Acetylcholine–dopamine balance hypothesis in the striatum: An update
Abstract
The imbalance between cholinergic activity and dopaminergic activity in the striatum causes a variety of neurological disorders, such as Parkinson's disease. During sensorimotor learning, the arrival of a conditioned stimulus reporting a reward evokes a pause response in the firing of the tonically active cholinergic interneurons in targeted areas of the striatum, whereas the same stimulus triggers an increase in the firing frequency of the dopaminergic neurons in the substantia nigra pars compacta. The pause response of the cholinergic interneurons begins with an initial depolarizing phase followed by a pause in spike firing and ensuing rebound excitation. The timing of the pause phase coincides well with the surge in dopaminergic firing, indicating that a dramatic rise in dopamine (DA) release occurs while nicotinic receptors remain unbound by acetylcholine. The pause response begins with dopamine D5 receptor-dependent synaptic plasticity in the cholinergic neurons and an increased GABAergic IPSP, which is followed by a long pause in firing through D2 and D5 receptor-dependent modulation of ion channels. Inactivation of muscarinic receptors on the projection neurons eventually yields endocannabinoid-mediated, dopamine-dependent long-term depression in the medium spiny projection neurons. Breakdown of acetylcholine-dopamine balance hampers proper functioning of the cortico-basal ganglia-thalamocortical loop circuits. In Parkinson's disease, dopamine depletion blocks autoinhibition of acetylcholine release through muscarinic autoreceptors, leading to excessive acetylcholine release which eventually prunes spines of the indirect-pathway projection neurons of the striatum and thus interrupts information transfer from motor command centers in the cerebral cortex. Geriatr Gerontol Int 2010; 10 (Suppl. 1): S148–S157.
Introduction
The cholinergic system in the brain can be classified into two categories: the interneuronal system, in which acetylcholine is provided via its own cholinergic interneurons; and the projection neuronal system, in which acetylcholine is provided through axons of cholinergic neurons whose somata are localized in other nuclei. Most brain regions, including the cerebral cortex, are in the latter category, whereas the former includes the striatum, the nucleus accumbens and the olfactory tubercle. These nuclei are richly fed with dopamine by the A8, A9, and A10 dopaminergic systems of Dahlström and Fuxe (1964).1 This remarkable abundance of acetylcholine and dopamine in the striatum strongly suggests that they play critical roles in the functioning of the basal ganglia.
The first hint that dopamine and acetylcholine compete and cooperate in the striatum was obtained in the 19th century when Jean Martin Charcot treated patients with Parkinson's disease (PD), which he himself named, with an anticholinergic hyoscine derived from Atropa belladonna. Parkinson's disease is a common neurodegenerative disorder that is caused primarily by the progressive loss of dopaminergic neurons of the substantia nigra pars compacta (SNc). Its clinical features include tremors at rest, bradykinesia, rigidity and postural instability. Until L-DOPA, a dopamine precursor that is transformed to dopamine in the brain, was developed and took the place of anticholinergics in the 20th century, the anticholinergics had been the only medical treatment for PD and are still in use. Despite the long history of the dopamine–acetylcholine balance hypothesis, we are just beginning to understand why and how dopamine depletion triggers a profound deterioration of basal ganglia circuit dynamics, such as overactivation of cholinergic system activity leading to motor and cognitive disturbances. In the present review, we describe recent discoveries related to physiological interactions between dopamine and acetylcholine in the striatum and what the imbalance between them causes in basal ganglia functioning.
Learning responses of striatal tonically active neurons and nigral dopamine neurons
Cholinergic interneurons as tonically active neurons
Extracellular unit recording techniques can discriminate between at least two cell types in the striatum, tonically active neurons (TAN) and phasically active neurons (PAN). PAN are usually very silent and spike phasically in relation to behavior, and are thought to contain projection neurons termed medium spiny (MS) neurons, though some of them might be GABAergic interneurons. In contrast, TAN always fire tonically and are thought to correspond to cholinergic interneurons. Indirect evidence that TAN are cholinergic interneurons (LA cells) was obtained in our previous study using primate striatum.2 First, TAN are preferentially located in the matrix, especially in the border regions between the matrix and striosomal compartments of the striatum, as are the cholinergic interneurons. Second, the density of TAN is very similar to that of cholinergic interneurons. Third, TAN are believed to be very large, because they can be detected at long distances (up to 500 µm) along an extracellular electrode track. These characteristics are reminiscent of those of the cholinergic interneurons termed “giant spiny neurons”. Fourth, TAN fire tonically in vivo with wide action potentials, just as cholinergic interneurons do in vivo, as well as in vitro.3 Finally, TAN are considered to be interneurons because they do not respond to antidromic stimulation of the globus pallidus.4
TAN spike tonically at 2–10 Hz and do not appear to fire in relation to body movements per se, but do respond to sensory stimuli that have been associated with reward.5 During acquisition of a sensorimotor association, TAN gradually develop typical conditioned responses (TAN pause response) consisting of an initial activation followed by a strong transient suppression of firing and a rebound activation (see Fig. 1).6 Histological reconstruction showed that the TAN that became responsive during learning were widely distributed throughout the striatum, suggesting that after conditioning the activity of TAN becomes synchronized to the conditioning stimulus in widespread portions of the striatum.7 A recent review by Apicella described TAN responses as reflecting stimulus detection, movement control and recognition of a specific behavioral context.8 TAN thus play significant roles in the selection of appropriate behavioral responses to environmental events.
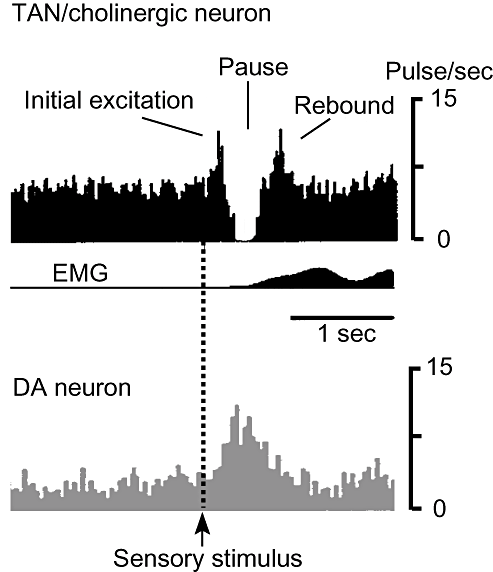
Schematic drawings of tonically active neurons (TAN) pause response and activity of dopamine neurons. Sensory stimulus reporting a reward or salient stimulus elicits a pause response in TAN (LA cells or cholinergic interneurons, upper) in the striatum and, simultaneously, burst discharges in dopamine neurons in the substantia nigra (lower). The pause response consists of an initial excitation, a pause and rebound excitation. Some TAN do not show the initial excitation phase. A pause in acetylcholine release amplifies the dopamine signal. The higher the frequency of dopaminergic cell firing, the more dopamine is released, whereas the lower the frequency of such firing, the less dopamine is released.
Learning responses of dopamine neurons
Interestingly, dopamine neurons also exhibit conditioned responses after sensorimotor learning. Schultz et al. showed that after learning to reach a small morsel of apple in a small box, dopamine neurons responded to the sight of primary food reward and to the conditioned stimulus associated with reward (door opening).9 These neurons then gradually lost responses to the primary reward and instead remained responsive to the conditioning stimulus. Their responses were, however, progressively reduced after overtraining resulted in automatic task performance. These findings have suggested that there might be a close relationship between TAN activity and dopamine cell firing during sensorimotor learning. This long-held hypothesis was experimentally confirmed by Morris et al. by paired recordings from a dopamine neuron in the substantia nigra and a TAN in behaving monkeys.10 One clear difference between them is that the conditioned response in dopaminergic neurons involves an increase in spike discharge, whereas a prominent pause is characteristic of the response to stimuli of TAN.
Loss of conditioned responses of TAN after dopamine depletion
After monkeys had been well trained to lick reward juice in response to a click sound, they were rendered hemi-parkinsonian by local injection of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), a selective neurotoxin of dopamine neurons, into the striatum.11 As expected, the proportion of responsive TAN was dramatically decreased in the depleted striatum, whereas the cells in the intact side remained responsive. When the dopamine receptor agonist apomorphine was systemically injected, the response returned in half of the non-responsive cells on the depleted side. Application of the dopamine receptor antagonist haloperidol to the intact side of the striatum made previously responsive cells unable to respond. These findings suggest that the behaviorally contingent activity of TAN in the striatum is strongly affected by nigrostriatal dopaminergic inputs. According to a recent review by Apicella,8 the characteristics the two types of neurons have in common are: (i) both respond to a reward stimulus, though they respond to a neutral stimulus if it is associated with a reward; (ii) the more unexpectedly the reward comes, the more strongly they respond; and (iii) even if the stimulus has no reward value, they respond to it if it is novel and strong. However, as it is given repetitively, they gradually adapt to it and lose responsiveness. Despite these similarities, they are different in other properties. Dopamine neurons do not appear to respond to aversive stimuli, though TAN sometimes do respond to them. The more strongly animals desire a reward, the more vigorously dopamine neurons fire. Therefore, if no reward is received, against expectations, they stop firing. In contrast, no correlation between expectancy and responsiveness is found for TAN. Furthermore, the activities of TAN depend on behavioral context, animal movement and location of stimuli, whereas those of dopamine neurons do not. How, then, do dopamine and acetylcholine interact with one another to produce dopamine-dependent TAN pause responses?
Cellular mechanisms of TAN pause response
A conditioned stimulus leads to transient suppression of the ongoing tonic activity of TAN, which is often (but not always) preceded by spike firing and followed by rebound activation. Cholinergic interneurons receive excitatory input from the frontal cortex and the centre median and parafascicular nuclei of the thalamus.12–14 Thalamic input shapes the pause response, which also requires activation of D2 and in part D1 class (probably D5) dopamine receptors.12,15–17 The cellular mechanisms underlying the pause response determined in vitro thus far can be summarized as follows.
Initial excitation phase
The question of whether the pause response of TAN results from a change in their own synaptic efficacy was tested with high-frequency stimulation (HFS) of the subcortical white matter and recording of postsynaptic potentials in the striatal cholinergic interneurons in rodent brain slice preparations.18 Postsynaptic potentials were composed of a small depolarizing excitatory postsynaptic potential (EPSP) and a subsequent hyperpolarizing component. The latter component was GABAergic, and was considered disynaptically-mediated, because the AMPA antagonist CNQX completely abolished not only the EPSP but also the GABA component. Tetanic stimulation of cortico- or thalamostriatal fibers evoked long-term potentiation (LTP) of monosynaptic EPSP and enhancement of disynaptic inhibitory postsynaptic potentials (IPSP) in the cholinergic interneurons. This induction of LTP was dependent on activation of D5 dopamine receptors and a postsynaptic rise in intracellular Ca2+ through voltage-sensitive Ca2+ channels and Ca2+-permeable AMPA receptors. Long-term enhancement of IPSP was for the most part presynaptically mediated, because the amplitude of each unitary IPSC was almost the same as before tetanus, whereas its frequency significantly increased after tetanus. LTP of monosynaptic EPSP thus facilitated spike generation at the time of cortico- or thalamostriatal synaptic input (initial excitation phase), whereas yet unidentified GABA neurons in the striatum increased firing probability briefly after tetanus to disynaptically inhibit the cholinergic interneurons. Although the duration of the TAN pause appeared to be much longer than that of the disynaptic IPSP, this dual synaptic plasticity might be of critical importance for the generation of the subsequent pause phase.
Pause phase
Using in vivo intracellular recording from rat striatum, Reynolds et al. showed that both subthreshold and suprathreshold depolarizations in cholinergic interneurons induced a prolonged afterhyperpolarization (AHP).19 The duration and amplitude of the AHP depended on the level of depolarization, so that enhanced EPSP as a result of LTP after tetanus prolonged the duration of AHP. Prolonged AHP occurred even in the absence of a preceding action potential, consistent with the TAN pause response observed in monkeys. They also found that paired HFS of substantia nigra and cortex induced potentiation of the cortically evoked EPSP and prolonged AHP duration, replicating in vivo the dopamine-dependent LTP in cholinergic interneurons.
What, then, is the ionic mechanism of AHP prolongation (pause)? Spontaneous tonic firing in cholinergic cells occurs in the absence of any synaptic input and is determined by the intrinsic membrane properties of these cells.20,21 Opening of tetrodotoxin (TTX)-sensitive fast Na+ channels generates the action potential that results in Ca2+ influx through voltage-dependent Ca2+ channels. Ca2+-dependent BK channels and voltage-dependent K+ channels (possibly A-type) work to produce repolarization, whereas another type of Ca2+-dependent SK channel produces the slow AHP. The AHP in turn activates hyperpolarization-activated cation current (Ih) that depolarize the membrane potential to a point at which the subthreshold Na+ current is activated and a spike is initiated. All of these channels contribute to the generation of the prolonged AHP (pause) response which is triggered by synaptic input in the presence of dopamine. During an action potential, D1-like dopamine receptor activation enhances L-type Ca2+ currents that augment Ca2+-activated K+ current and ultimately increase the duration of AHP.22–24 Furthermore, Wilson showed that two hyperpolarization-activated currents are responsible for the generation of the pause.25 Hyperpolarization produced by either inhibitory inputs or AHP that follow a single or multiple action potential is regeneratively amplified by inwardly rectifying K+ channels (Kir), thus forming a prolonged AHP (pause). The prolonged AHP is terminated by hyperpolarization-activated non-specific cation current (HCN or Ih). In addition to the Kir, prolongation of the AHP is achieved by modulation of voltage-dependent Na+ channels and Ih through activation of dopamine D2 receptors. Maurice et al. showed that voltage-dependent Na+ channels in cholinergic interneurons show greater depolarized fast-inactivation voltage dependence (8–10 mV) than most regular-spiking neurons. These unique properties of the Na+ currents yield the inward depolarization that brings the membrane potential to spike threshold and makes cholinergic cells act as slow pacemakers. Importantly, D2 dopamine receptor activation leads to a reduction in the Na+ currents, which is mediated by enhancement of channel entry into a slow-inactivated state through a Gβ signaling pathway that activates protein kinase C. This eventually reduces the rebound discharge. Deng et al. also showed that dopamine D2 receptor, but not D1-like receptor activation, blocks Ih through a protein kinase A-independent cyclic AMP pathway, thereby prolonging the pause.26
What, then, causes hyperpolarization to trigger the regenerative amplification of Kir? Injection of hyperpolarizing pulses to mimic IPSP did not induce prolongation of AHP,19,26 indicating that inhibitory inputs alone are not sufficient for induction of regenerative amplification. Dopamine D5 receptor activation alone also did not induce a prolonged AHP, and instead only a large-amplitude membrane depolarization of long duration was induced in vitro and in vivo.17,19 Stimulation of cortico- or thalamostriatal pathways invariably elicited disynaptic IPSP that shaped the evoked action potential and, together with D5 receptor activation, HFS of the pathway evoked LTP of EPSP as well as lasting enhancement of GABA release, which was followed by a prolonged AHP.18 Furthermore, D5 dopamine receptor activation is known to enhance a Zn2+-sensitive component of GABAergic currents in cholinergic interneurons.16 GABAergic inputs onto cholinergic interneurons are derived from fast-spiking (FS) GABAergic interneurons (unpubl.). Activation of cholinergic interneurons excites nearby GABAergic interneurons through nicotinic acetylcholine receptors (nAChR), which instantaneously returns GABAergic IPSP to cholinergic cells and MS cells.27,28 This type of neuron has been shown to be excited by dopamine D5 receptor activation and exhibit LTP and long-term depression (LTD) after HFS of glutamatergic corticostriatal pathways.29,30 It thus appears likely that both LTP of EPSP and the ensuing polysynaptic IPSP trigger generation of the pause response.
Rebound excitation phase
The pause is terminated by Ih and the Na+ currents, which have been blocked by dopamine D2 receptor activation. Rebound depolarization induces resumption of tonic spontaneous firing of action potentials that occur in the absence of synaptic inputs and do induce AHP but not prolonged ones, probably because the release of dopamine wanes during this phase.
In summary, dopamine strongly regulates the activity of cholinergic cells during conditioning. Through activation of D5 receptors, it induces LTP of EPSP, and enhancement of L-type calcium channel opening and polysynaptic GABAergic IPSP that activate Kir and Ca2+-dependent K+ conductances. Prolongation of the AHP is made possible by the activation of D2 receptors that block Ih and Na+ channel currents.
Physiological significance of TAN pause response
Cholinergic neurons spike tonically, even in the absence of synaptic inputs. This implies that acetylcholine is always released into the striatum as background activity. Dopamine neurons are also tonically active, releasing dopamine to some extent. Behaviorally relevant synaptic inputs induce a further increase in the frequency of the firing of dopamine neurons and a prolonged pause in cholinergic interneurons that are scattered throughout wide portions of the striatum. An increase in dopamine release and cessation of acetylcholine release thus occur simultaneously during the pause. Therefore, although it might appear paradoxical, it is the inactivation of nicotinic and muscarinic acetylcholine receptors that is of physiological significance in the pause response.
Dramatic increase in dopamine release by nicotinic acetylcholine receptor inactivation
It has long been known that nicotinic acetylcholine receptors (nAChR) are expressed on nerve terminals of dopamine neurons. Exogenous nicotinic agonists and endogenous cholinergic activity potently regulate dopamine release through nAChR containing the β2 subunit in the striatum.31 Agonists of nAChR activate instantaneously, but soon desensitize the receptor channels, so that nicotine bath application is almost equivalent in its effect on dopamine neurons to the application of antagonists of nAChRs. Unexpectedly, however, using fast-scan cyclic voltammetry, two groups independently found that if nAChR were desensitized by nicotine or blocked by the nAChR antagonist mecamylamine, dopamine release was more enhanced at higher stimulation frequencies (25 and 100 Hz, phasic firing) but more suppressed at lower frequencies (1, 5, 10, 20 Hz, tonic firing) in corticostriatal slices from guinea pig32 and mouse.33 These observations led Cragg to hypothesize that TAN pauses might powerfully enhance the contrast in dopamine signals offered by reward-related bursts of dopamine neurons, assuming that the TAN pause, that is, the cessation of ACh release, is equivalent to the inactivated state of nAChR.34 During the pause phase, an increase in firing frequency of dopamine neurons dramatically increases dopamine release, whereas depression of the firing of these neurons in response to error signals significantly decreases dopamine release. The number of TAN responsive to a conditioning stimulus increased from 17% to 50–70% after learning.6 This indicates that larger responsive areas and smaller unresponsive areas appear in the striatum after conditioning, and that in the responsive areas many TAN exhibit synchronized responses. In addition, TAN are preferentially located in the border regions between the striosome and matrix compartments. Because these two neurochemically different compartments differ in input–output connections and concomitant specialization of their neurotransmitters and neuromodulators, a role for TAN in inter-compartmental communication has been suggested.35 In short, the pause response provides not only a time window but also space for synaptic plasticity in the striatum.
Synaptic plasticity in striatal MS projection neurons and muscarinic receptor inactivation
Striatal MS projection neurons are largely divided into two subpopulations: neurons that target the substantia nigra pars reticulata (SNr) or the globus pallidus pars interna (Gpi; direct pathway), and neurons that project to the globus pallidus pars externa (GPe; indirect pathway). Both types of neurons release GABA, and the two types have similar physiological properties. However, they differ in many respects as well. Neurons in the direct pathway possess dopamine D1 receptors and release substance P and dynorphin, whereas those in the indirect pathway have D2 dopamine receptors and adenosine A2 receptors and release enkephalin. They also differ in muscarinic acetylcholine receptors (mAChR). Muscarinic receptors are G-protein-coupled and subdivided into two classes; M1-class (M1, M3, M5), which is coupled to Gq/11, and M2-class (M2, M4), which is mainly coupled to Gi/o proteins. Among them, virtually all MS cells possess M1 receptors, whereas M4 receptors are preferentially expressed on striatonigral (direct pathway) neurons.36–40 Several functions are ascribed to the mAChR in MS cells, ranging from suppression of several types of Ca2+ channels (L, N, and P/Q types by M1 and N and P/Q types by M4)2,36,41,42 and several types of K+ channels (KCNQ, A channel, SK channel, and inward rectifier K channel)43–46 to enhancement of N-methyl-d-aspartic acid (NMDA) currents.47 Regarding the roles of mAChR in the TAN pause response, Wang et al. provided evidence that inactivation of mAChR might eventually induce LTD of corticostriatal EPSP in the MS projection neurons.48 Dopamine release during the pause inhibits acetylcholine release from cholinergic cells through D2 receptor activation, which makes M1 receptors located on spines of MS cell dendrites inactive, resulting in disinhibition of L-type Ca2+ (Cav1.3) channels and subsequent Ca2+ inflow. Simultaneously, glutamate release from corticostriatal terminals activates metabotropic glutamate receptors 1 and 5, which also increase intracellular Ca.2+ Elevation of Ca2+ results in enhanced production of endocannabinoids which, acting at the CB1 receptors at corticostriatal synaptic terminals, inhibit glutamate release, thereby inducing LTD. The involvement of mAChR in the synaptic plasticity of MS cells was supported by a recent study by Pakhotin and Bracci, in which tonic firing of cholinergic interneurons was found to suppress corticostriatal EPSC in MS cells by 34% through mAChR activation, suggesting that, during the pause response, EPSC amplitude returns to a normal level.49
Synaptic plasticity of MS cells has long been studied, since Calabresi et al. first showed in 1992 that HFS of corticostriatal inputs and coactivation of dopamine D1 and D2 receptors induced LTD in vitro.50 Multiple forms of synaptic plasticity of MS cells have been reported.51 The finding that both D1 and D2 dopamine receptors are required for the induction of synaptic plasticity was inconsistent with the anatomical finding that each MS cell has mostly either D1 or D2 receptors. Recently, Kreitzer and Malenka found that HFS induced endocannabinoid-mediated LTD that required dopamine D2 receptor activation only in the indirect-pathway MS cells and none in the direct-pathway MS cells.52 Furthermore, Shen et al. added another form of synaptic plasticity, a Hebbian form of spike-timing-dependent plasticity (STDP), in which, when presynaptic activity precedes postsynaptic spiking (positive timing), LTP is induced in both direct- and indirect-pathway MS cells, whereas reversing this order induces LTD in the indirect-pathway MS cells but not in the direct-pathway MS cells.53 The finding of Wang et al. that M1 muscarinic receptors on MS cells and D2 receptors on cholinergic interneurons together enable induction by direct-pathway MS cells of endocannabinoid-mediated LTD might thus unravel the long mystery that both indirect- and direct-pathway MS cells often exhibit LTD, whereas each MS cell has mostly either D1 or D2 receptors.
Acetylcholine-dopamine imbalance in Parkinson's disease
Basic organizational principles: cortic–basal ganglia–thalamocortical loop circuits
We devote the remaining space to a discussion of how acetylcholine–dopamine imbalance causes motor deficits in PD. Before considering the details, we first consider the basic organizational principles of the cortico–basal ganglia–thalamocortical loop circuits.
The striatum is the input station of the basal ganglia, and is thought to be engaged in motor control, reinforcement learning and drug addiction. It receives glutamatergic excitatory corticostriatal inputs from pyramidal neurons mainly in layer V of the cerebral cortex (see Fig. 2a). Another excitatory input to the striatum arises from neurons in the thalamus. The striatum also receives dopaminergic fibers from the ventral tegmental area, substantia nigra and retrorubral area, among which dopaminergic neurons in the pars compacta of the substantia nigra (SNc) are the major source for the dorsal striatum and the very neurons that exhibit degeneration in PD. As previously stated, the projection neurons in the striatum are MS neurons that can be further classified into two subtypes, striatonigral “direct” pathway neurons and striatopallidal “indirect” pathway neurons. MS cells are inhibitory and utilize GABA as a neurotransmitter. In addition to GABA, MS cells in the direct pathway release neuropeptide transmitter substance P and dynorphin, whereas those in the indirect pathway release enkephalin. Dopamine receptors are classified into two categories, D1-like and D2-like receptors. D1-like receptors include D1 and D5 dopamine receptors, and are positively coupled to the cAMP cascade, whereas D2-like receptors consist of D2, D3 and D4 receptor subtypes, activation of which inhibits the cAMP cascade. Importantly, direct-pathway MS neurons express dopamine D1 receptors, whereas indirect-pathway neurons contain D2 receptors, although a small population of cells expresses both receptor subtypes.54 Direct-pathway MS neurons project directly to the output nuclei of the basal ganglia, such as the substantia nigra pars reticulata (SNr) and the internal segment of the globus pallidus (GPi; entopeduncular nucleus in rodents), whereas information carried by indirect-pathway MS neurons is conveyed to the external segment of the globus pallidus (GPe; globus pallidus in rodents) and the subthalamic nucleus (STN). Neurons in GPe, GPi and SNr are also GABAergic, whereas those in STN are glutamatergic and therefore excitatory. In this sense, STN is a unique nucleus in the basal ganglia. Like striatum, STN also directly receives glutamatergic inputs from the cerebral cortex. In contrast, STN sends information back to the GPe and forward to GPi and SNr (not shown in Fig. 2a). As a result, inhibitory inputs from direct-pathway MS neurons of the striatum and excitatory inputs from indirect-pathway STN neurons converge in the output structures of the basal ganglia.55 The combined outputs of GPi and SNr are sent to the thalamus, which in turn innervates the striatum and cerebral cortex, thus forming a cortico–basal ganglia–thalamocortical loop circuit. It has been suggested that there are at least five loop circuits which are organized in parallel and remain largely segregated anatomically and functionally from one another.56 The “motor” circuit begins with the primary motor, premotor and supplementary motor cortices. The “oculomotor” circuit starts from the frontal and supplementary eye fields. The two “prefrontal” circuits are separately derived from the dorsolateral prefrontal and lateral orbitofrontal cortices. The “limbic” circuit originates from the anterior cingulate area and medial orbitofrontal cortex. These loop circuits target separate but often contiguous nuclei in the basal ganglia and thalamus.
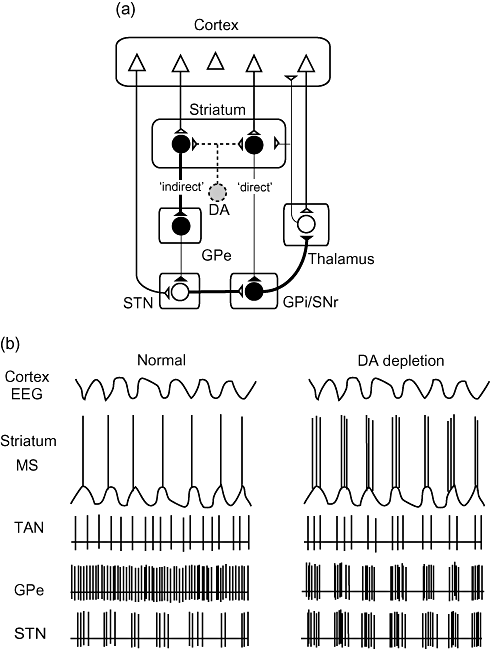
Simplified models of Parkinson's disease. (a) Classical rate model. Excitatory corticostriatal terminals synapse on dopamine (DA) D1 receptor-bearing medium spiny (MS) neurons in the striatum. These direct-pathway MS cells are inhibitory and innervate GABAergic cells in the globus pallidus pars interna (GPi)/substantia nigra pars reticulata (SNr). D2 receptor-bearing indirect-pathway MS cells also receive corticostriatal terminals, but innervate GABAergic cells in the globus pallidus pars externa (GPe) that in turn send axons to excitatory cells in the subthalamic nucleus (STN). STN makes excitatory synapses on cells in GPi/SNr. The balance between inhibitory inputs from the direct pathway and excitatory inputs from the indirect pathway determines the strength of outputs from GPi/SNr. Cells in GPi/SNr then inhibit thalamic cells that innervate the cerebral cortex. Loss of DA cells diminishes direct-pathway cell activity but enhances indirect-pathway activity, thereby inhibiting thalamocortical pathways. (b) Schematic drawings of activities of cerebral cortex and basal ganglia nuclei based on firing pattern model. In normal state, striatal MS cells show slow rhythmic oscillations (up and down states) that are originally generated by the cerebral cortex and produce action potentials during the up state. DA depletion leads to rhythmic oscillations in all basal ganglia nuclei. Activities of cortex and striatal MS cells are shown as electroencephalogram (EEG) and intracellular voltage traces, respectively. Tonically active neurons (TAN) in the striatum and cells in the other nuclei are shown as extracellular current traces.
Rate model
Based on the organizational principles of the loop circuits stated above, Alexander, Crutcher and DeLong proposed a model of movement disorders of basal ganglia origin (Fig. 2a).56 Inhibitory inputs from direct-pathway MS neurons of the striatum and excitatory inputs from indirect-pathway STN neurons converge in the output structures of the basal ganglia. It is now hypothesized that the direct pathway might convey cortical commands to execute desired movements, whereas the indirect pathway might monitor ongoing movements, and suppress unwanted and irrelevant cortical information. In hypokinetic disorders, such as PD, inactivation of inhibitory D2 dopamine receptors leads to excessive activation of indirect-pathway MS neurons that induces sequentially excessive inhibition of GPe GABAergic neurons, disinhibition of STN glutamatergic neurons, and then overactivation of GPi/SNr outputs. In contrast, inactivation of excitatory D1 dopamine receptors leads to reduced activity of the direct-pathway MS neurons, which send reduced inhibitory input to GPi/SNr. As a result, the net effect of dopamine depletion on the activities of GPi/SNr is overexcitation of inhibitory inputs to the thalamic output to the cerebral cortex, which causes motor dysfunction.
From mean rates to patterns of activity of basal ganglia nuclei: rhythmic oscillations in the STN-GPe network in PD
The rate model of movement disorders explained various neurological signs and symptoms well enough to be accepted in both clinical neurology and basic neuroscience. The next big leap in understanding of the pathophysiological mechanisms of PD was the discovery of rhythmic oscillations in the STN-GPe network (see Fig. 2b).57 In the classical rate model, the connections between GPe and STN were simplified, with GPe innervating STN unidirectionally with a constant mean rate of activity. However, anatomical studies using bidirectional transport of neuronal tracers showed that functionally related regions of excitatory STN and inhibitory GPe are reciprocally connected, an anatomical relationship that could support oscillatory activity and act as a pattern generator. In fact, the STN and GPe neurons themselves show spontaneous, rhythmic firing activity even when isolated in brain slices or organotypic co-cultures.58 Normally, the STN-GPe network exhibits complex and rarely correlated spatiotemporal patterns of firing. The low-frequency rhythmic oscillation seen in STN is dependent on and phase-locked to cortical oscillation. In PD, however, STN and GPe neurons exhibited more strongly correlated, synchronous and rhythmic patterns of activity. Dopamine depletion makes the burst-like discharges of STN more intense, and makes the firing of GPe also oscillatory and phase-locked to the cortical rhythm (Fig. 2b). Consistent with this, withdrawal of L-dopa treatment in PD patients uncovers rhythmic discharges in the tremor frequency range (4–10 Hz) and in α (8–13 Hz) and β ranges (14–30 Hz) in the cortex, STN and GPe.57,59 Blockade of low-frequency oscillations in the STN-GPe network by silencing the activity of STN by subthalamotomy or deep-brain stimulation has been proven to be very effective in normalizing movement.60
Hypercholinergic state and its outcome in PD
Loss of dopamine eliminates the TAN pause response and synaptic plasticity of MS neurons. Tonic irregular firing of TAN is altered to synchronous oscillation at around 16 Hz (3–19 Hz) after dopamine depletion (Fig. 2b).61,62 This cholinergic synchronous oscillation, which is a feature of the synchronized networks in the cortico–basal ganglia–thalamocortical loop circuits, results in periodic barrages of ACh release into the striatum. Although ACh, if released tonically and irregularly in normal conditions, is readily hydrolyzed to a certain extent by nearby acetylcholinesterase, synchronized and periodic surges in ACh release throughout the striatum might boost ACh concentration.
Classically, the cellular mechanism of elevation of ACh release in PD was believed to involve dopamine D2 receptors on cholinergic neurons acting as key regulators of ACh release. However, cholinergic neurons also contain D5 receptors, the activities of which are opposite those of D2 receptors and the overall firing frequency of cholinergic neurons was found not to significantly change after DA depletion.11,17,61 In contrast, using brain slices from M2 and M4 receptor single knockout mice, Zhang et al. found that autoinhibition of ACh release was predominantly mediated by M4 receptors in the striatum.63 Ding et al. further showed that this autoinhibition by M4 receptors was disrupted by upregulation of RGS4 (regulators of G protein signaling 4) proteins, the expression of which is negatively regulated by protein kinase A (PKA), which is usually upregulated by the activation of dopamine D5 receptors on cholinergic neurons.64 As a result, cholinergic cells continue releasing ACh without feedback inhibition by M4 receptors in PD.
It appears that elevated ACh release selectively affects the indirect-pathway MS cells. Differences between the indirect- and direct-pathway MS cells have recently been reported one after another by Kreitzer and Malenka,52 and Surmeier's lab.53,65–69 Indirect-pathway MS cells appear to be more excitable than the direct-pathway cells for the following reasons: (i) indirect-pathway synapses have a higher probability of transmitter release and a higher density of NMDA receptors than direct-pathway synapses;52 (ii) they have significantly smaller dendritic trees than do direct-pathway cells, so they are more responsive to intrasomatic current injection;66 (iii) they have more Kir2.3 subunits in the Kir2 K+ channels on dendritic spines and DA depletion increases the number of subunits further. M1 muscarinic receptor activation potently reduces Kir2 K+ channel opening, which enhances dendritic excitability in the indirect-pathway cells;65 (iv) Cav1.3 L-type Ca2+ channels are linked to glutamatergic synapses by the scaffolding protein Shank70 and are specifically inhibited by dopamine D2 receptor activation in the D2 dopamine receptor-bearing indirect-pathway neurons.69 Loss of dopamine, therefore, disinhibits Cav1.3 L-type Ca2+ channels on dendritic spines of the indirect-pathway cells; and (v) back-propagating action potentials invade more distal dendritic regions of indirect-pathway neurons than direct-pathway neurons.67 After DA depletion, this tendency is further enhanced in distal dendrites and spines in indirect-pathway neurons. All of these features lead to increased dendritic Ca2+ entry, which triggers an adaptive reduction in the density of dendritic spines in D2 receptor-bearing indirect-pathway MS neurons in PD.
Conclusion
The classical ACh-DA balance hypothesis based on the inhibitory effects of D2 dopamine receptors on ACh release from cholinergic interneurons has become more dynamic with the incorporation of recent physiological and anatomical breakthroughs. Normally, dopamine-dependent synaptic plasticity in striatal MS projection neurons is made possible by a dramatic increase in dopamine release triggered by a cessation of ACh release during the TAN pause response. The nigrostriatal dopamine depletion results in multifaceted alterations in functions of the cortico–basal ganglia–thalamocortical loop circuits. The synaptic plasticity of MS projection neurons is lost after dopaminergic denervation and synchronized, rhythmic, oscillatory activities in nuclei of the loop circuits prevent appropriate information transfer. Rhythmic firing of the cholinergic interneurons and breakdown of autoinhibition of ACh release by M4 muscarinic receptors results in the unregulated release of ACh, which, through multiple pathways enhances dendritic Ca2+ entry into dendritic spines of indirect-pathway MS neurons and prunes them through unknown mechanisms. Although many missing pieces remain in the puzzle of PD pathophysiology, understanding of the pathophysiology of PD has already laid the foundations for the development of better therapeutic strategies.
Acknowledgments
This work was supported by the Grant-In-Aid for Scientific Research of Japan.
Conflicts of interest
This study was financially supported by a grant from the Ministry of Health, Labour and Welfare in Japan (Medical Frontier Strategy Research-H13-Chihou/Kossetu-019). The authors confirm that there is no commercial interest of the authors in the findings presented in this study.




