HAP1 facilitates effects of mutant huntingtin on inositol 1,4,5-trisphosphate-induced Ca2+ release in primary culture of striatal medium spiny neurons
Abstract
Huntington's disease is caused by polyglutamine expansion (exp) in huntingtin (Htt). Htt-associated protein-1 (HAP1) was the first identified Htt-binding partner. The type 1 inositol (1,4,5)-trisphosphate receptor (InsP3R1) is an intracellular Ca2+ release channel that plays an important role in neuronal function. Recently, we identified a InsP3R1–HAP1A–Htt ternary complex in the brain and demonstrated that Httexp, but not normal Htt, activates InsP3R1 in bilayers and facilitates InsP3R1-mediated intracellular Ca2+ release in medium spiny striatal neurons [MSN; T.-S. Tang et al. (2003) Neuron, 39, 227–239]. Here we took advantage of mice with targeted disruption of both HAP1 alleles (HAP1 –/–) to investigate the role of HAP1 in functional interactions between Htt and InsP3R1. We determined that: (i) HAP1 is expressed in the MSN; (ii) HAP1A facilitates functional effects of Htt and Httexp on InsP3R1 in planar lipid bilayers; (iii) HAP1 is required for changes in MSN basal Ca2+ levels resulting from Htt or Httexp overexpression; (iv) HAP1 facilitates potentiation of InsP3R1-mediated Ca2+ release by Httexp in mouse MSN. Our present results indicate that HAP1 plays an important role in functional interactions between Htt and InsP3R1.
Abbreviations
-
- ACSF
-
- artificial cerebrospinal fluid
-
- DHPG
-
- 3,5-dihydroxyphenylglycine
-
- DMEM
-
- Dulbecco's modified Eagle's medium
-
- EGFP
-
- enhanced green fluorescent protein
-
- EGFR
-
- epidermal growth factor receptor
-
- exp
-
- expansion
-
- FBS
-
- fetal bovine serum
-
- HAP1
-
- Htt-associated protein-1
-
- HD
-
- Huntington's disease
-
- Htt
-
- huntingtin
-
- InsP3R1
-
- type 1 inositol (1,4,5)-trisphosphate receptor
-
- MSN
-
- medium spiny striatal neurons
-
- NMDA
-
- N-methyl-d-aspartate
-
- PBS
-
- phosphate-buffered saline
-
- polyQ
-
- polyglutamine
-
- SDS–PAGE
-
- sodium dodecyl sulphate–polyacrylamide gel electrophoresis
Introduction
Huntington's disease (HD) is an autosomal-dominant neurological disorder caused by polyglutamine (polyQ) expansion (exp) in the amino-terminus of huntingtin (Htt), a ubiquitously expressed cytoplasmic protein (Nasir et al., 1996; Ross et al., 1999). It is characterized by selective, progressive neurodegeneration that primarily occurs in the striatum (Vonsattel et al., 1985; Vonsattel & DiFiglia, 1998) leading to death 15–20 years after onset of symptoms, which include chorea and psychiatric disturbance with gradual but inexorable intellectual decline (Vonsattel & DiFiglia, 1998). Despite significant progress, the molecular and cellular mechanisms that link Httexp with the pathogenesis of HD remain a mystery (Tobin & Signer, 2000).
A growing body of evidence suggests that HD-causing polyQ expansion of Htt (Httexp) is a gain of function mutation that leads to abnormal interactions of Httexp with other proteins. A number of proteins have been identified that bind with the amino-terminus of Htt (Kalchman et al., 1997; Gusella & MacDonald, 1998; Singaraja et al., 2002). The Htt-associated protein-1 (HAP1) was the first identified Htt-binding partner (Li et al., 1995, 1998b, 2000; Gutekunst et al., 1998; Page et al., 1998; Martin et al., 1999). Importantly, HAP1 is enriched in the brain and its binding to Htt is promoted by polyQ expansion (Li et al., 1995, 1998b). In rodents, two HAP1 protein isoforms differing in their carboxy-termini are expressed via alternative splicing − HAP1A and HAP1B − both of which bind Htt (Li et al., 1995; Nasir et al., 1998). Only one HAP1 isoform has been identified in humans and this is most similar to rodent HAP1A (Li et al., 1998b). HAP1 is abundantly expressed in the hypothalamus, and targeted disruption of the HAP1 gene in mice results in postnatal death due to depressed feeding behaviour (Chan et al., 2002; Li et al., 2003). The lack of HAP1 causes defective epidermal growth factor receptor (EGFR) signalling, which may contribute to neuronal degeneration in the hypothalamus (Li et al., 2003).
Recently, we identified a ternary type 1 inositol (1,4,5)-trisphosphate receptor (InsP3R1)–HAP1A–Htt complex in the brain (Tang et al., 2003b). We discovered that InsP3R1, an intracellular Ca2+ release channel that plays an important role in neuronal Ca2+ signalling, is associated with HAP1A and Htt both in vitro and in vivo. The association of InsP3R1 carboxy-terminal with Htt promoted by polyQ expansion of Htt enhanced InsP3R1 activity in planar lipid bilayers and InsP3R1-mediated intracellular Ca2+ release in medium spiny striatal neurons (MSN; Tang et al., 2003b). These findings identified a novel molecular link between Httexp and InsP3R1-mediated neuronal Ca2+ signalling (Tang et al., 2003b). Here we took advantage of mice without HAP1 (HAP1 –/–; Chan et al., 2002) to investigate the role of HAP1 in functional interactions between Htt and InsP3R1.
Materials and methods
HAP1 knockout mice
Generation and breeding of HAP1 knockout mice (mixed 129/ICR background) was previously described (Chan et al., 2002). The brains of E14.5–E15.5 embryos from HAP1 +/– intercross matings were collected and shipped from Vancouver, Canada to Dallas, TX, USA on wet ice in Hibernate media. The embryos were genotyped by polymerase chain reaction as previously described (Chan et al., 2002). All experiments in this paper were performed with HAP1 +/+, HAP1 +/– and HAP1 –/– littermates.
Plasmids and antibodies
GST-IC10 = F2627-A2749 of rat InsP3R1 (Mignery et al., 1990) in pGEX-KG vector was previously described (Tang et al., 2003b). The full-length rat HAP1A construct in pCMV-HA mammalian expression vector and pGEX-KG bacterial expression vector were previously described (Tang et al., 2003b). Full-length Htt plasmids Htt-23Q (HD-FL-23Q) and Htt-82Q (HD-FL-82Q) in pRc/CMV expression vector (Invitrogen) were kindly provided by Dr Christopher A. Ross (Cooper et al., 1998). The GST-Htt-N expression constructs are Htt-N-15Q/138Q = M1–K158 of human Htt were previously described (Tang et al., 2003b).
Monoclonal antibodies: anti-GAD65 from BD Pharmingen, anti-HAP1 from BD Transduction, anti-Htt from Chemicon, anti-DARRP-32 from Cell Signalling Technology, antiβ-actin from Sigma. Polyclonal anti-InsP3R1 antibody T443 was previously described (Kaznacheyeva et al., 1998). Secondary horseradish peroxidase-conjugated anti-rabbit and anti-mouse antibodies were from Jackson ImmunoResearch.
Primary cultures of mice MSN
The mice MSN cultures were established as described previously for rat MSN (Chesselet et al., 1993; Mao & Wang, 2001) with some modifications. Briefly, striata were dissected from brains of HAP1 +/+, HAP1 +/– and HAP1 –/– embryonic mice in ice-cold dissection solution (1 × divalent cation-free Hank's balanced salt solution, 15 mm HEPES, 10 mm NaHCO3 and 100 units/mL penicillin/streptomycin, pH 7.2). The striata from mouse with identical genotypes were pooled together and treated with 0.25% trypsin for 7 min at 37 °C. After enzyme inhibition with 10% heat-inactivated fetal bovine serum (FBS, Invitrogen) in Dulbecco's modified Eagle's medium (DMEM, Invitrogen), the tissue was dissociated with trituration solution (1 × divalent-free Hank's balanced salt solution, 1.0% DNase I, pH 7.2; Goslin et al., 1998) using a series of reducing bore-size Pasteur pipettes. The cells were washed and plated at a density of 1 × 106 cells/mL on poly-d-lysine (MW = 30,000–70 000 g/mol; 0.01% final concentration) precoated 12-mm round coverslips in plating medium containing 60% DMEM, 30% Neurobasal media, 10% FBS, 100 units/mL penicillin/streptomycin (Invitrogen) and incubated at 37 °C in 5% CO2. Twenty-four hours later, the cultures were replaced by culture medium [containing 65% DMEM, 30% Neurobasal media, 1 × B27 (Gibco), 5% FBS, 100 units/mL penicillin/streptomycin (Invitrogen)]. Cytosine arabinoside (4 µm, AraC, Sigma) was added at 2–4 DIV to inhibit glial cell growth if necessary. The cultures were fed with fresh culture medium every 7 days.
Immunostaining of MSN cultures
At 12 DIV MSN cultures from wild-type mouse (HAP1 +/+), HAP1 heterozygotes (+/–) and HAP1 knockout mice (–/–) were washed once in phosphate-buffered saline (PBS), fixed for 30 min in 4% paraformaldehyde and 4% sucrose in PBS on ice, and permeabilized for 5 min at room temperature in 0.25% Triton X-100 in PBS. Non-specific binding sites were blocked by incubation of cells for 60 min in 5% bovine serum albumin (BSA, Sigma, fraction V). Neurons were covered by primary GAD65 monoclonal antibodies diluted in blocking solution, washed three times with PBS, and incubated with anti-mouse FITC-conjugated secondary antibodies (Jackson Immunoresearch). Slides were extensively washed with PBS and mounted in Moweol-488 (Polysciences). FITC-fluorescent and bright-field images were collected with a Olympus microscope with 40 × objectives using Cascade650 camera (Rhoper Scientific) and Metafluor software (Universal Imaging), and prepared for presentation using Adobe Photoshop.
Western blotting of MSN culture lysates
Primary MSN cultures in 24-well plates without coverslips were set up as described above. At 16 DIV, MSN from the wild type mouse (+/+), HAP1 heterozygotes (+/–) and HAP1 knockout mice (–/–) were washed with ice-cold PBS and solubilized for 60 min at 4 °C in extraction buffer A (in mm: 1% CHAPS, NaCl, 137; KCl, 2.7; Na2HPO4, 4.3; KH2PO4, 1.4, pH 7.2; EDTA, 5; EGTA, 5 and protease inhibitors). Extracts were clarified by centrifugation for 20 min at 100 000 g (TL-100), and analysed by Western blotting with monoclonal antibodies against HAP1, Htt, DARPP-32 and β-actin and polyclonal antibodies against InsP3R1. Extract from ∼ 105 cells (∼ 8 µg total protein) was loaded on sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) for each genotype.
Planar lipid bilayer experiments
Single-channel recordings of recombinant InsP3R1 (RT1) expressed in Spodoptera frugiperda (Sf9) cells by baculoviral infection were performed as previously described (Tu et al., 2002; Tang et al., 2003a) at 0 mV transmembrane potential using 50 mm Ba2+ (trans) as a charge carrier. The cis (cytosolic) chamber contained 110 mm Tris dissolved in HEPES (pH 7.35), 0.5 mm Na2ATP, pCa 6.7 (0.2 mm EGTA + 0.14 mm CaCl2; Bezprozvanny et al., 1991). InsP3R1 were activated by addition of 100 nm InsP3 (Alexis) to the cis chamber as indicated in the text. Htt-N-15Q, Htt-N-138Q and HAP1A proteins were expressed in BL21 Escherichia coli, purified on glutathione beads, eluted with glutathione, and dialysed overnight against cis recording buffer (110 mm Tris/HEPES, pH 7.35). Equal amounts of Htt-N-15Q and HAP1A or Htt-N-138Q and HAP1A proteins were mixed together and added in 1 µL volume (0.3 mg/mL total protein with addition of 0.02 mm ruthenium red) directly to the cis side of the bilayer containing InsP3R1 without stirring. Exposure of InsP3R1 to the test proteins was terminated 2–3 min after addition by stirring the cis chamber for 30 s (1 : 3000 dilution of test protein stocks). The InsP3R1 single-channel currents were amplified (Warner OC-725), filtered at 1 kHz by low-pass eight-pole Bessel filter, digitized at 5 kHz (Digidata 1200, Axon Instruments) and stored on computer hard drive and recordable optical discs. For off-line computer analysis (pClamp 6, Axon Instruments) currents were filtered digitally at 500 Hz. For presentation of the current traces, data were filtered at 200 Hz.
MSN transfection and Ca2+ imaging experiments
The wild-type (HAP1 +/+) and HAP1 knockout (HAP1 –/–) mouse MSN cultures at 16–18 DIV were transfected by the calcium-phosphate method with enhanced green fluorescent protein (EGFP)-C3 plasmid (Clontech) or a 1 : 3 mixture of EGFP : Htt plasmids, as previously described for rat MSN (Tang et al., 2003b). At 48 h after transfection, the MSN neurons were loaded with 5 µm Fura2-AM (Molecular Probes) in artificial cerebrospinal fluid (ACSF, in mm) NaCl, 140; KCl, 5; MgCl2, 1; CaCl2, 2; HEPES, 10, pH 7.3 for 45 min at 37 °C. For imaging experiments the coverslips were mounted onto a recording/perfusion chamber (RC-26G, Warner Instruments) maintained at 37 °C (PH1, Warner Instruments), positioned on the movable stage of an Olympus IX-70 inverted microscope, and perfused with ACSF media by gravity flow. The transfected MSNs were identified by GFP imaging. Following GFP imaging, the culture was washed extensively with Ca2+-free ACSF (omitted CaCl2 from ACSF and supplemented with 100 µm EGTA). In Ca2+ imaging experiments, the MSN cells were intermittently excited by 340 nm and 380 nm UV light (DeltaRAM illuminator, PTI) using a Fura-2 dichroic filter cube (Chroma Technologies) and 60 × UV-grade oil-immersed objective (Olympus). The emitted light was collected by an IC-300 camera (PTI), and the images were digitized by ImageMaster Pro software (PTI). Baseline 1–3 min measurements were obtained prior to bath application of 10 µm 3,5-dihydroxyphenylglycine (DHPG) (Tocris) dissolved in Ca2+-free ACSF. The DHPG solutions were prewarmed to 37 °C before application to MSNs. Images at 340 nm and 380 nm excitation wavelengths were captured every 5 s, and 340/380 image ratio traces were recorded. Background fluorescence was determined according to manufacturer's (PTI) recommendations and subtracted.
Results
HAP1 is expressed in mouse MSN
HAP1 is abundantly expressed in the hypothalamus, and genetic ablation of HAP1 results in hypothalamic dysfunction (Chan et al., 2002; Li et al., 2003). HAP1 is also highly expressed in the accessory olfactory bulb, colliculi, pedunculopontine nucleus, and brain stem of mouse, rat and human (Li et al., 1995, 1998a, 2000, 2003; Gutekunst et al., 1998; Page et al., 1998; Chan et al., 2002). Northern blot, in situ hybridization and immunostaining analyses revealed moderate levels of HAP1 expression in the striatum (Li et al., 1995; Gutekunst et al., 1998; Page et al., 1998). HD selectively affects MSN. To determine if HAP1 was expressed in MSN, we established primary MSN cultures from E14.5–15.5 embryos of HAP1 +/+, HAP1 +/– and HAP1 –/– mouse (Chan et al., 2002; see Materials and methods). We found that neuronal cultures from the wild-type (HAP1 +/+), HAP1 +/– and HAP1 –/– mouse had similar density (Fig. 1). The identity of established cultures was confirmed in immunostaining experiments with GAD65 monoclonal antibodies. For all three genotypes, most of the cells in our MSN cultures were GAD65-positive (Fig. 1), consistent with previous observations (Chesselet et al., 1993; Mao & Wang, 2001).
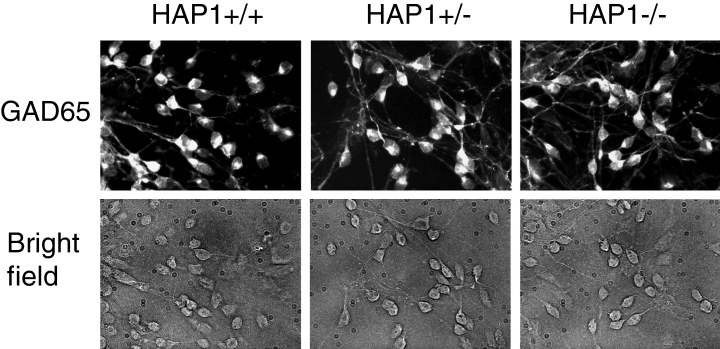
Primary MSN cultures from HAP1 +/+, HAP1 +/– and HAP1 –/– mice. Primary MSN cultures from HAP1 +/+, HAP1 +/– and HAP1 –/– mice were fixed, permeabilized and stained for GAD65 at 12 DIV, as described in Materials and methods (top row). The corresponding bright-field images are also shown (bottom row). More than 90% of neurons in our cultures are GAD65-positive.
At 16 DIV, these MSN cultures were collected, solubilized in 1% CHAPS and analysed by Western blotting with anti-HAP1 monoclonal antibodies. Both HAP1A and HAP1B were clearly detected in HAP1 +/+ and HAP1 +/– lysates (Fig. 2, top row). No HAP1 signals were detected in lysates from HAP1 –/– MSN, confirming the absence of both HAP1 isoforms in these mice (Fig. 2, top row). Our experiments demonstrate the expression of HAP1 protein in mouse MSN cultures. This could not be due to contaminating glial cells in the cultures, as HAP1 transcripts are associated with neurons and not glia (Li et al., 1996). We next explored the effects of HAP1 ablation on expression levels of InsP3R1 and Htt in MSN. Analysis of MSN lysates from HAP1 +/+, HAP1 +/– and HAP1 –/– mice by Western blotting with anti-Htt monoclonal antibodies and anti-InsP3R1 polyclonal antibodies revealed that absence of HAP1 expression had no significant effect on expression levels of InsP3R1 (Fig. 2, second row) or Htt (Fig. 2, third row). Western blotting experiments with monoclonal anti-DARPP-32 antibodies (Fig. 2, fourth row) and anti-β-actin antibodies (Fig. 2, fifth row) were performed to demonstrate that equal amounts of cellular lysate were loaded on the gel for each genotype.
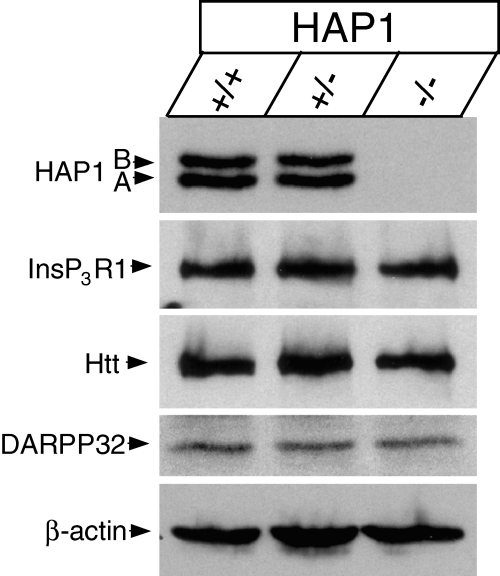
Western blotting analysis of cultured mouse MSN. 16 DIV MSN cultures from HAP1 +/+, HAP1 +/– and HAP1 –/– mice were collected and solubilized in 1% CHAPS. Lysates from ∼ 105 cells (∼ 8 µg total protein) were loaded on the gel for each genotype. Lysates were separated by SDS–PAGE and blotted by mAb against HAP1, pAb against type 1 inositol (1,4,5)-trisphosphate receptor (InsP3R1), mAb against Htt, mAb against DARPP-32, and mAb against β-actin as indicated.
HAP1 facilitates activation of InsP3R1 by Htt and Httexp in planar lipid bilayers
In the previous study (Tang et al., 2003b) we demonstrated that Httexp, but not Htt, activates InsP3R1 in planar lipid bilayers. To examine the role of HAP1 in mediating functional effects of Htt on InsP3R1, we expressed InsP3R1 in Sf9 cells and reconstituted recombinant InsP3R1 in planar lipid bilayers as previously described (Tu et al., 2002; Tang et al., 2003a). Addition of 100 nm InsP3 to the cis (cytosolic) chamber induced low levels of InsP3R1 activity (Fig. 3A, second trace, Fig. 3B). Addition of premixed Htt-N-15Q and HAP1A GST-fusion proteins directly to the bilayer enhanced InsP3R1 activity (Fig. 3A, third trace and Fig. 3B). In parallel experiments, we found that addition of premixed Htt-N-138Q and HAP1A GST-fusion proteins resulted in significantly greater facilitation of InsP3R1 activity (Fig. 3C, third trace and Fig. 3D). On average, InsP3R1 open probability was equal to 0.020 ± 0.008 (n = 16) in the presence of 100 nm InsP3, 0.15 ± 0.08 (n = 3) after addition of HAP1A + Htt-N-15Q, and 0.31 ± 0.14 (n = 3) after addition of HAP1A + Htt-N-138Q. In the previous planar lipid bilayer experiments we found that addition of HAP1 alone has no effect on InsP3R1 activity (Tang et al., 2003b). Thus, we concluded that HAP1A facilitates activation of InsP3R1 by Htt and Httexp, most likely by promoting Htt association with the carboxy-terminus of InsP3R1 (Tang et al., 2003b). This conclusion is consistent with our previous analysis of functional effects of HAP1 and Htt on InsP3R1 (Tang et al., 2003b).
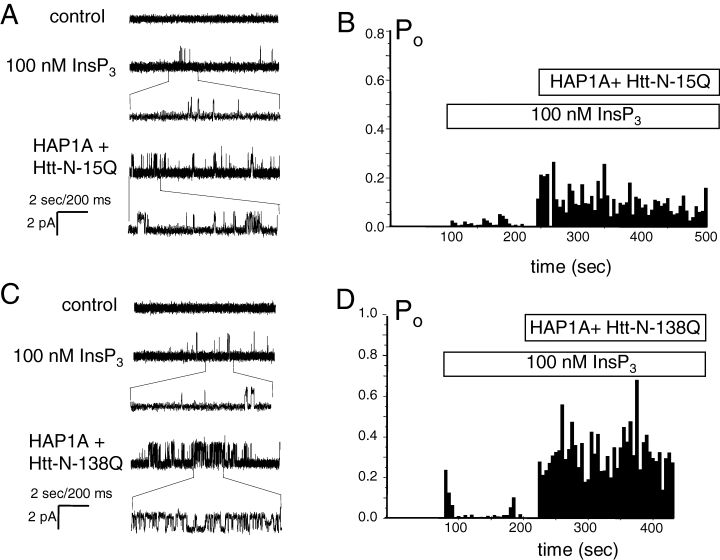
FFunctional effect of Htt-associated protein-1 (HAP1)/Htt-N complex on type 1 inositol (1,4,5)-trisphosphate receptor (InsP3R1) in planar lipid bilayers. In the previous planar lipid bilayer experiments we found that addition of HAP1 alone or Htt-N-15Q alone had no effect on InsP3R1 activity (Tang et al., 2003b). (A) Effects of HAP1A + Htt-N-15Q complex on activity of recombinant InsP3R1 in planar lipid bilayers at 100 nm InsP3. Each current trace corresponds to 10 s (2 s for expanded traces) of current recording from the same experiment. (B) The average InsP3R1 open probability (Po) in the presence of 100 nm InsP3 is calculated for a 5-s window of time and plotted for the duration of an experiment. The times of InsP3 and HAP1A + Htt-N-15Q additions are shown above the Po plot. Data from the same experiment are shown in A and B. Similar results were obtained in three independent experiments. (C and D) Effects of HAP1A + Htt-N-138Q complex on activity of recombinant InsP3R1 in planar lipid bilayers at 100 nm InsP3. The data are presented and analysed as described for A and B. Similar results were obtained in three independent experiments.
Httexp sensitizes InsP3R1-mediated Ca2+ release in mouse MSN
In the previous study (Tang et al., 2003b) we demonstrated that overexpression of Httexp, but not Htt, sensitizes InsP3R1-mediated DHPG-induced Ca2+ release in rat MSN. We now performed Htt transfection and Ca2+ imaging experiments with MSN from the wild-type (HAP1 +/+) mouse. In these experiments the wild-type mice MSN at 16–18 DIV were transfected with full-length Htt-23Q or Htt-82Q expression plasmids. To identify transfected cells, the Htt plasmids were co-transfected with an EGFP-expressing plasmid. To ensure that every GFP-positive cell was transfected with Htt-expressing plasmid, the Htt : EGFP plasmid ratio was kept at 3 : 1 during transfection. In control experiments, MSNs were transfected with the EGFP plasmid alone. Only GFP-positive cells were compared in our analysis of different Htt constructs. Because MSN abundantly express phospholipase C (PLC)-linked mGluR1/5 receptors (Tallaksen-Greene et al., 1998; Mao & Wang, 2001, 2002), DHPG, a specific mGluR1/5 receptor agonist (Schoepp et al., 1999) was used to stimulate InsP3R1-mediated Ca2+ mobilization in MSNs. To exclude the contribution of N-methyl-d-aspartate (NMDA) receptors and L-type Ca2+ channels to the observed Ca2+ signals and to simplify the analysis, the Ca2+ imaging experiments were performed in Ca2+-free media containing 100 µm EGTA (see Materials and methods for details).
The local Ca2+ concentration in these experiments is estimated from the ratio of Fura-2 emission signals at 340 nm and 380 nm excitation wavelengths. Prior to 340/380 ratio recording, GFP images were taken to identify the Htt-transfected MSN. Basal Ca2+ levels in transfected MSN were recorded for 1–3 min prior to bath application of 10 µm DHPG. The basal 340/380 ratio was equal to 0.51 ± 0.01 (n = 9) for MSN transfected with EGFP (ig. 3. , 6), 0.55 ± 0.015 (n = 11) for MSN transfected with EGFP + Htt-23Q (4, 6), and 0.6 ± 0.01 (n = 20) for MSN transfected with EGFP + Htt-82Q (4, 6). The statistical analysis reveals that basal Ca2+ levels were significantly (P < 0.05, unpaired t-test) higher in Htt-23Q or Htt-82Q transfected MSN as compared with EGFP-transfected MSN (Fig. 6A). These findings are consistent with elevated basal Ca2+ levels observed in our previous rat MSN Htt-transfections (Tang et al., 2003b) and in experiments with hippocampal neurons from YAC72 transgenic HD mice (Hodgson et al., 1999).
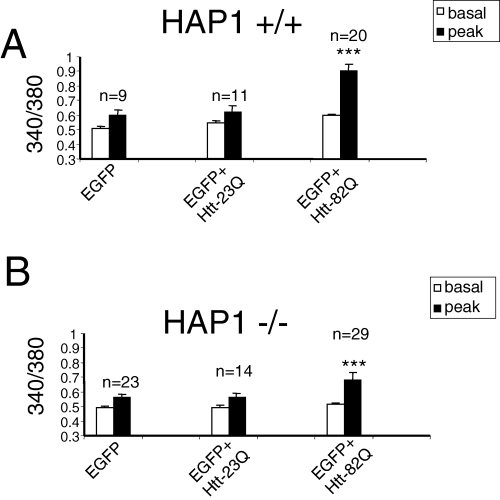
Quantitative analysis of wild-type and Htt-associated protein-1 (HAP1) –/– MSN Ca2+ imaging experiments. Summary of Ca2+ imaging experiments with wild-type (HAP1 +/+) (A) and HAP1 knockout (HAP1 –/–) (B) mouse MSN transfected with Htt. Average basal and 10 µm DHPG-evoked peak 340/380 ratios from three independent transfections are shown as mean ± SEM (n = number of cells). The DHPG-induced peak 340/380 ratios in both wild-type and HAP1 –/– MSN transfected with enhanced green fluorescent protein (EGFP) + Htt-82Q were significantly (P < 0.05, unpaired t-test) higher than the DHPG-induced peak 340/380 ratios in MSN of the same genotype transfected with EGFP or EGFP + Htt-23Q. The DHPG-induced peak 340/380 ratios for HAP1 –/– mouse MSN transfected with EGFP + Htt-82Q were significantly lower (P < 0.05, unpaired t-test) than the DHPG-induced peak 340/380 ratios in wild-type mouse MSN transfected with the same plasmid combination. The basal Ca2+ levels are significantly higher (P < 0.05, unpaired t-test) in wild-type mouse MSN transfected with Htt-23Q and Htt-82Q plasmids than in EGFP-transfected wild-type MSN. The basal Ca2+ levels in HAP1 –/– mouse MSN were not significantly different (P < 0.05, unpaired t-test) for EGFP, EGFP + Htt-23Q and EGFP + Htt-82Q transfections.
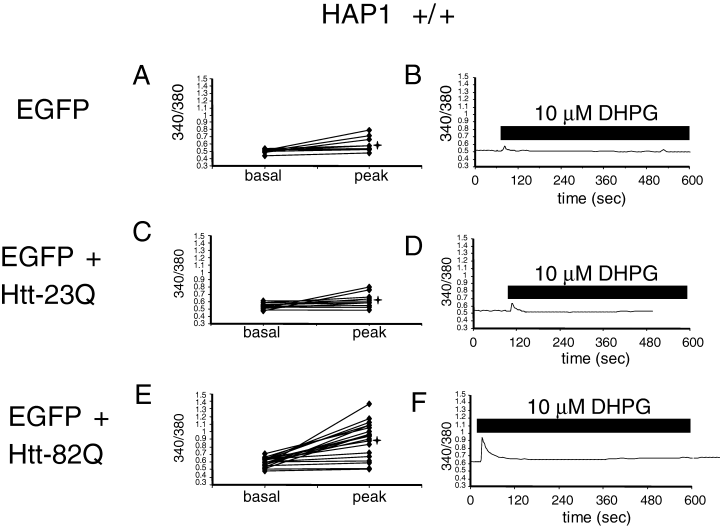
Httexp facilitates 3,5-dihydroxyphenylglycine (DHPG)-induced Ca2+ release in wild-type mice MSN. Basal and peak 340/380 ratios are shown for individual wild-type mouse MSN neurons [Htt-associated protein-1 (HAP1) +/+] transfected with enhanced green fluorescent protein (EGFP) (A, B), EGFP + Htt-23Q (C, D), EGFP + Htt-82Q (E and F). Only GFP-positive MSN were considered for quantitative analysis for each group of cells. The basal ratios were determined 1–3 min prior to application of 10 µm DHPG. The peak ratios were determined from the maximal signals observed within 30 s after DHPG application. 340/380 ratio traces for representative cells (marked *) are shown in B, D and F. Time of DHPG application is shown. Similar results were obtained in three independent transfections.
We found that similar to our previous results with rat MSN (Tang et al., 2003b), 10 µm DHPG, a threshold concentration for mGluR1/5 receptor activation in MSN neurons, induces small intracellular Ca2+ responses in control MSN transfected with EGFP plasmid alone (4, 6) and in MSN transfected with EGFP + Htt-23Q plasmids (4, 6). In contrast, significantly higher Ca2+ responses were induced by application of 10 µm DHPG in MSN transfected with EGFP + Htt-82Q plasmids (4, 6). On average, the peak 340/380 ratio was equal to 0.6 ± 0.04 (n = 9) for MSN transfected with EGFP (Fig. 6A), 0.62 ± 0.05 (n = 11) for MSN transfected with EGFP + Htt-23Q (Fig. 6A), and 0.9 ± 0.05 (n = 20) for MSN transfected with EGFP + Htt-82Q (Fig. 6A). The DHPG-induced peak Ca2+ levels in Htt-82Q + EGFP transfections were significantly (P < 0.05, unpaired t-test) higher than in EGFP or EGFP + Htt-23Q transfected MSN, whereas DHPG-induced peak Ca2+ levels were not significantly different (P < 0.05, unpaired t-test) between EGFP + Htt-23Q and EGFP transfected MSN (Fig. 6A). Thus, similar to our previous findings with rat MSN (Tang et al., 2003b), overexpression of full-length Httexp (Htt-82Q) but not normal Htt (Htt-23Q) sensitizes InsP3R1-mediated Ca2+ release in mouse MSN.
HAP1 potentiates effects of Httexp on InsP3R1-mediated Ca2+ release in mouse MSN
To determine a role of HAP1 in functional effects of Httexp on InsP3R1-mediated Ca2+ release in MSN, we repeated Htt transfections and Ca2+ imaging experiments with MSN from HAP1 knockout (HAP1 –/–) mice. Similar to experiments with rat MSN (Tang et al., 2003b) and wild-type mouse MSN (4, 6), HAP1 –/– MSN were transfected with EGFP, EGFP + Htt-23Q and EGFP + Htt-82Q plasmids, and analysed by Fura-2 Ca2+ imaging. In contrast to rat MSN (Tang et al., 2003b) and wild-type mouse MSN (4, 6), expression of Htt had no effect on the basal Ca2+ levels in HAP1 –/– MSN. On average, the basal 340/380 ratio was equal to 0.49 ± 0.01 (n = 23) for HAP1 –/– MSN transfected with EGFP (Figs 5A and B, and 6B), 0.49 ± 0.01 (n = 14) for HAP1 –/– MSN transfected with EGFP + Htt-23Q (5, 6), and 0.51 ± 0.01 (n = 29) for HAP1 –/– MSN transfected with EGFP + Htt-82Q (5, 6). Thus, the presence of HAP1 is necessary for mediating the effects of Htt on basal Ca2+ levels in mouse MSN.
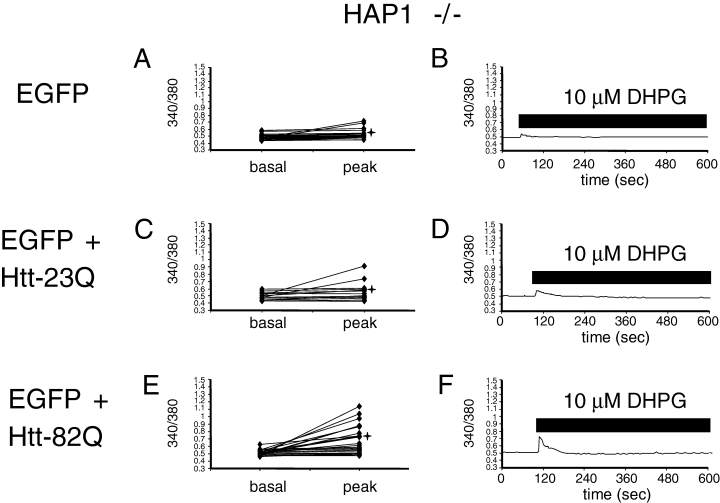
Reduced facilitation of 3,5-dihydroxyphenylglycine (DHPG)-induced Ca2+ release by Httexp in Htt-associated protein-1 (HAP1) –/– mouse MSN. Basal and peak 340/380 ratios are shown for individual HAP1 –/– MSN neurons transfected with enhanced green fluorescent protein (EGFP) (A, B), EGFP + Htt-23Q (C and D), EGFP + Htt-82Q (E and F). The data are presented and analysed as described in the legend to Fig. 4. Similar results were obtained in three independent transfections.
We found that peak Ca2+ levels resulting from 10 µm DHPG application were equal to 0.56 ± 0.02 (n = 23) in HAP1 –/– MSN transfected with EGFP (5, 6), 0.56 ± 0.03 (n = 14) for HAP1 –/– MSN transfected with EGFP + Htt-23Q (5, 6), and 0.68 ± 0.05 (n = 29) for HAP1 –/– MSN transfected with EGFP + Htt-82Q (5, 6). Statistical analysis revealed that the peak Ca2+ levels in HAP1 –/– MSN transfected with EGFP + Htt-82Q were significantly higher (P < 0.05, unpaired t-test) than in HAP1 –/– MSN transfected with EGFP or EGFP + Htt-23Q plasmids (Fig. 6B), but the peak Ca2+ levels in HAP1 –/– MSN transfected with EGFP or EGFP + Htt-23Q plasmids were not different (P < 0.05, unpaired t-test). Thus, similar to experiments with rat MSN (Tang et al., 2003b) and wild-type mouse MSN (4, 6), overexpression of full-length Httexp (Htt-82Q) but not normal Htt (Htt-23Q) sensitizes InsP3R1-mediated Ca2+ release in mouse HAP1 –/– MSN. Additional statistical analysis also revealed that the peak Ca2+ levels in HAP1 –/– MSN transfected with EGFP + Htt-82Q were significantly lower (P < 0.05, unpaired t-test) than the peak Ca2+ levels in wild-type mouse MSN transfected with EGFP + Htt-82Q (Fig. 6). On average (peak-basal), the difference in 340/380 ratios for neurons co-transfected with EGFP + Htt-82Q plasmid combination was equal to 0.304 ± 0.05 (n = 20) for HAP1 +/+ MSN and 0.168 ± 0.04 (n = 29) for HAP1 –/– MSN. The difference in (peak-basal) values between HAP1 +/+ and HAP1 –/– MSN was statistically significant, with P = 0.03 < 0.05 (unpaired t-test). These results indicated that HAP1 is not required for functional effects of Httexp on InsP3R1-mediated Ca2+ release in MSN, but that the presence of HAP1 potentiates the functional effects of Httexp on InsP3R1-mediated Ca2+ release in mouse MSN.
Discussion
The present study extends our previous discovery of functional interactions between Htt, HAP1 and InsP3R1 (Tang et al., 2003b). In this study we focused on the role played by HAP1 in mediating functional effects of Htt and Httexp on InsP3R1 in vitro and in vivo. Here we demonstrate that: (i) HAP1 is expressed in MSN; (ii) HAP1A facilitates functional effects of Htt and Httexp on InsP3R1 in planar lipid bilayers; (iii) HAP1 is required for changes in MSN basal Ca2+ levels resulting from Htt or Httexp overexpression; and (iv) HAP1 facilitates potentiation of InsP3R1-mediated Ca2+ release by Httexp in mouse MSN. These results indicate an involvement of HAP1 in derangements of Ca2+ signalling in MSN neurons that may occur early in the pathogenesis of HD.
HAP1 was the first identified Htt-binding partner in yeast two-hybrid screens (Li et al., 1995; Gutekunst et al., 1998; Page et al., 1998). The HAP1–Htt interaction is increased by expansion of the polyQ tract in the amino-terminus of Htt, implying that HAP1 function may be affected in HD. A number of possible molecular functions of HAP1 have been proposed: HAP1 is involved in neuronal vesicular transport via its interactions with microtubule-based transporters and vesicles (Block-Galarza et al., 1997; Engelender et al., 1997; Li et al., 1998b). HAP1 was also found to be involved in the endosomal trafficking and signalling of the EGFR (Li et al., 2002, 2003). Evidence from targeted disruption of HAP1 suggests an important role of HAP1 in hypothalamic function (Chan et al., 2002; Li et al., 2003). However, the main locus of HD pathology is the striatum, and not the hypothalamus, and the role played by HAP1 in striatal neuronal function and the pathogenesis of HD has remained poorly understood.
In our previous study we identified a protein complex containing InsP3R1, Htt and HAP1 (Tang et al., 2003b). This complex was initially discovered through the identification of HAP1A as a binding partner of the InsP3R1 carboxy-terminal tail in the yeast two-hybrid system. We further found that Htt could directly interact with the InsP3R1 carboxy-terminus and that binding of Htt to the InsP3R1 carboxy-terminus was dependent on both the presence of HAP1A and polyQ expansion. Thus, Httexp can bind to InsP3R1 carboxy-terminus either directly or indirectly through HAP1A. Consistent with these biochemical data and with our previous functional analysis (Tang et al., 2003b), we here demonstrate that HAP1 facilitates functional effects of Htt and Httexp on InsP3R1 in planar lipid bilayers (Fig. 3). We further demonstrate that Httexp, but not normal Htt, sensitizes InsP3R1-mediated Ca2+ release in HAP1 +/+ and HAP1 –/– mouse MSN (4, 5, 6). Thus, Httexp can exert effects on InsP3R1 in vivo in the absence of HAP1, consistent with the direct association between Httexp and the carboxy-terminus of InsP3R1 in biochemical experiments (Tang et al., 2003b). In contrast to Htt-82Q, the presence or absence of HAP1 did not affect peak Ca2+ responses observed in the presence of Htt-23Q (Fig. 6). Interestingly, functional effects of Httexp on InsP3R1-mediated Ca2+ release were attenuated in HAP1 –/– mouse MSN when compared with wild-type HAP1 +/+ mouse MSN (Fig. 6). Thus, HAP1 potentiates functional effects of Httexp on InsP3R1 function in vivo. These results are consistent with our previous biochemical analysis (Tang et al., 2003b) and planar lipid bilayer experiments (Fig. 3; Tang et al., 2003b). Moreover, effects of Htt or Httexp on basal Ca2+ levels in MSN neurons required HAP1 (Fig. 6), suggesting that Htt-dependent sensitization of InsP3R1 to basal InsP3 levels in vivo requires formation of an InsP3R1–HAP1–Htt complex. An alternative explanation for this result is that Htt or Httexp overexpression increases basal Ca2+ levels in MSN in a HAP1-dependent manner by a different mechanism, for example by upregulating EGFR-mediated signalling (Li et al., 2002, 2003).
The pathophysiological mechanisms underlying neuronal death in HD are still unclear. Perturbed Ca2+ homeostasis is one of the key steps during the initiation of the apoptotic programme in affected neurons (Mattson & Chan, 2001). Possible connections between HD and aberrant neuronal Ca2+ signalling have been reported (Chen et al., 1999; Sun et al., 2001; Panov et al., 2002; Zeron et al., 2002; Tang et al., 2003b). Importantly, increases in neuronal Ca2+ represent early events in the pathogenesis of HD (Chen et al., 1999; Sun et al., 2001; Panov et al., 2002; Zeron et al., 2002; Tang et al., 2003b). InsP3R1 sensitivity to InsP3 has been found to be potentiated by Httexp but not normal Htt in MSN, leading to excessive Ca2+ release from endoplasmic reticulum (ER) at threshold levels of mGluR1/5 stimulation (Tang et al., 2003b; present manuscript). Along with the evidence that Httexp preferentially enhances the NR1A/NR2B NMDA receptor in MSN, hyper-responsive Ca2+ signalling mediated by InsP3R1 may be a primary cause of selective MSN death in HD. Our present results indicate that HAP1 facilitates functional effects of Httexp on InsP3R1 and suggest a role for HAP1 in altered neuronal Ca2+ signalling, which potentially leads to selective neuronal loss in HD.
Acknowledgements
We thank Dr Christopher Ross for HD-FL-23Q and HD-FL-82Q plasmids, Nan Wang for technical assistance and Phyllis Foley for administrative assistance. I.B. is supported by the Robert A. Welch Foundation, Huntington's Disease Society of America, Hereditary Disease Foundation, and NIH R01 NS38082. M.R.H. is supported by the Canadian Institutes of Health Research, Hereditary Disease Foundation, and Huntington's Disease Society of America, and holds a Canada Research Chair in Human Genetics.




