Death of dopaminergic neurones in the rat substantia nigra can be induced by damage to globus pallidus
Abstract
Parkinson's disease is a debilitating disorder that results from the death of dopaminergic neurones in the substantia nigra. Subthalamic nucleus neurones use glutamate as their neurotransmitter and send excitatory projections to the substantia nigra. Changes in both the mean firing rate and firing pattern of neurones of the subthalamic nucleus have been found in patients with this disease. This has led to the suggestion that hyperactivity of the subthalamic nucleus may be involved in the pathology of the dopaminergic neurones. Subthalamic nucleus lesions or treatment with glutamatergic antagonists can be neuroprotective in animal models of Parkinson's disease but until now there has been no direct evidence that hyperactivity of subthalamic nucleus neurones can lead to downstream cell death. Here we show that lesions of the rat globus pallidus (a treatment that has been shown to increase subthalamic nucleus neuronal activity) result in a significant reduction of the number of dopaminergic neurones in the substantia nigra.
Abbreviations
-
- DA
-
- dopamine
-
- GFAP
-
- glial fibrillary acidic protein
-
- GP
-
- globus pallidus
-
- OX42
-
- monoclonal antibody to type 3 complement receptor
-
- PPN
-
- pedunculo-pontine nucleus
-
- SN
-
- substantia nigra
-
- SNc
-
- substantia nigra pars compacta
-
- STN
-
- subthalamic nucleus
-
- TH
-
- tyrosine hydroxylase
Introduction
Amongst the nuclei that form the basal ganglia, the subthalamic nucleus (STN) is the only one containing neurones that use glutamate as a neurotransmitter. Under normal circumstances STN activity can modulate the firing patterns of the dopamine (DA) cells in the substantia nigra pars compacta (SNc) (Iribe et al., 1999; Kang & Futami, 1999). Indeed, tetanic stimulation of STN neurones has been shown to induce N-methyl-d-aspartate receptor-dependent long-term potentiation at synapses on to DA cells (Overton et al., 1999).
It has been known for a long time that glutamate can be toxic to neuronal cells (Lucas & Newhouse, 1957; Olney et al., 1971; Coyle, 1983). It is possible, in situations where the firing rates of STN neurones are increased or their pattern has changed to become more bursty, that the subsequent increased release of glutamate into the SNc by STN neurones could become toxic to the DA cells there (Rodriguez et al., 1998). Several studies have shown that N-methyl-d-aspartate receptor antagonists can be neuroprotective in both the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 6-hydroxydopamine models of Parkinson's disease (reviewed in Rodriguez et al., 1998).
In keeping with the possible involvement of the STN in this excitotoxic cell death, lesions of the rat STN before a neurotoxic challenge with 6-hydroxydopamine can be neuroprotective (Piallat et al., 1998), although not all authors agree on this (Baunez & Robbins, 1999a). Conversely, when glutamate release from the STN is increased by an acute injection of ibotenic acid at the same time as a 6-hydroxydopamine injection then the destruction of DA cells is increased (Phillips et al., 1998). Thus, a glutamatergic action from STN terminals in the SNc may be involved in the progressive destruction of nigral dopaminergic cells seen in patients with Parkinson's disease. At post-mortem, patients who had Parkinsonism caused by self-administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (Langston et al., 1999) still had signs of active degeneration in the SNc even though it was up to 16 years after the last exposure to the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine that had caused the initial DA cell death. Excitotoxicity due to STN hyperactivity provides an attractive explanation for these observations as this could also cause oxidative stress and induce inflammatory processes, two processes also suggested as causes of the delayed degeneration (Langston et al., 1999).
Many authors have made acute pharmacological manipulations of STN activity levels in vivo (Robledo & Feger, 1990; Feger & Robledo, 1991; Murer & Pazo, 1993; Chergui et al., 1994; Hauber, 1998; Baunez & Robbins, 1999b; Kearney & Albin, 2000) but the actions which might damage DA cells in Parkinson's disease would be chronic. Lesions of the globus pallidus (GP) in rats (equivalent to the external pallidum in humans) have been shown to produce a relatively long-lasting (at least 2 weeks) increase in the resting firing rate of STN neurones to approximately 20% above their normal level (Palmer et al., 1987; Hassani et al., 1996; Périer et al., 2000). The experiments presented here were designed to test whether the relatively small increase in STN neurone firing rate that is produced by a GP lesion would be enough to lead to damage of the dopaminergic neurones in the SNc.
Materials and methods
Twenty-one male Sprague Dawley rats weighing 300–320 g (Harlan, UK) were anaesthetized with halothane, placed in a stereotaxic frame and, using co-ordinates from Paxinos & Watson (1986), injected into the left GP with 0.6 µL of a 10 µg/1 µL solution of ibotenic acid made up in phosphate-buffered saline, pH 7.4. The co-ordinates used were 2.8 mm lateral to the midline, 1.1 mm posterior to bregma and 5.7 mm vertical to the cortical surface. The ibotenic acid was injected over a period of 5 min and the needle was left in position for a further 5 min to limit diffusion back along the needle tract. To help the rats through any seizures in the first few hours after the lesion, ketamine (20 mg/kg) and xylazine (10 mg/kg) were injected i.p. All procedures involving animals were carried out under the control of UK Home Office Licences, with local ethical review process approval.
Three or six weeks after the GP lesions these rats, plus nine age-matched controls (five at 3 weeks, four at 6 weeks), were anaesthetized with pentobarbitone and perfused transcardially with 0.9% saline containing 2000 U/L of heparin followed by 4% paraformaldehyde and 0.05% glutaraldehyde in 0.1 m phosphate buffer, pH 7.4. The brain tissue was stored at 4 °C in the same fix diluted 50 : 50 with 20% aqueous sucrose for a minimum of 24 h. Free-floating 50-µm coronal sections to be used for immunohistochemical processing were collected in 0.05 m phosphate-buffered saline, pH 7.4, containing 0.3% Triton-X100. Alternate sections were stained for an antibody to tyrosine hydroxylase (TH) with the other group being split and stained with antibodies to neuronal nuclear protein, glial fibrillary acidic protein (GFAP) or type 3 complement receptor (OX42).
Immunohistochemistry
All tissue was processed using the Vectastain ABC peroxidase methods (Vector Laboratories Ltd) using 3,3-diaminobenzidine as chromogen to give an insoluble brown reaction product. Sections for TH and GFAP were incubated for 24 h in rabbit anti-TH (1 : 4000; Affiniti Research Prod. Ltd) or rabbit anti-GFAP (1 : 2000; DAKO Cytomation, Denmark) followed by 1 h in biotinylated goat anti-rabbit secondary antibody, while those stained with mouse monoclonal anti-neuronal nuclear protein (1 : 1000; a gift from Dr Staines, University of Ottawa, Canada) and OX42 (1 : 2000; Serotec) were incubated for 24 h followed by 1 h in biotinylated horse anti-mouse secondary antibody. All sections were incubated for 1 h in elite AB complex followed by 10 min in 3,3-diaminobenzidine. Between each of the incubations, sections were washed with phosphate-buffered saline-Triton-X100. The sections were mounted on gelatine-coated slides, dried, dehydrated and coverslipped in DPX.
Immunohistochemical staining for monoclonal antibody to neuronal nuclear protein, GFAP and OX42 in the GP and substantia nigra (SN) was assessed qualitatively. Monoclonal antibody to neuronal nuclear protein staining was used to visualize the ibotenic acid lesions which could be seen easily down the microscope as an area devoid of any stained nuclei. The lesion size and position were examined in each animal and those in which > 70% of the GP remained were treated as a separate group in the subsequent analysis. All of the chemicals except the antibodies as listed were from Sigma.
Stereology
In order to make an accurate count of the total number of neurones stained with the antibody to TH (TH positive) in the SN, systematic random sampling techniques were used. From a random starting point, the set of sections from the SN was used for unbiased stereological counting of TH-positive cells on both sides of the brain using the optical fractionator method (West et al., 1991) from the stereo investigator program on a Neurolucida computer-controlled microscopy system (Microbrightfield Inc., VT, USA). On each section a contour was drawn outlining the SN both on the ipsilateral and contralateral sides to the GP lesion. Counting frames (150 × 150 µm) were placed at the intersections of a grid (frame size 700 × 300 µm) that had been randomly placed over the section (Fig. 1). Only counting frames for which at least a part of the frame fell within the contour of the SN were used for counting. Cells were marked if they were TH positive and were in focus within the counting area. In this way, the total number of TH-positive cells in the SN was counted. The reliability of this estimate was assessed by calculation of the coefficient of error according to the formulae described in West & Gundersen (1990) where a coefficient of error of less than 0.1 is taken as an indication of reliable cell counts. As well as estimating the total number of TH-positive cells in the SN the outlines of the SN were used to gain an independent estimate of the total SN volume using the Cavalieri method (Coggeshall, 1992).
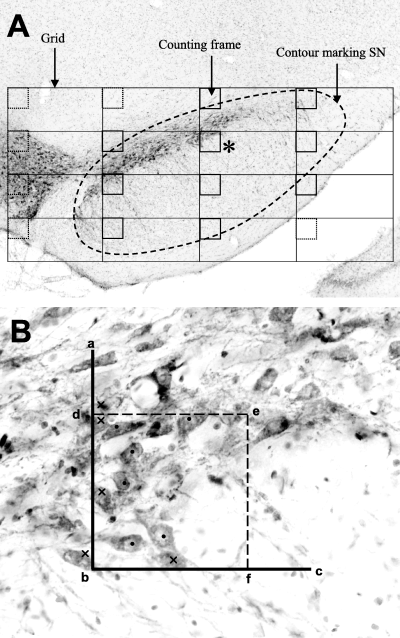
Unbiased counting of dopamine cells. The photomicograph in A shows a single section of the substantia nigra (SN). The broken line outlines the nucleus with a counting grid superimposed on it. The rectangles in the grid are 700 × 300 µm and the counting frames (represented by squares in the corner of each grid section) are 150 µm square. Counting frames outside the nigral outline (dotted) are not used in the estimate of cell number. B shows one counting frame (* in the lower power view in A). Stained cells touching the solid line (a, d, b, f and c) are not counted (marked with a cross) while the others within the frame (marked with a dot) are included even if they cross the dotted line (d, e and f). Note that only those cells in focus are marked, the final count would include all those cells that could be brought into focus in the section.
The cell numbers in the STN on both sides of the brains were also estimated by the optical fractionator method but in sections stained with monoclonal antibody to neuronal nuclear protein, which stains neuronal nuclear protein, a protein only present in neuronal nuclei. In these counts the tops of stained nuclei were counted as they came into focus in counting frames within the nucleus.
Results
Lesion area
Immunohistochemical staining with monoclonal antibody to neuronal nuclear protein enabled confirmation of the size and position of the GP lesions by inspection of the areas of brain in which there were no stained neurones on the ipsilateral compared with the contralateral side (Fig. 2). The GP lesions were large and included the majority of the GP and usually a small part of the adjacent striatum. As well as the 16 animals with successful lesions, there were three rats in which the striatum alone was lesioned, while in two others less than 30% of the GP was damaged along with a lesion in the striatum (Fig. 3). These five animals were used to assess the effect of damage to the surrounding striatum.
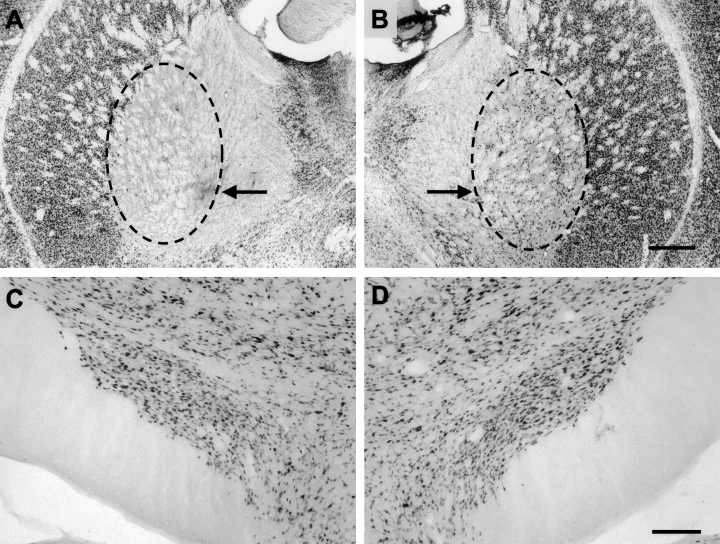
Neuronal nuclear protein-stained sections used to check neuronal integrity. (A) In the region of the lesion the outline marks the area devoid of neuronal nuclei and, in this case, includes all of the globus pallidus (GP). (B) The contralateral GP is intact and is shown for comparison. Sections like those in C and D were used to make quantitative estimates of the total number of neurones in the subthalamic nucleus on both sides. The whole nucleus was sampled for quantitative measurements although only one section is illustrated here to emphasize the intact neuronal distribution on both sides. Scale bar in B represents 500 µm in both A and B while that in D represents 200 µm in C and D.
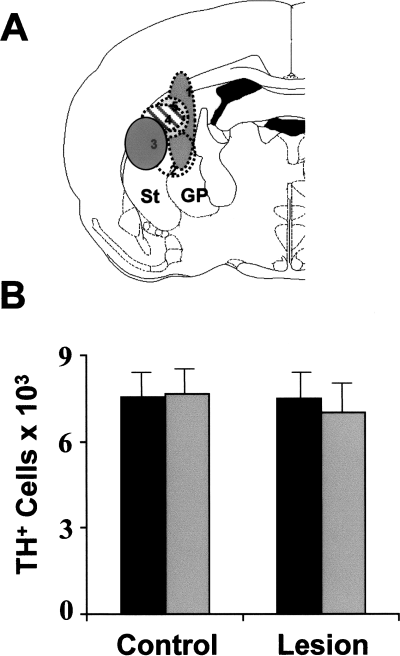
Nearby lesions that spare the globus pallidus (GP) do not change dopamine (DA) cell numbers. The group of lesions in which > 70% of the GP was intact is illustrated in A. The different shading represents the maximum area of the lesion projected onto the plane in the stereotaxic atlas that best represents the position of the successful lesions. In B the counts of the number of dopamine cells in these five animals are compared with the numbers observed in unlesioned control animals. There are no significant differences between sides in these animals nor between the DA cell numbers in these and in control animals. ST, striatum; TH, tyrosine hydroxylase.
Glial responses
There was an increase in staining for OX42 in the SN ipsilateral to the GP lesion at 3 weeks after the lesion (Fig. 4A). High magnification views of the area of increased OX42 staining revealed clusters of microglia around presumed dead or dying neuronal cell bodies (Fig. 4A, x and y). These changes were paralleled by a marked increase in the numbers of GFAP-staining astrocytes in the SN ipsilateral to the GP (Fig. 4B). There was no obvious difference between the staining of any of the glial markers in the SN contralateral to the lesion compared with staining in normal animals.
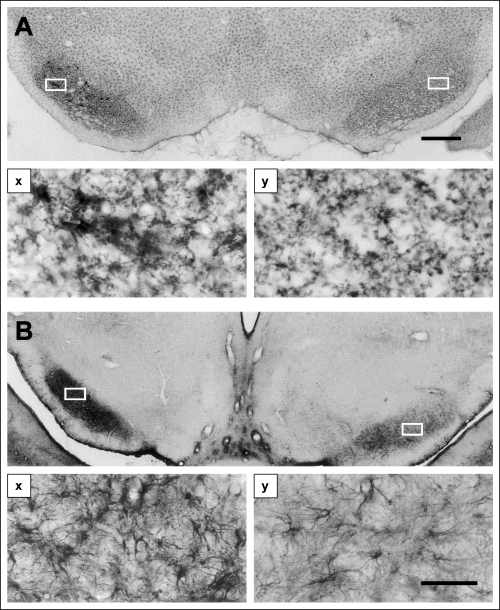
Evidence of an inflammatory response in the substantia nigra. The photomicrograph in A illustrates the pattern of staining with monoclonal antibody to type 3 complement receptor 3 weeks after a lesion of the globus pallidus (on the left in the section). In the substantia nigra, microglia clump in groups that can be seen in the enlargement in (Ax) to be collected around large cell bodies with the morphology of dopamine cells. No such clumping is visible on the other side of the brain (Ay). (B) Staining with an antibody to glial fibrillary acidic protein. The marked increase in the number and density of staining in the astrocytes on the lesion side is clear in both the low-power micrograph (B) and at high power in (Bx) and (By) which correspond to the white boxes in B. Scale bar in A represents 500 µm in A and B while that in (By) represents 50 µm in (Ax and y and Bx and y).
Six weeks after the GP lesions the side-to-side difference, which had been seen in both the GFAP- and OX42-stained sections at 3 weeks after the lesion, was no longer evident. In agreement with this, there were no longer any clusters of activated microglia in either the ipsilateral or contralateral SN.
Neuronal cell counts and volume measurements
At 3 weeks post-lesion the mean (± SD) estimated total number of TH-positive cells on either side in each animal was 6424.93 ± 633.87 on the side ipsilateral to the lesion and 7602.81 ± 736.90 on the contralateral side (Fig. 5). This represents a 15.48% decrease in the total number of cells on the ipsilateral side compared with the side contralateral to the lesion. A two-tailed paired t-test indicated that this ipsilateral–contralateral difference was statistically significant (P < 0.01, n = 9). At the same time there was no significant difference in the number of neuronal nuclei stained in the STN on either side of the same brains.
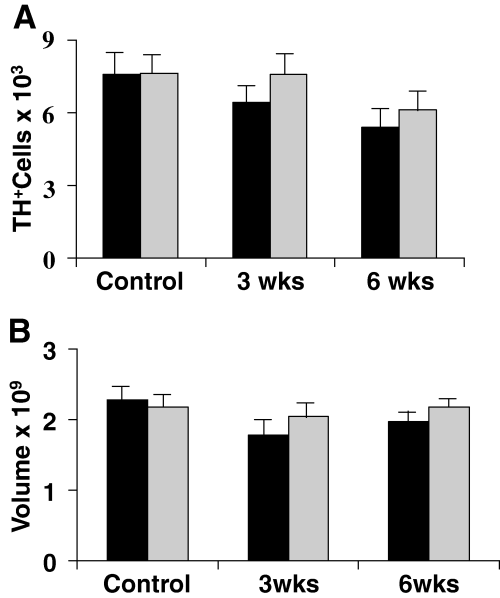
Stereological results in the substantia nigra. The histograms in A show the mean (± SD) estimated total number of dopamine cells on the two sides of the brains of control rats (pooled from those studied at different ages to match the lesioned animals). ▮, lesioned side of the brain in animals lesioned in the globus pallidus either 3 or 6 weeks before the counts were made. The lesioned side is statistically less well populated with cells in both 3 and 6 week sets of animals but the other side also shows a reduced number of tyrosine hydroxylase (TH)-positive cells at 6 weeks after the lesion (P < 0.05 for all comparisons, anova with Tukey's; n = 9 at 3 weeks and in the combined control group and n = 7 at 6 weeks). The volume of the substantia nigra is represented with the same convention as above in B. It was reduced on the side of the lesion in both groups of rats (paired t-test, P < 0.05). anova showed that the volume was also reduced on the control side at 3 weeks but not at 6 weeks after the lesion (P < 0.05, Tukey's).
The volume of the SN used for counting was 1.78 ± 0.21 mm3 on the ipsilateral side and 2.04 ± 0.18 mm3 on the contralateral side (Fig. 5B). The ipsilateral volume was 13% smaller than the contralateral volume. A two-tailed paired t-test indicated that this difference was statistically significant (P < 0.01, n = 9).
At 6 weeks post-lesion the mean (± SD) estimated total number of TH-positive cells in the SN was 5404.21 ± 948.34 on the side ipsilateral to the lesion and 6149.06 ± 990.04 on the contralateral side (Fig. 5). This is a 12% decrease in the number of DA cells on the ipsilateral side compared with the side contralateral to the lesion. A paired t-test indicated that this ipsilateral–contralateral difference was statistically significant (P < 0.001, n = 7). At 6 weeks there was still no sign of any difference in the number of cells in the STN on the two sides.
The volume of the SN used in the counts was 1.97 ± 0.12 mm3 on the ipsilateral side and 2.18 ± 0.18 mm3 contralaterally (Fig. 5B). The 11% difference in volume between the two sides was statistically significant (P < 0.01, n = 7; paired t-test).
There were no significant differences between cell numbers and nuclear volumes in the two control groups and therefore in the anova they were treated as a single group. Compared with this control group there were statistically significant differences between the total numbers of TH-positive cells in the ipsilateral SN at both 3 and 6 weeks after the lesions (anova, P < 0.001). In comparison, the contralateral SN cell counts at 3 weeks were not significantly different from the controls. However, by 6 weeks the contralateral cell numbers were similar to the ipsilateral count at 3 weeks and both were reduced compared with the control data (anova followed by Tukey's test, P < 0.05 in each case).
There were no differences in glial response, DA cell numbers or volume of the SN in any of the ‘striatal-lesioned’ animals, whether or not there was some minor damage to the GP (Fig. 3B).
Discussion
The data presented above show that 3 weeks after lesions of the GP there were significantly fewer TH-positive cells in the ipsilateral SN than there were in the contralateral SN. This difference in the numbers of TH-positive cells was accompanied by increases in immunohistochemical staining for OX42 and GFAP in the ipsilateral SN. These immunohistochemical changes are consistent with the activation of microglia and astrocytes that accompanies local tissue damage in the brain (Soltys et al., 1901; Tzeng & Wu, 1999; Tzeng et al., 1999; Rao et al., 2000). The decrease in TH-positive cell numbers on the lesioned side of brain seen at 3 weeks after the GP lesion was more marked at 6 weeks after the lesion, although at this time point the difference between the sides of the brain was slightly smaller. However, at 6 weeks post-lesion there is also evidence for bilateral reduction in the TH-positive cell numbers, compared with intact animals. Such a result suggests that there may be an excitotoxic action of the STN, which is capable of causing serious damage to the SNc even after relatively small, prolonged increases in activity in this glutamatergic projection.
Tyrosine hydroxylase is the rate-limiting enzyme in the DA synthesis pathway; all dopaminergic neurones express TH and can be marked with antibodies against it. Nevertheless, it should not be automatically assumed that the loss of TH-positive cells that we have measured represents the death of dopaminergic neurones. Electrolytic lesions of the rat caudate nucleus, which destroy nigrostriatal axon terminals, can lead to a 40% reduction in TH activity in the SN at 7 days after the lesion that is almost entirely reversed by 21 days (Reis et al., 1978). Similar effects have been seen in neurones using transmitters other than DA. For example, cholinergic neurones (Lams et al., 1988) and noradrenergic neurones (Ross & Reis, 1981) stop expressing choline acetyl transferase or dopamine-β-hydroxylase, respectively, in a reversible manner after axotomy. Although our data do not allow us to rule out this possibility completely, it is of note that all the studies that show reversible changes in TH expression point out that this reduced expression is a precursor to cell death and that recovery comes only if the initial insult is small (Reis et al., 1978). Evidence that cell death is likely to be the cause of the reduced numbers of TH-positive cells in the present experiments comes from the staining for GFAP and OX42. The levels of both of these markers were increased on the ipsilateral side at 3 weeks after the GP lesion. When astrocytes are activated by local damage, their expression of GFAP increases (Block & Schwarz, 1994). OX42 is an antibody against the type 3 complement receptor that is expressed by activated microglia (Tzeng et al., 1999). Increases in OX42 staining are suggestive of activation of the microglia and invasion of the area by macrophages from elsewhere (Tzeng & Wu, 1999). Such activation usually occurs when neurones are dying and peaks at around 3 days after an insult. The activation of astrocytes and microglia is part of an inflammatory response that occurs to remove debris from areas where cells have died. That levels of both of these markers showed activation in the SN 3 weeks after the GP lesions suggests that there were indeed degenerative processes occurring at this time.
There are a number of possible mechanisms by which cell death in the SN may follow an ibotenic acid lesion of the GP. A simple explanation is that the ibotenic acid injected in order to lesion the GP was able somehow to travel to the SN. If this was the case then it would be expected that the other nuclei through which the ibotenic acid would pass en route would also exhibit cell loss. The ibotenic acid would have to pass the STN to get to the SN. These nuclei showed no signs of cell loss at 3 weeks after the GP lesion. Another route for the ibotenic acid to travel to the dopaminergic cells in the SN would be from the striatum. In the present experiments many of the ibotenic acid lesions of the GP included a small part of the striatum. It is possible that dopaminergic axon terminals in the striatum may have taken up some ibotenic acid and transported it retrogradely to the SN. There are two lines of evidence to suggest that this is not the case. Firstly, it is widely believed that ibotenic acid is not taken up by axon terminals or by axons of passage. Secondly, in the present series of experiments there were five rats in which the ibotenic acid lesion was largely confined to the striatum. These rats showed no side-to-side difference in TH-positive cell numbers in the SN (Fig. 3).
If the ibotenic acid injected into the GP was not the direct cause of the loss of TH-positive cells in the SN then there must be an indirect cause.
There is evidence that ibotenic acid can cause indirect damage to axons of passage (Coffey et al., 1990). This damage is thought to be mediated through the inflammatory response that usually accompanies tissue damage. It is possible that the inflammatory response in the GP damaged the dopaminergic fibres that pass through this nucleus. Mechanical axonal damage to dopaminergic cells can cause the cell to stop expressing TH and eventually die (Reis et al., 1978). However, most of the damage to axons of passage by ibotenic acid involves demyelination. Dopaminergic fibres are unmyelinated in the rat and so would not be damaged by any mechanism that involved demyelination. Another possible mechanism for the loss of the TH-positive nigral cells would be if the GP was an important source of growth factors to these neurones. Lesioning the GP may have destroyed the source of these growth factors thus causing the subsequent death of any cells that relied on them. However, the GP is not a major target of the nigral dopaminergic cells (Lindvall & Bjorklund, 1979) and so it would be surprising to find that it was their main source of growth factors. On the other hand, the striatum is a major source of glial-derived neurotrophic factor which is important for the development of DA cells (Burke, 2003). Lesions of the striatum in adult animals do cause some ‘transynaptic’ degeneration in the substantia nigra pars reticulata (DeGiorgio et al., 1998) but do not cause damage in the SNc (Macaya & Burke, 1992; Macaya et al., 1994; Kelly & Burke, 1996; Oo et al., 2003).
Lesions of the GP render the STN hyperactive such that the resting firing rates of its neurones are increased (Hassani et al., 1996), they fire more bursts (Ryan & Clark, 1992) and their response to cortical stimulation is amplified (Ryan & Clark, 1992; Fujimoto & Kita, 1993). Such hyperactivity in the STN would lead to an increased level of glutamate release in its target nuclei, which includes the SN. These increases, although small, may cause a slowly mounting level of excitotoxic damage to the dopaminergic neurones of the SN over time. The ‘transynaptic’ neuronal damage in the SN which follows large lesions of both the striatum and GP (DeGiorgio et al., 1998) is reduced by previous lesion of the STN (Saji et al., 1996) suggesting that, in this circumstance too, STN hyperactivity is enough to damage the post-synaptic cells.
As well as increasing the activity in the STN, the lesion of the GP will also have reduced the inhibition on the DA cells directly, through the pathway from the GP to the SNc. However, reduction of inhibition by the GP of substantia nigra pars reticulata neurones would lead to an increase in the inhibition of DA cells from the substantia nigra pars reticulata (Iribe et al., 1999), which may compensate for the loss of direct GP inhibitory input to the SNc.
At 6 weeks after the GP lesions there was evidence that the loss of TH-positive cells was bilateral. This is in agreement with studies showing that, after unilateral electrical stimulation of the STN, there is a bilateral increase in glutamate levels in the SNc (Windels et al., 2000). There is no evidence that the GP sends projections bilaterally to the STN. There is also no evidence for bilateral projections from the STN to the SNc. However, there is a bilateral projection from the glutamatergic pedunculo-pontine nucleus (PPN) to the STN (Canteras et al., 1990). The PPN has reciprocal connections with the STN and so may pass excitation across the midline to the other STN and thence to the SNc. There may also be direct PPN to SNc connections. Indeed, damage to the PPN has been shown to protect SNc neurones from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine toxicity in monkeys (Takada et al., 2000). This bilateral brainstem projection seems to provide the only possibility for downstream activation after a unilateral GP lesion having bilateral consequences. Of course, there may be other mechanisms for the spread of damage across the midline; for example, the inflammatory response may spread but such an explanation is not supported by the apparent normalization of OX42 and GFAP staining in the SNc of animals 6 weeks after the lesions.
In summary, there is a substantial unilateral loss of dopaminergic cells in the SNc 3 weeks after a unilateral ibotenic acid lesion of the GP. This cell loss appears to be even greater at 6 weeks post-lesion. The most likely mechanism through which this loss of cells could occur is excitotoxicity as a consequence of the GP lesion rendering the neurones in the STN hyperactive. The bilateral nature of the DA cell loss at 6 weeks after the lesion also suggests an involvement of the PPN at later stages.
Acknowledgements
J.F.A. was the holder of an MRC PhD Studentship in The University of Edinburgh Centre for Neuroscience. A Wellcome Trust grant no. 054303 and Parkinson's Disease Society grant no. 4031 also supported the work. We are grateful to both the Neuroscience Department and the Department of Preclinical Veterinary Sciences, Edinburgh University for facilities.




