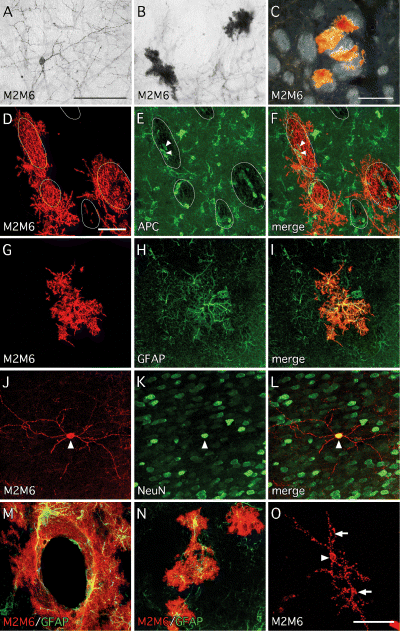
Differentiation potential of foetal- and adult-derived Sox1–GFP cells in vivo. (A–C) Six weeks after transplantation of GFP+ve cells isolated from E13.5 Sox1–GFP+/– mouse brains (CTX, MGE, LGE or brainstem), grafted cells were identified using the mouse-specific marker M2M6. In all grafts, cells with morphological features of (A) neurons and (B and C) glia could be identified. The darkfield image C illustrates that some glial cells were intimately associated with striatal fibre bundles. Double immunohistochemical labelling of grafts of E13.5-derived GFP+ve cells with M2M6 and phenotypic markers revealed the presence of (D–E) APC+ve oligodendrocytes (arrowheads denote cells confirmed as double-positive for APC+ve and M2M6+ve through confocal imaging; white lines indicate the location of striatal fibre bundles), (G–I) GFAP+ve astrocytes and (J–L) NeuN+ve neurons (arrowhead denotes M2M6–NeuN double-positive cell). (M and N) Grafts derived from GFP+ve cells isolated from the walls of the lateral ventricles of adult Sox1–GFP+/– mice contained predominately glial cells, (M) many of which were found to be intimately associated with blood vessels, and (N) some of which are GFAP+ve. (O) Unlike grafts derived from E13.5 Sox1–GFP+ve cells, which contain many neurons, grafts derived from adult Sox1–GFP+ve cells contain only rare examples of M2M6+ve neurons (arrow denotes neuronal cell body; arrowheads denote dendrites). Scale bars, 100 µm (in A for A and B; C), 50 µm (in D for D–N; O).
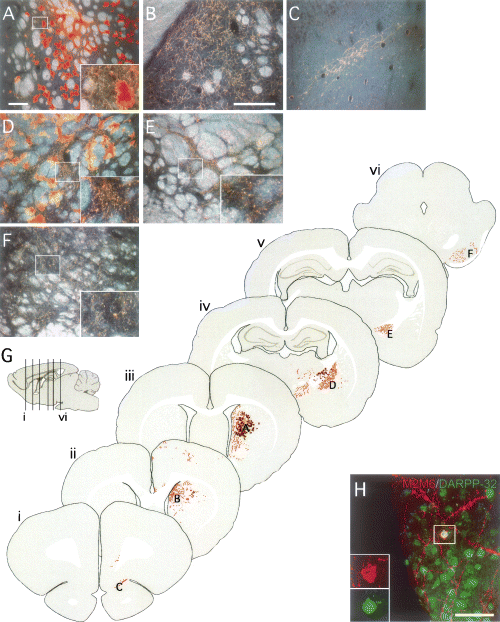
Grafts of foetal Sox1–GFP cells derived from the LGE. (A–F) Six weeks after transplantation of GFP+ve cells isolated from the LGE of E13.5 Sox1–GFP+/– mice, grafted cells were identified using the mouse-specific marker M2M6. (A) The graft cores were identified at the site of transplantation, within the host striatum, and contained intensely M2M6+ve clusters of glial cells as well as many M2M6+ve neuronal processes (high magnification inset from boxed area shows a cluster of glial cells along with fine dendritic processes). M2M6+ve neuronal processes could also be identified within (B) the dorsomedial striatum and (C) traversing through the dorsal transition zone into the olfactory bulb (not shown). A number of glial cells migrated some distance from the graft and were found in (D) the globus pallidus, associated with M2M6+ve fibres which were seen coursing through white matter and also ramifying within the palladial parenchyma (see inset from boxed area). This pattern of M2M6+ve fibre staining was also seen within (E) the entopeduncular nucleus (see inset from boxed area) and (F) the substantia nigra pars reticular (see inset from boxed area). (G) The pattern of graft-derived fibre staining seen in host brains is illustrated in schematic coronal sections from a representative case (glial cells are represented as brown clusters, neuronal processes as orange fibres). The rostrocaudal positioning of coronal sections (i–vi) is indicated in the sagitial drawing. The positions from which photographs A–F were taken are also indicated in the schematic. (H) A number of the M2M6+ve neurons in the grafts were seen to coexpress DARPP-32 (inset is from boxed area). Scale bars, 200 µm (A; and in B for B–F), 50 µm (H).
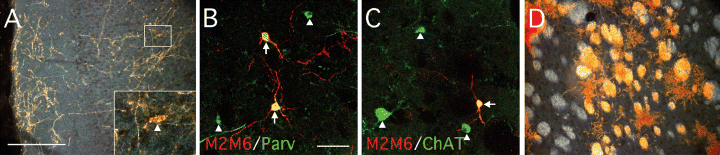
Grafts of foetal Sox1–GFP cells derived from the brainstem and MGE. Six weeks after transplantation of GFP+ve cells isolated from either (A–C) the MGE or (D) brainstem of E13.5 Sox1–GFP+/– mice, grafted cells were identified using the mouse-specific marker M2M6. (A) In animals grafted with MGE-derived cells, many M2M6+ve neurons appeared to have migrated some distance from the graft core and were found throughout the host forebrain, including in the frontal cortex (inset is from boxed area; arrowhead indicates M2M6+ve neuronal cell body). Many of these cells coexpressed markers typical for interneurons including (B) parvalbumin (Parv; arrows denote graft-derived M2M6+ve, parvalbumin+ve cells, arrowheads denote host-derived M2M6–ve, parvalbumin+ve cells) and (C) ChAT (arrows denote graft-derived M2M6+ve, ChAT+ve cells, arrowheads denote host-derived M2M6–ve, ChAT+ve cells). (D) Grafts of brainstem-derived Sox1–GFP+ve cells contained many M2M6+ve glia associated with host striatal fibre bundles. Scale bars, 200 µm (in A for A and D) 50 µm (in B for B and C).
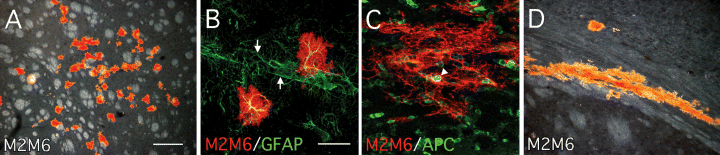
Grafts of Sox1–GFP cells derived from neurosphere cultures. Six weeks after transplantation of GFP+ve cells isolated from expanded (passage 5) neurosphere cultures originating from the LGE of E12.5 Sox1–GFP+/– mice, grafted cells were identified using the mouse-specific marker M2M6. (A) The grafts were identified at the site of injection, in the host striatum, and contained only cells with morphological features of glia. Many of these cells were immunopositive for (B) GFAP (arrows denote a host blood vessel) and (C) some cells were APC+ve. (D) The APC+ve cells were typically associated with host white matter such as the corpus callosum. Scale bars, 200 µm (in A for A and D) 50 µm (in B for B and C).
In order to provide low-magnification overviews of graft-derived fibre innervation throughout the host brain, fibre patterns were accurately traced (Canvas v9.0.4) over digital photomontages from coronal sections immunohistochemicaly labelled with M2M6.
Results
GFP was expressed in germinal zones of the developing brain
In this study, we have made use of a transgenic mouse that has GFP knocked into the Sox1 locus (Aubert et al., 2003). In these mice, the GFP reporter is widely expressed throughout the developing nervous system (Fig. 1A–C) and also in the developing lens, in a pattern closely mimicking that of the endogenous Sox1 protein (Aubert et al., 2003). Notably, however, GFP expression is completely absent from the developing ventral mesencephalon. This is consistent with immunohistochemistry for the Sox1 protein, which is also not expressed in the dopamine precursor domain of the ventral mesencephalon (not shown). However, we have observed that the related Sox family member, Sox2, is expressed in this region (E. Andersson, M. Parmar and L. Thompson, unpublished observations).
Double immunohistochemistry using antibodies raised against the GFP and Sox1 proteins demonstrates that virtually all the Sox1-positive cells in the developing forebrain are also GFP-positive (Fig. 1D–I). At both E12.5 (Fig. 1D–F) and E16.5 (Fig. 1G–I), Sox1 and GFP are highly expressed in the VZ of the ventral telencephalon. Low levels of Sox1 and GFP can also be detected in the developing cortex (see high magnification images from Fig. 1F). As GFP is a relatively stable protein, its expression can also be detected in cells that have down-regulated Sox1. Thus, GFP was expressed at relatively high levels in Sox1+ve cells within the VZ but also at lower levels in some Sox1–ve cells in the SVZ. This is reflected in cell counting from acutely dissociated E12.5 forebrain, which shows that the percentage of GFP+ve cells is slightly greater than that of Sox1+ve cells (Fig. 1J). High levels of GFP expression were also detected in the region of the developing hippocampus (Fig. 1D). In the adult brain we observed GFP expression in a number of areas including the subgranular layer of the dentate gyrus, consistent with a previous report (Aubert et al., 2003), as well as in the walls of the lateral ventricles, the other major neurogenic area of the adult brain (Fig. 1K–M). As for the foetal brain, the GFP expression domain surrounding the lateral ventricles appeared somewhat broader than that of Sox1 protein expression.
To determine the extent of overlap between Sox1 and GFP in different areas of the developing forebrain, we subdissected the proliferative regions (which included both the VZ and SVZ) from cortex, LGE and MGE of E12.5 Sox1–GFP embryos. The cells were dissociated, plated and, 2 h later, stained with anti-GFP and anti-Sox1 antibodies. From all three areas, the majority of the cells expressed both GFP and Sox1 (Fig. 1J): 91.4 ± 2.1% of the cortical cells were GFP+ve and 84.7 ± 2.3% of these also expressed Sox1; 83.1 ± 1.8% of the LGE cells expressed GFP and out of those 76.1 ± 2.4% also expressed Sox1; 80.3 ± 3.3% of the MGE cells were GFP+ve, and 70.0 ± 2.4% of the MGE cells were both GFP- and Sox1+ve.
GFP expression overlapped with other known markers for neural stem and progenitor cells
To further characterize the telencephalic cells expressing GFP, we performed immunohistochemistry for GFP in combination with other known markers of neural stem and progenitor cells: the family member Sox2 (Zappone et al., 2000), the intermediate filament protein Nestin (Lendahl et al., 1990) and the RNA binding protein Musashi1 (Sakakibara et al., 1996; Sakakibara and Okano, 1997). We found that Sox2 is expressed in the forebrain VZ in a dorsal to ventral gradient opposite to that of Sox1 and Sox1–GFP, i.e. with the highest expression dorsally, in the developing cortex (Fig. 2A). Cell counting in acutely dissociated tissue showed that the majority of GFP-expressing cells also expressed Sox2, but that there was a small (< 10%) population of cells that expressed Sox2 but were GFP–ve (Fig. 2B). There was also considerable overlap between GFP and Nestin (Fig. 2C) and between GFP and Musashi1 (Fig. 2E). However, in both cases, a small population of GFP-expressing cells that did not express Nestin or Musashi1 was also present (Fig. 2D and F). Further, we analysed to what extent GFP expression overlapped with that of PSA-NCAM. At this gestational stage, PSA-NCAM was only present in the mantle region of the LGE and the MGE where GFP is no longer expressed (Fig. 2G). Quantification confirmed that virtually all GFP-expressing cells were PSA-NCAM–ve (Fig. 2H).
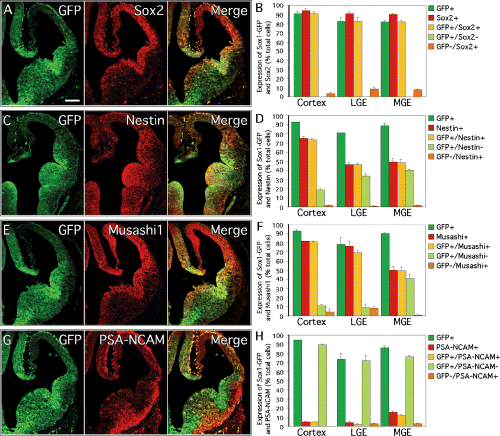
Comparison of Sox1–GFP expression with other markers of neural precursor cells in the developing brain. Double immunohistochemistry on coronal sections through the telencephalon of E12.5 transgenic mice showing the expression patterns of (green) GFP and (red) the neural progenitor cell markers (A) SOX2, (C) Nestin, (E) Musashi1 and (G) PSA-NCAM. Results of cell counting, showing (green bars) the percentage of Sox1–GFP-expressing cells and (red bars) the neural progenitor markers (B) SOX2, (D) Nestin, (F) Musashi1 and (H) PSA-NCAM in the cortex, LGE and MGE of E12.5 Sox1–GFP+/– mice. The percentage of cells positive for both GFP and the progenitor cell marker is represented in yellow, and the percentage of cells exclusively expressing either GFP or the stem and progenitor cell marker is represented in pale green or orange, respectively. Scale bar, 200 µm.
The Sox1–GFP population was expandable in vitro and contained all self-renewing and multipotent cells
Neurosphere culture is a commonly used method for expanding neural stem and progenitor cells in vitro (Reynolds and Weiss, 1992). Cells isolated from E12.5 forebrain of Sox1–GFP mice and plated in serum-free medium containing epidermal growth factor and basic fibroblastic growth factor formed neurospheres at the same frequency as from forebrain dissections of age-matched WT mice (M. Parmar, unpublished data). In the neurosphere cultures established from the forebrain of the Sox1–GFP mice, virtually all spheres contained GFP-expressing cells and flow cytometric analysis showed that primary neurosphere cultures contained 34.8 ± 2.2% (n = 3) GFP+ve cells (Fig. 3A). After passaging, all spheres examined still contained GFP-expressing cells but the proportion of GFP-expressing cells decreased to ≈ 5%, where it remained constant throughout the culture period: 5.2 ± 0.2% at passage 2 (Fig. 3B; n = 3) and 5.1 ± 0.8% at passage 5 (Fig. 3C; n = 3).
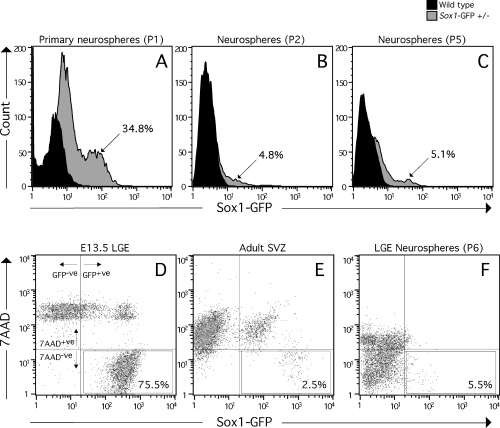
Fluorescence-activated cell sorting of Sox1–GFP cells. Flow cytometric analysis of cell populations generated from Sox1–GFP+/– mice. (A) Primary and (B and C) passaged neurosphere cultures generated from whole forebrains of E12.5 WT (solid histograms) and Sox1–GFP+/– mice (shaded histograms). Sorting with WT cells sets the lower limit for GFP intensity (x-axis) when defining GFP-positive cells sorted from Sox1–GFP cultures. The count (y-axis) indicates relative cell numbers and the percentages indicates the fraction of total cells sorted that are GFP+ve (n = 3). For transplantation experiments, cells were sorted from (D) foetal or (E) adult Sox1–GFP+/– mice and also from (F) expanded neurosphere cultures generated from the developing LGE of these mice. The vertical lines in these panels delimit the threshold for GFP expression as determined by analysis of WT tissue. The horizontal lines define the threshold level for 7AAD+ve cells as determined by analysis of cells where 7AAD was not included. The boxed regions indicate the gate settings used for isolation of cells which are GFP+ve and 7AAD–ve, and the corresponding fraction of cells this represents as a percentage of the total population is also indicated.
From secondary spheres we defined three subpopulations of cells based on their relative GFP intensity (see Fig. 4A and B). The resulting GFPHigh,GFPLow and GFPNegative populations contained ≈ 1.5, 3.5, and 80% of the total cell population, respectively (Fig. 4B). Seven days after sorting and plating at clonal density (Hulspas et al., 1997), the number of spheres formed from each subpopulation was quantified (Fig. 4C). The GFPHigh population represented only 1.5% of the total cells, yet the majority of the neurosphere-forming cells (1.7 ± 0.1% of the GFPHigh cells) were found in the GFPHigh subpopulation. Some neurosphere-forming cells (0.5 ± 0.1%) were also present in the GFPLow subpopulation that made up 3.5% of the total cells, whereas no spheres were generated from the GFPNegative subpopulation. In a control population (all live cells gated, i.e a mixture of all three subpopulations) < 0.04 ± 0.02% of the cells formed neurospheres. Thus, the number of spheres formed from the GFPHigh population represented a > 40-fold enrichment compared to the control cells. Because preparation and handling of foetal neural stem and progenitor cells for FACS reduces the sphere-forming ability of treated cells by at least 6-fold (M. Parmar, unpublished observation; also see Kawaguchi et al., 2001), it can be estimated that ≈ 10% of the GFPHigh expressers are neurosphere-forming stem cells.
To confirm the stem cell characteristics of the spheres generated from the GFP-expressing cells, their self-renewal capacity and multilineage potential were tested in a clonal in vitro stem cell assay (outlined in Fig. 4D). Thirty-three individual spheres formed after FACS sorting (21 from the GFPHigh and 12 from the GFPLow population) were picked under a light microscope. The self-renewal properties of the GFPHigh and the GFPLow sorted cells were analysed by subcloning of the individual spheres. We found that all (21/21) spheres formed from the GFPHigh population, but only 66% (8/12) of the spheres formed from the GFPLow population, formed new second-generation spheres. When these second-generation spheres again were individually picked and subjected to the same sphere-forming assay, more spheres from the GFPHigh than from the GFPLow population formed new tertiary spheres (70% vs. 47%, n = 108). The individual third-generation spheres (originating from the GFPHigh and GFPLow populations) were picked and plated under differentiation conditions. After 7 days, all spheres had generated cells with morphological characteristics of astrocytes and many clones also contained neurons and oligodendrocytes, as detected by expression of β-III-tubulin and CNPase, respectively (Fig. 4E). Of the 24 third-generation spheres generated from the GFPHigh population, all but two generated both neurons and glia: 13 clones (54.2%) generated all three cell types (neurons, astrocytes and oligodendrocytes), nine clones (37.5%) generated both astrocytes and neurons and two clones (8.3%) contained only astrocytes (Fig. 4F). In contrast, only one of the 11 third-generation spheres from the GFPLow population generated both neurons and glia, and the other 10 generated only astrocytes after differentiation in vitro (Fig. 4F).
Sox1–GFP cells isolated from the embryonic and adult brain gave rise to neurons and glia in vivo
We further analysed the Sox1–GFP+ve cells for their capacity to generate different cell types in vivo, following intracerebral transplantation. Thus we FACS-isolated the GFP-expressing cells from the E13.5 LGE, MGE, cortex or brainstem of Sox1–GFP mice and transplanted them into the striatum of neonatal rats. The FACS analysis showed that the proportion of GFP+ve cells in these regions was ≈ 93% (brainstem), 80% (cortex), 88% (MGE) and 75% (LGE). Notably, the vast majority of the GFP–ve cells did not survive the dissociation and FACS procedure as evidenced by 7AAD incorporation (LGE, Fig. 3D; other regions not shown). Six weeks after transplantation, native GFP fluorescence was no longer detectable in any of the grafted animals. Grafted cells were identified in the rat hosts through immunohistochemistry for the mouse-specific marker M2M6. Cells with both neuronal (Fig. 5A) and glial (Fig. 5B) morphology could be identified and a number of the glial cells appeared closely associated with striatal fibre bundles (Fig. 5C). These cells expressed phenotypic markers characteristic of oligodendrocytes, including APC (Fig. 5D–F) and CNPase (not shown). Labeling for other phenotypic markers confirmed the presence of graft-derived astrocytes (GFAP; Fig. 5G–I) and neurons (NeuN; Fig. 6J–L). The presence of multiple cell types from different neural lineages was a consistent feature of all grafts regardless of the embryonic brain region from which the cells were isolated.
Compared to the high proportion of GFP+ve cells in foetal tissue, the SVZ dissected from adult brains of Sox1–GFP+/– mice contained relatively few GFP+ve cells (< 15%; Fig. 3E). The majority of low-expressing GFP+ve cells as well as GFP–ve cells did not survive the FACS procedure, as indicated by 7AAD incorporation (Fig. 3E). The cells expressing relatively high levels of GFP survived the dissociation and FACS procedure (representing ≈ 2.5% of the total cell population) and were taken for transplantation. Six weeks following transplantation, the grafted M2M6+ve cells were distributed in the striatal parenchyma, often found to be intimately associated with host blood vessels (Fig. 5M), and were almost exclusively of a glial phenotype with astrocytic morphology. A number of these cells were also seen to express GFAP (Fig. 5N). Notably, the grafts were almost completely devoid of neurons, although single examples of cells with neuronal morphology were occasionally seen in all animals analysed (Fig. 5O). Furthermore, no white matter-associated oligodendroglial cells were noted. As stem cells in the adult SVZ are known to generate neurons destined for the olfactory bulb, we also examined the host olfactory bulbs for the presence of M2M6+ve cells; however, none were observed.
Sox1–GFP cells from the developing brain were regionally specified
Although acutely dissociated Sox1–GFP cells from all four embryonic regions analysed were capable of generating neurons, astrocytes and oligodendrocytes after transplantation, certain other features of the grafts were dependent on where the cells were originally derived from. For example, grafts of Sox1–GFP cells derived from LGE or cortex were much larger than those derived from MGE or brainstem, with the cortical grafts being the largest and MGE grafts the smallest. Furthermore, the fibre outgrowth and neuronal phenotypes found in the grafts also differed depending on the cell source.
LGE-derived grafts
To illustrate the pattern of graft-derived fibre innervation, schematic coronal sections from an animal transplanted with Sox1–GFP cells originating from the LGE are shown in Fig. 6G, i–vi. A notable feature was the presence of M2M6+ve long-distance projections innervating the host globus pallidus (Fig. 6D), entopeduncular nucleus (Fig. 6E) and substantia nigra pars reticulata (Fig. 6F). Graft-derived fibre outgrowth was also observed rostral to the graft core in mediodorsal regions of the striatum (Fig. 6B), as well as coursing through the ventral striatum and the dorsal transition zone (Fig. 6C) and into the olfactory bulb (not shown). Many graft-derived glial cells could be found in and around the graft core (Fig. 6A) and these cells also appeared to migrate some distance from the graft in association with M2M6+ve neuronal projections in the globus pallidus (Fig. 6D). Although the dense glial M2M6+ve signal makes it difficult to identify neuronal cell bodies within the graft core, M2M6+ve neurons could clearly be identified scattered around the graft. Many of these cells displayed features typical of striatal projections neurons: they had many spiny dendrites and expressed DARPP-32 (Fig. 6H).
Cortex-derived grafts
Cortical Sox1–GFP+ve cells gave rise to the largest grafts, with many intensely M2M6+ve glial cells identifiable in and around the graft core (Fig. 7A). Although it was difficult to identify M2M6+ve neuronal cell bodies against the background of M2M6+ve glial cells, double staining with M2M6 and NeuN revealed that the graft cores did in fact contain many neurons (data not shown). The pattern of M2M6+ve fibre outgrowth typically associated with these grafts is illustrated in schematic coronal sections (Fig. 7E, i-vi). Graft-derived fibres could be found throughout the striatum, including the most anterior aspects rostral to the graft core (Fig. 7B) and in a particularly dense pattern in the dorsomedial striatum (Fig. 7C). Fibres were also seen traversing through the corpus callosum and in the dorsomedial striatum of the contralateral hemisphere (Fig. 7D). Caudal to the graft core, there was a robust innervation of the host thalamus, particularly ventral thalamic nuclei (Fig. 7F), and scattered M2M6+ve fibres could also be seen as far back as the subthalamic nucleus (Fig. 7G).
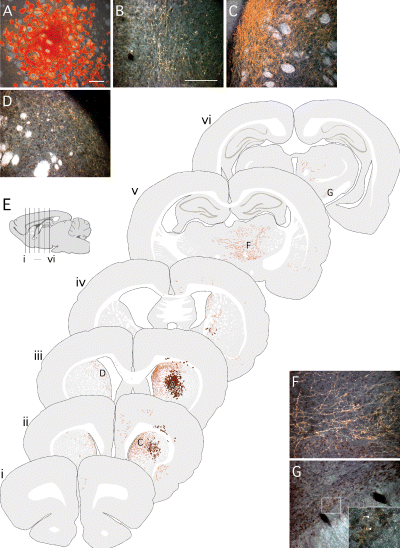
Grafts of foetal Sox1–GFP cells derived from the cortex. (A–D, F and G) Six weeks after transplantation of GFP+ve cells isolated from the cortex of E13.5 Sox1–GFP+/– mice, grafted cells were identified using the mouse-specific marker M2M6. (A) The graft cores were identified at the site of transplantation, within the host striatum, and contained large numbers of intensely M2M6+ve glial. Many M2M6+ve neuronal processes were seen throughout the host stiatum, both (B) rostrally and (C) at the level of the graft, particularly in the dorsomedial striatum. (D) A number of M2M6+ve fibres were also seen traversing through the corpus callosum and in the dorsolateral striatum contralateral to the graft. Caudal to the graft site, M2M6+ve fibres extended into (F) the thalamus and (G) the subthalamic nuclei, where the staining pattern was indicative of terminal ramification (inset from boxed area; arrowheads denote M2M6+ve fibres). (E) The pattern of graft-derived fibre staining seen in host brains is illustrated in schematic coronal sections from a representative case (glial cells are represented as brown clusters, neuronal processes as orange fibres). The rostrocaudal positioning of coronal sections (i–vi) is indicated in the sagitial drawing. The positions from which photographs A–D, F and G were taken are also indicated. Scale bars, 200 µm (A; and in B for B–D, F and G).
MGE- and brainstem-derived grafts
Compared to the LGE- and cortically derived grafts, grafts derived from MGE or brainstem Sox1–GFP cells were generally smaller and lacked long distance fibre projections into host brain structures. There were, however, other unique properties associated with these graft types. A characteristic feature of MGE-derived grafts was the appearance of neuronal cell bodies at relatively long distances away from the graft site. In all other grafts (derived from LGE, cortex or brainstem), identification of neuronal cell bodies was limited to areas in the host striatum in and around the graft. In contrast, in animals grafted with Sox1–GFP cells originating from the MGE, M2M6+ve neurons could be found throughout the striatum and also scattered through the frontal cortex (Fig. 8A). Many of these cells expressed either parvalbumin (Fig. 8B) or ChAT (Fig. 8C). A conspicuous feature of the brainstem-derived grafts, on the other hand, was the large number of oligodendroglial cells. Relative to LGE-, cortical or MGE-derived grafts many more of the glial cells in the brainstem-derived grafts were associated with striatal fibre bundles (Fig. 8D) and expressed the oligodendrocytic marker APC (not shown).
Neurosphere expanded Sox1–GFP cells had a more limited differentiation potential in vivo
Compared to the Sox1–GFP cells from acutely dissected foetal tissue, Sox1–GFP cells isolated from expanded neurosphere cultures were shown to have a more limited differentiation potential in vivo. Specifically, these cells lose the ability to generate neurons after transplantation. GFP-positive cells isolated from LGE derived neurosphere cultures survived the dissociation and FACS comparatively well (Fig. 3F), but the sorted cells only gave rise to glial cells following transplantation (Fig. 9A). The glial population included GFAP-expressing astrocytes (Fig. 9B) and APC-expressing oligodendrocytes (Fig. 9C). The oligodendrocytic phenotype was associated with the positioning of the transplanted cells in host white matter tracts such as the internal capsule and corpus callosum (Fig. 9D). Similar results were obtained after grafting Sox1–GFP cells isolated from expanded E13.5 cortical tissue (not shown).
Discussion
The transcription factor Sox1 is one of the earliest known markers for neural stem and progenitor cells. Its expression can already be detected in the neuroectoderm, and it is widely expressed in the ventricular zone throughout development in the central nervous system (Pevny et al., 1998). The Sox1–GFP knock-in mouse used here allowed for an analysis of the properties of pure populations of Sox1–GFP cells both in vitro and in vivo.
We show that GFP expression almost completely mimics that of endogenous Sox1 in the forebrain of Sox1–GFP knock-in mice. The GFP reporter was highly expressed in almost all cells throughout the foetal VZ. At the foetal stages analysed (E12.5, E16.5), we detected a lower level of GFP expression in the SVZ of the LGE and MGE. In the acutely dissociated cell fractions analysed from E12.5 LGE and cortex, 76–85% of the GFP+ve cells stained positively for Sox1. Although the SVZ also contains a population of dividing precursor cells, Sox1 is not expressed in this region. This discrepancy between GFP and Sox1 protein expression is most probably due to a slower turnover of the GFP protein relative to that of the endogenous Sox1 protein. Therefore, GFP marks a somewhat broader population than that defined by Sox1, including both the Sox1-expressing VZ precursors and also some of their progeny in the SVZ that no longer express Sox1. Many of the GFP-expressing cells also colocalized with Sox2, another member of the Sox family expressed by neural stem and progenitor cells (Zappone et al., 2000), and most expressed the neural stem or progenitor markers Nestin and Musashi1 (Lendahl et al., 1990; Sakakibara and Okano, 1997). A fraction of the GFP+ve cells, however, did not express Nestin or Musashi1. Many of the GFP+ve, Nestin- or Musashi1–ve cells most probably represent GFP-expressing SVZ cells that have down-regulated Nestin, Musashi1 or Sox1 but retain some level of the relatively stable GFP protein. Alternatively, because Sox1 expression is already initiated at the time of neural induction (Pevny et al., 1998) but Nestin expression is not initiated until the time of neural tube closure (Lendahl et al., 1990), it cannot be ruled out that a fraction of these GFP-positive cells represent NSCs at a stage of development that is earlier than the onset of Nestin. Further support for this comes from the work of Tanaka et al. (2004), where it is reported that the Nestin-enhancer elements contain a Sox1-binding motif.
Although it has previously been reported that Sox1 is expressed in all germinal areas of the developing brain (Pevny et al., 1998), we found that the GFP reporter (and Sox1) is conspicuously absent from the ventral aspects of the midbrain and rostral hindbrain at mid-embryogenesis. In the ventral midbrain, GFP (as well as endogenous Sox1 expression) was completely absent from the area that contains the precursors for the dopaminergic neurons. This implies that Sox1–GFP expression cannot be used to identify and isolate NSCs from all areas of the developing CNS. For the midbrain dopaminergic neurons, for example, other reporter systems (such as GFP expressed in the Sox2 locus) are likely to be required for identification and isolation of the corresponding ventral midbrain precursors.
The expression pattern of GFP in the forebrain of Sox1–GFP mice suggests that it can be used as putative marker for NSCs in this region. We show that the Sox1–GFP-expressing cells isolated from the embryonic forebrain are expandable in neurosphere culture, a method commonly used for in vitro expansion of neural stem and progenitor cells (Reynolds and Weiss, 1996). Further analysis of the cells in clonal stem cell assays showed that all self-renewing and multipotent neurosphere-forming cells are contained within the GFP-expressing population but are most frequent in cells expressing high levels of GFP. Compared to unsorted forebrain cells at the same stage of development, high Sox1–GFP expression can be used to enrich for NSCs by 40-fold. We estimate that ≈ 10% of the GFPHigh expressors are neurosphere-forming stem cells. However, this estimate is based entirely on the neurosphere assay and it is not clear whether it is a reliable tool for assessing stem cell numbers, or whether the number of stem cells may be underestimated using this technique (see Temple, 2001, for discussion). It seems possible therefore that the proportion of neural stem cells in the Sox1–GFPHigh cell population may be higher than the estimated 10%. It is also worth noting that, while neurospheres initiated from Sox1–GFPHigh cells were able to give rise to both neurons and glia, neurospheres generated from Sox1–GFPLow cells were more restricted in their potential and gave rise almost exclusively to astrocytes. This finding is most probably related to the relative stability of GFP compared to the Sox1 protein, such that the Sox1–GFPHigh cells represent genuine Sox1-expressing precursors while the Sox1–GFPLow cells represent more mature (mostly glial) progenitors that have down-regulated Sox1 expression.
As Sox1 is the earliest known marker for committed neural precursors in the proliferating neuroepithelium, Sox1–GFP expression is also likely to identify an earlier subset of neural precursors than are other similar reporter systems such as Nestin–GFP (Sawamoto et al., 2001; Mignone et al., 2004). Accordingly, while neurosphere-forming cells are exclusively found in the GFP+ve population when isolated from the Sox1–GFP mice, neurospheres can be generated from both GFP+ve and GFP–ve cells when Nestin–GFP is used as a reporter (Mignone et al., 2004). These GFP–ve sphere-forming cells from the Nestin–GFP mouse are likely to be the early Sox1+–Nestin– precursors. In addition to marking earlier neural precursors, Sox1–GFP is much more limited in expression relative to Nestin in neurosphere cultures. While almost all cells within expanded neurospheres express Nestin (Reynolds et al., 1992; Parmar et al., 2002), only ≈ 5% of these cells express Sox1–GFP. This figure falls within the same range as the number of sphere-forming cells previously reported to be present within neurospheres (Reynolds and Weiss, 1996).
When Sox1–GFP cells isolated from various regions of the developing brain were grafted to the striatum of neonatal rats they gave rise to differentiated cells belonging to all three neural lineages: neurons, astrocytes and oligodendrocytes. Many of the grafted cells were found to have phenotypic characteristics appropriate for the brain region from which they were originally derived. The spiny dendritic morphology and DARPP-32 expression seen in neurons within the LGE grafts are typical features associated with the striatal projection neurons derived from the developing LGE. Furthermore, graft-derived fibre innervation was found in host brain regions normally innervated by striatal projection neurons including globus pallidus, entopeduncular nucleus and substantia nigra. In animals grafted with cortical Sox1–GFP cells, the distinct pattern of innervation of host brain structures such as striatum, thalamic nuclei and the subthalamic nucleus is indicative of the presence of cortical projection neurons. The extensive migration of grafted MGE-derived Sox1–GFP cells, as well as the expression of parvalbumin and ChAT, are typical features of the interneurons that normally develop in the MGE (Marin et al., 2000), while the high proportion of oligodendrocytes in grafts of brainstem Sox1–GFP cells is in line with the large number of oligodendrocytes normally generated in this embryonic brain region (Pringle et al., 1996; Rowitch, 2004). Thus, many of the cells isolated from the embryonic brain using the Sox1–GFP reporter appear to be sufficiently specified with positional information to generate cell types reflecting the region from which they were isolated and yet are immature enough to survive the FACS and transplantation procedure.
In contrast to the many neurons generated from grafts of embryonic Sox1–GFP tissue, grafts of Sox1–GFP cells isolated from the adult SVZ consisted almost exclusively of glia. Although olfactory interneurons are known to be generated from stem cells residing in the postnatal SVZ (Wichterle et al., 2001; Stenman et al., 2003), we saw only few examples of graft-derived neurons following transplantation of SVZ Sox1–GFP cells into the neonatal striatum and no cells migrating towards the olfactory bulb. One possible explanation for this result is that these Sox1–GFP cells are not able to generate neurons when placed ectopically in the striatum but, rather, they require further extrinsic cues from the SVZ and rostral migratory stream in order to achieve a neuronal phenotype upon differentiation. The fate adopted by neural precursor cells upon differentiation is known to be dependent upon both extrinsic signalling from the local environment and the on-going transcriptional activity within the cells that defines intrinsic signalling patterns. Hence, in the present study, when grafting Sox1–GFP+ve cells from foetal or adult tissue into the same non-neurogenic environment (the postnatal striatum), differences in the phenotype of the grafted cells are likely to be dependent on the intrinsic proporties of the GFP+ve cell fraction. Consistent with this, Abramova et al. (2005) have recently used a microarray-based approach in order to demonstrate that neural precursor cells isolated by FACS using the cell-surface marker Lewis X (also known as SSEA1 and CD15) display inherently different gene-expression profiles depending on whether they are taken from foetal or adult tissue. We also considered that it may be the relatively low number of cells transplanted relative to the foetal grafts described here; however, similarly small foetal grafts consistently and robustly give rise to neurons (M. Parmar and L. Thompson, unpublished observations).
Previous studies have documented that cells expanded as neurosphere cultures have only a limited capacity for the generation of neurons following transplantation (Winkler et al., 1998; Eriksson et al., 2003). We report here that an enrichment based on expression of the Sox1–GFP reporter, and thus an enrichment of the early stem and/or progenitor pool within the spheres, does not improve the outcome in terms of neuronal differentiation in vivo following transplantation. Thus, although neurospheres clonally derived from expanded Sox1–GFP cells are capable of generating neurons in vitro, the Sox1–GFP cells appear to lack the degree of intrinsic specification required for the formation of neuronal progenitors and subsequent neuronal differentiation when placed in the postnatal striatum.
In conclusion, these results show that GFP expressed under the control of Sox1 regulatory sequences identifies neural stem and progenitor cells from the developing forebrain. Furthermore, we demonstrate the utility of the Sox1–GFP reporter system in the isolation of neural stem and progenitor cells from highly mixed cell populations, thus allowing for the characterization of these cells under defined conditions. However, despite several advantages over similar reporter systems, Sox1–GFP expression is still selective for rather than exclusive to NSCs. Thus, to exclusively identify and isolate the NSCs, sorting based on Sox1–GFP still has to be combined with other putative stem cell markers. Furthermore, although Sox1 has been described as being expressed throughout the developing CNS as a ubiquitous marker for neural precursors (Pevny et al., 1998), we report here that Sox1 expression is absent from certain developing brain regions such as the ventral midbrain. The selective isolation of neural stem and progenitor cells from these regions must therefore be based on other markers, and might best be achieved through the use of alternative reporter systems based on expression of genes specific to the particular progenitors of interest. Finally, we report that, while the Sox1–GFP reporter identifies transplantable neuronal precursors from the foetal brain, this is not the case for other cell sources such as adult brain or expanded neurospheres, where the Sox1–GFP cell populations display inherently different properties, generating almost exclusively glia following transplantation.
Acknowledgements
We thank Anna Fossum, Birgit Haraldsson, Ulla Jarl, Anneli Josefsson, Elsy Ling, Zhi Ma and AnnaKarin Olden for technical assistance, Josephine B. Jensen and Beatrice Navarro-Galve for help with transplantations, Dr A. Smith for the Sox1–GFP mice and Drs R. Lovell-Badge and H. Okano for their gift of antibodies. This work was supported by grants from the Swedish Research Council (04X-3874 and 99SN-14480) and by EuroStemCell (EU Framework 6 project LHSB-CT-2003–503005). Lund Stem Cell Center is supported by a Center of Excellence Grant from the Swedish Foundation for Strategic Research. L.T. is supported in part by a fellowship from the Wenner–Gren foundation.
Abbreviations
-
- 7AAD
-
- 7-aminoactionomycin-D
-
- APC
-
- adenomatous polyposis coli
-
- ChAT
-
- choline acetyltransferase
-
- CNPase
-
- 2′,3′-cyclic nucleotide-3′-phosphodiesterase
-
- DARPP-32
-
- dopamine- and cAMP-regulated phosphoprotein of 32 kDa
-
- E
-
- embryonic day
-
- FACS
-
- fluorescence-activated cell sorting
-
- GFAP
-
- glial fibrillary acidic protein
-
- GFP
-
- green fluorescent protein
-
- LGE
-
- lateral ganglionic eminence
-
- MGE
-
- medial ganglionic eminence
-
- NeuN
-
- neuronal nuclei
-
- NSCs
-
- neural stem cells
-
- PBS
-
- phosphate-buffered saline
-
- PFA
-
- paraformaldehyde
-
- PSA-NCAM
-
- polysialylated neural cell adhesion molecule
-
- RT
-
- room temperature
-
- SVZ
-
- subventricular zone
-
- VZ
-
- ventricular zone
-
- WT
-
- wild-type




