Assessment of sampling approaches for a multi-taxa invertebrate survey in a South African savanna-mosaic ecosystem
Abstract
Invertebrate diversity is seldom included in conservation assessments, primarily because information is lacking. Broad surveys may be too costly, difficult or ineffective. Here we assess a ‘shopping basket’ approach, targeting 17 taxa using a range of methods. We sampled 43 one-hectare sites stratified within 560 km2 of heterogenous African savanna. We achieved up to 80% sampling completeness for epigaeic fauna, but generally much lower completeness (around 50%) for plant-dwelling and flying taxa. For the former we identified duplication of methods, and for the latter, addition of methods and increased temporal variation rather than effort would improve completeness. Within a taxon, sampling 75% of species present required, on average, about 784 individuals. When considering the local richness, 75% completeness required about 27 individuals per species, but these figures require validation in other areas. About 58 sites were required to achieve 75% sampling completeness, translating to about one site per 10 km2. The percentage of species sampled only in a particular month ranged between 4% and 46%, with greater temporal effects recorded for flying taxa than for epigaeic ones. The trend was similar for species unique to a particular year, with the most extreme case being 67% of the butterfly species sampled one year not previously recorded. We demonstrated and evaluated the feasibility of a simultaneous multi-taxon survey approach to produce data useful for conservation planning and monitoring. We strongly recommend a quantified approach for surveys and inventories, with details such as specific methods decided based on the biome sampled, and taxonomic expertise available for identification.
INTRODUCTION
Invertebrates are important components of biodiversity because of their high diversity and critical role in ecosystem functioning (Kellert 1993). In terrestrial ecosystems, invertebrate distribution patterns manifest at different scales to vertebrates or vascular plants (Ferrier et al. 1999), and they are also more sensitive, and react more rapidly, to environmental changes than plants or vertebrates (Kremen et al. 1993). In addition, large numbers of insect species have gone extinct or are highly likely to go extinct because of their narrow distribution or because of their relationship with other species that go extinct (Dunn 2005). Invertebrates should be included in biodiversity assessments for conservation planning (Kremen et al. 1993) and in biodiversity monitoring programmes (Kremen et al. 1993; Andersen et al. 2004; Rohr et al. 2007). However, for most invertebrate taxa a large proportion of species have not been discovered, and data on species distributions are far from complete (Ward & Larivière 2004). For effective conservation planning and monitoring, surveys are necessary to address such gaps (Rohr et al. 2007). Survey inventories are useful because they provide information on species distributions and areas of endemism (Kremen et al. 1993), and can be used to measure rarity and diversity (Ward & Larivière 2004). Further, inventories provide essential baseline data for monitoring (Rohr et al. 2007). However, surveys should allow comparison among areas, at different spatial and temporal scales, and provide some measure of inventory completeness if the data are to have a wide range of uses (Kremen et al. 1993).
Invertebrates are hyperdiverse and it is not feasible to include all taxa in a survey (Oliver & Beattie 1996; Ward & Larivière 2004). While surveys of a single taxon are useful, a single group may fail to serve as a biodiversity surrogate (see references in Ward & Larivière 2004). To overcome this problem the multi-taxa or ‘shopping basket’ approach can be used (Oliver & Beattie 1996; Kotze & Samways 1999; Slotow & Hamer 2000), in which a set of taxa meeting a number of criteria are selected for inclusion in a survey or monitoring programme. Oliver et al. (1999) list the following criteria: (i) functionally important in ecosystems; (ii) ubiquitous or with wide distributions on a continental scale; (iii) represented in any one locality by a substantial but not excessive number of species; (iv) identifiable at species level; (v) easy to collect and sample; and (vi) responsive to habitat variables at a convenient scale. A sixth criterion was offered by Kremen et al. (1993) suggesting that all taxa selected should be easily observed.
Once taxa have been selected, an appropriate, effective and efficient suite of methods is selected for survey. There are numerous sampling methods for the collection of invertebrates (New 1998; Southwood & Henderson 2000;), but protocols for multi-taxa surveys in different habitats are not standardized or thoroughly assessed, and these are likely to differ with habitat or biome and with the target taxa selected for survey. Most assessments of multi-taxa sampling strategies have been limited to forest ecosystems (e.g. Lowman et al. 1996; Oliver & Beattie 1996; Kotze & Samways 1999; Kitching et al. 2001; Rohr et al. 2007). One exception assessed quantitative methods for sampling Diplopoda, Chilopoda and Scorpionida in a South African savanna (Druce et al. 2004a), but assessment of broader invertebrate survey methods in savannas has been neglected.
Temporal variation is particularly relevant to invertebrates (e.g. Didham & Springate 2003), but its impact on invertebrate survey results is poorly understood. Some investigations of temporal changes in individual taxa in South African biomes have been published (e.g. Boonzaier et al. 2007), but only Druce et al. (2004a) and Hamer et al. (2006) have examined changes in savanna, and even then, these studies were limited to taxa with longer lived adults and they were not intensive.
While the invertebrate fauna of South Africa is considered to be relatively well known (McGeogh 2002), there are no published results of quantified, multi-taxa surveys of invertebrates at broad (regional) scales. Surveys that have resulted in inventories generally focus on one taxon in a particular area, for example those by Haddad et al. (2006) on the arachnids of Ndumu Game Reserve; by Whitmore et al. (2002) on the spiders of the Greater Makalali Conservancy in Limpopo; and by Davis et al. (1999) on dung beetles, or they may cover a limited number of taxa within a small area, such as that by Druce et al. (2004b), on three taxa in the Makalali Conservancy. In most cases either inventories lack explicit quantified methods (e.g. Dippenaar-Schoeman et al. 2005; Dippenaar-Schoeman 2006), which means that they do not provide information on relative abundance of species, and they do not allow comparisons of diversity between areas, or, in the case of many community studies, sampling is quantified, but specimens were not identified beyond morphospecies level (e.g. Kotze & Samways 2001; Foord et al. 2003; Witt & Samways 2004), which means that these studies do not contribute to understanding species distributions or endemism or the threat status of the species. Published studies that combine explicit quantified sampling methods and identifications of several taxa to species level are rare, and even rarer still are those covering a range of taxa, and this is the case not only in South Africa, but globally (Ward & Larivière 2004; Lewinsohn et al. 2005).
Savanna, the most widespread biome in Africa and South Africa, is not only economically important (Scholes 1997) but is also emerging as important for the conservation of a diverse invertebrate fauna (e.g. Whitmore et al. 2002; Druce et al. 2004b; Hamer et al. 2006). Savanna invertebrates are poorly known (but see references above and the publication edited by Coe et al. (1999) on the survey of Mkhomazi Game Reserve in Tanzania, and Marais and Kirk-Spriggs (2000) on the Namibian Brandberg inventory). Importantly, with the exception of Druce et al. (2004a), sampling methods for savanna invertebrates have not been assessed, and there is no information available on sampling effort required to inventory different invertebrate taxa. This type of information is critical for ensuring effectiveness and efficiency of invertebrate surveys. Data for conservation planning, and management and monitoring of ecosystem functioning in this important biome are largely unavailable, and future work does not have a foundation for effectiveness.
Here we evaluate sampling methods and effort required for savanna invertebrates within the context of an integrated survey for target taxa using the ‘shopping basket’ approach. Our specific objectives were to: (i) contrast species assemblages sampled by selected methods; (ii) determine sampling effectiveness for target taxa; (iii) assess inventory completeness; (iv) determine the minimum number of sites required for an inventory; and (v) undertake a preliminary assessment of temporal effects on surveying. Although our results derive from a savanna system, they are relevant and applicable to surveys within other biomes, and we make general recommendations based on our results.
METHODS
Study site
We conducted fieldwork in the Mkhuze Game Reserve (27.67°S, 32.27°E; 400 km2), Phinda Private Game Reserve (27.78°S; 32.35°E; 140 km2) and False Bay Park (27.94oS, 32.38°E; 25 km2) in north-eastern KwaZulu-Natal, South Africa (Fig. 1). The reserves are situated in the diverse Maputaland centre of endemism, which consists of a mosaic of mainly extensive savanna communities arranged in complex patterns (van Wyk 1996), and which falls within the Maputaland-Pondoland-Albany global hotspot (Steenkamp et al. 2004). We stratified sites across vegetation types and underlying geology to capture the local variation. The following vegetation types, classified according to Mucina and Rutherford (2006), were sampled: Lowveld Riverine Forest (two sites only), Makatini Clay Thicket, Maputaland Coastal Belt, Sandforest (five sites), Southern Lebombo Bushveld, Western Maputaland Clay Bushveld, Western Maputaland Sandy Bushveld and Zululand Lowveld. The majority of these sites (36) fell within vegetation classified broadly as savanna. Sampling sites were 1-ha plots of uniform vegetation type. We sampled 43 different sites between November 2002 and March 2005 (summer months), 23 of which were sampled once only, 12 were sampled twice, four three times and two each four and five times (Fig. 1). For those sites that were sampled repeatedly, some were sampled in different months during the summer season and/or across years, giving a total of 77 sampling events.
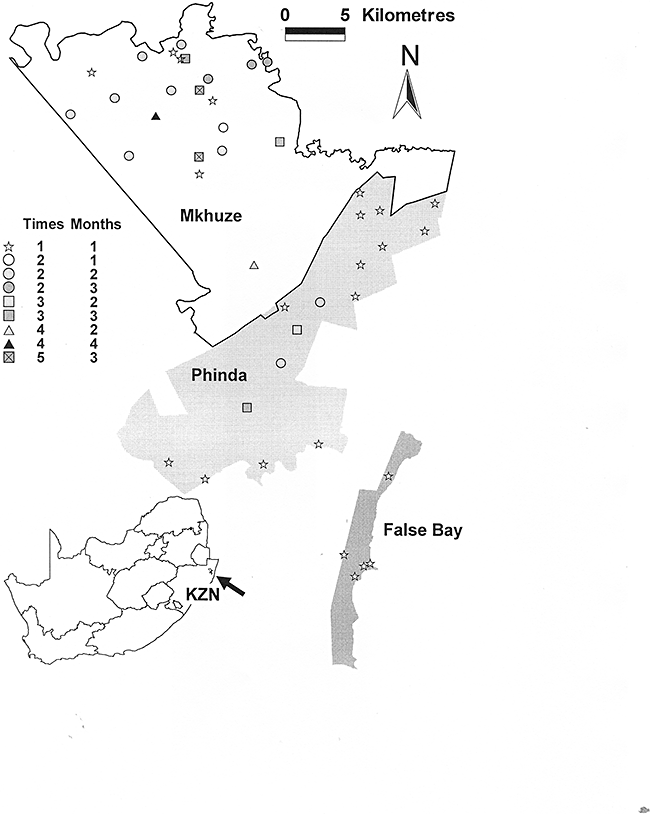
Location of the 1ha study sites within the three reserves in northern Kwa Zulu-Natal indicating the replicated survey site and the months in which surveys were undertaken.
Sampling methods
We selected 17 taxa that generally meet the criteria of Oliver and Beattie (1996) (Table 1). We included a range of functional groups, life histories, habitat specialists, body sizes and taxa with at least some conspicuous species. Selected taxa, with the exception of the Blattodea, could be identified to species level by taxon experts. We selected a range of sampling methods based on the following criteria: (i) must sample more than one focal taxon; (ii) must be relatively simple to implement, even by non-scientists; (iii) equipment required must be easily obtained and affordable; (iv) must be effective and feasible to sample eight or more sites within a 9 day sampling trip (based on our logistical constraints); (v) sorting and processing all samples collected must be possible within 6 months; (vi) must provide quantified data; and (vii) must have minimal impact on the local fauna. We suspected that some of the methods we selected were sampling the same fauna (are redundant), and this question formed part of the investigation. We sampled one site per day between 07.00 and 13.00 hours using a 10 to 14 person team.
| Order and common name of target group | Family | Functional group† | Activity group‡ | Life cycle§ | Expert and repository¶ |
|---|---|---|---|---|---|
| Lepidoptera (butterflies) | Pollinator | Flying | Complex | S. Lovell, UKZN | |
| Hymenoptera (bees) | Apoidea†† | Pollinator | Flying | Complex | C. Eardley, ARC |
| Orthoptera (grasshoppers, locusts) | Herbivore | Plant/Flying | Simple | A. Armstrong, UKZN | |
| Blattodea (cockroaches) | Detritivore | Epigaeic | Simple | S. Lovell, UKZN | |
| Odonata (dragonflies, damselflies) | Predator | Flying | Complex | T. Crouch, UKZN | |
| Hemiptera (leafhoppers) | Cicadelloidea†† | Sap-sucker | Plant | Simple | M. Stiller, ARC |
| Isoptera (termites) | Detritivore | Epigaeic | Complex | L. Uys, ARC | |
| Coleoptera (fruit chafers, dungbeetles) | Cetoniinae‡‡ | Scavenger/Pollinator | Flying | Complex | R. Perissinotto, UKZN |
| Scarabaeinae‡‡ | Scavenger/Detritivore | Epigaeic | Complex | A. Davis, UP | |
| Diptera (bee flies, robber flies) | Bombyliidae | Pollinator | Flying | Complex | D. Greathead, NERC |
| Asilidae | Predator | Flying | Complex | J. Londt, NM | |
| Neuroptera (lacewings, owl flies) | Predator | Flying | Complex | M. Mansell, ARC | |
| Araneae (lynx spiders, crab spiders, araneids) | Oxyopidae | Predator | Plant | Simple | A. Dippenaar-Schoeman, ARC |
| Thomisidae | Predator | Plant | Simple | ||
| Araneidae | Predator | Plant | Simple | ||
| Scorpionida (scorpions) | Predator | Epigaeic | Simple | L. Prendini, AMNH/NM | |
| Chilopoda (centipedes) | Predator | Epigaeic | Simple | M. Hamer, NM | |
| Diplopoda (millipedes) | Detritivore | Epigaeic | Simple | M. Hamer, NM | |
| Gastropoda (slugs, snails) | Herbivore/Predator | Epigaeic | Simple | D. Herbert, NM | |
| Annelida (earthworms) | Detritivore | Epigaeic | Simple | D. Plisko, NM |
- † The dominant function that taxa perform in an ecosystem.
- ‡ ‡ Where the majority of activity occurs.
- § Complex = metamorphic invertebrates; Simple = non-metamorphic.
- ¶ ¶ Expert who identified species; Repository: AMNH, American Museum of Natural History; ARC, Agricultural Research Council; NERC, National Environment Research Council Centre for Population Biology, UK; NM, Natal Museum; UKZN, University of KwaZulu-Natal; UP, University of Pretoria.
- †† †† Superfamily.
- ‡‡ ‡‡ Subfamily.
In order to minimize impact, for active sampling methods of millipedes, molluscs and butterflies, we retained representative samples with the balance released at the collection site after identification by taxon expert and recording. Reference specimens were frozen or placed in killing jars containing ethyl acetate, then preserved in 70% ethanol or pinned. We sorted to morphospecies, before sending specimens to taxon experts for species identification (as indicated in Table 1).
We identified Blattodea as morphospecies because no taxonomic expert was available. Because of time and capacity constraints, we identified three families of Araneae (Oxyopidae, Araneidae and Thomisidae) and two of Diptera (Asilidae and Bombyliidae). Reference collections are currently housed in the School of Biological and Conservation Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa, or in formal collections that focus on that particular taxon (see Table 1 for details).
Sampling methods were described in detail in Lovell et al. (2007), and are summarized here, with details relevant to sampling effort presented here.
We sampled epigaeic (ground-dwelling) invertebrates using three different active searching methods referred to here as plot, quadrat and random searches. Active searching targeted Scarabaeinae, Blattodea, Isoptera, Scorpionida, Diplopoda, Chilopoda, Mollusca and Annelida. Two 20 m × 20 m plots each searched for 1 h were sampled per 1-ha site. Quadrat sampling was more intensive than plots, with 2 m × 10 m, divided into five 2 m × 2 m blocks being thoroughly searched, and two of these quadrats being sampled at each site. Random searches involved 1 h of searching within the site, but without defined boundaries. This method was not used at all sites, but where it was carried out, we did two random searches.
To sample micro-molluscs, we collected 5 L leaf litter samples from each of the 20 m × 20 m plots and within each of the random searching areas. We initially collected four leaf litter samples per site, but this was later increased to eight to better determine species accumulation curves.
We sampled flying insects and plant-dwelling invertebrates (Lepidoptera, Apoidea, Diptera, Neuroptera, Odonata, Cicadellidae, Cetoniinae, Orthoptera and Araneae) using transect walks, colour pan traps, baited butterfly traps, sweep netting and tree beating. Transect walks involved sampling along a 50 m tape in a straight line with a 10 m strip (5 m on either side of the transect line) sampled by three people. We collected Orthoptera, Lepidoptera, Odonata, Neuroptera, Diptera and Apoidea, with each specimen placed in a bottle at point of capture for later recording. The distance along the transect at which the specimen was collected was recorded. We sampled two to four transects per site.
We set an equal number of blue- and yellow-coloured pan traps in two parallel lines for 24 h. Traps targeted Diptera, Apoidea and Cicadellidae. For 12 sampling events we set five of each colour pan (total 10), but we doubled this for 65 sampling events to 20 (10 yellow, 10 blue) pans in order to determine whether the additional effort improved the measurement of species density.
We set fruit-baited butterfly traps for 24 h at each site. We recorded Cetoniinae, Diptera, Lepidoptera and Apoidea.
We sampled low-level vegetation (grass, forbes, small shrubs) by sweep netting with two sets of 10 sweeps collected at each site. We targeted Araneae, Cicadellidae and Diptera.
We used tree beating to sample Cicadellidae, Blattodea, Araneae, Mollusca and Diplopoda. One tree was struck with a heavy stick 10 times and we sampled 20 trees at each site.
Data analysis
To assess species assemblages sampled by each method, we constructed Bray-Curtis similarity matrices (presence–absence) across sampling events for each sampling method, followed by analysis of similarity (anosim) and non-metric multi-dimensional scaling (MDS) plots using PRIMER version 5.2.9 (Clarke & Warwick 2001). From anosim, if R > 0.75 groups are well separated, if R > 0.5 groups are overlapping but clearly different, and if R < 0.25 groups are barely separable (Clarke & Gorley 2001). Redundant methods were identified as those where there was a complete overlap in the species assemblages sampled, as identified using anosim and visualized using MDS plots. To assess the effect of pan trap colour on the community sampled, we calculated the number of unique species sampled by each colour for each taxon.
Species density (Sd) is defined as being the number of species per specified collection area or unit (Magurran 2004), which we defined as the mean number of species sampled during one sampling event (1-ha site) using a defined sampling effort, calculated separately for each taxon. We compared these using Friedman tests (data were non-parametric). Where we obtained significant differences across methods (from Friedman tests), or where only two methods were compared, we used a Wilcoxon signed ranks test (with Bonferroni adjustment for multiple tests) to identify which specific methods were different.
When methods sampled different communities (determined by the anosim and MDS), we determined the extent to which each method sampled target taxa using species accumulation curves for each taxon at each sampling event. We used the Species–Area plot routine in PRIMER, in which the sample order was randomized using 999 permutations (Clarke & Warwick 2001). Where methods were not significantly different they were combined and we calculated a species density curve. We then calculated a mean species accumulation curve for each taxon using each method or combination of methods. We extrapolated the predicted mean species density at a sampling event, for each method, for each taxon using the Michaelis–Menten (MM) equation (using EstimateS 7.5), which is the most stable extrapolation procedure across all sample sizes (Palmer 1990; Colwell & Coddington 1994; Chazdon et al. 1998; Longino et al. 2002).
We determined effectiveness of the sampling methods at each sampling site by calculating the observed species density as a percentage of the estimated species density. We estimated efficiency of sampling methods by plotting the number of species sampled per unit effort using species accumulation curves for each taxon at each sampling event as described above. These were estimated for the minimum sampling effort, and separately where we had included additional sampling effort for a particular method at a particular site (see methods above). The slope of the curve indicated the rate at which species were added (efficiency) and the extent to which they sampled the fauna (effectiveness).
We calculated the number of species sampled per hour using each method where effort was constant across sites. We used the following time allocations: (i) two plot searches, two random searches and 20 tree beats took an estimated 2 h to complete each method; (ii) two 2 m × 10 m quadrat searches took an estimated 10 h; (iii) four 5 L leaf litter samples took an estimated half hour to sample; (iv) two 50 m transects took an estimated 1 h; (v) the set-up and sampling duration for 10 baited and 10 pan traps took approximately 24.5 h for each method (because we had to return to the same site the next day, after sampling a different site, which included time and travel costs); and (vi) two sweep samples took an estimated half hour. The time required for processing the samples was not considered.
We calculated the extent of completeness of sampling for individual taxa at sites (sampling events) as the mean observed species density as a percentage of the estimated species density. We did this separately for both the minimum and supplemented sampling efforts (calculated using the MM equation).
We defined species richness (S), the total number of species (Magurran 2004), as the total number of species sampled across all sampling events. We assessed completeness as the observed species richness as a percentage of the estimated species richness (from MM extrapolation of individual-based species accumulation curves for each taxon), and using only the minimum sampling effort. For comparative purposes, we also calculated the Chao 2 richness estimator, incidence-based coverage estimator and the second-order Jackknife species richness estimator using EstimateS.
To estimate the number of individuals required to achieve 70%, 75% and 80% sampling completeness for a survey at this spatial and temporal scale we plotted the percentage completeness against the number of individuals sampled for different taxa. The number of individuals sampled may influence the observed number of species for a particular taxon. We accounted for this potential confounding effect by dividing the number of individuals by the observed number of species, and plotting the percentage sampling completeness for each taxon, against that ratio.
We determined the minimum number of sampling events (sites) required to sample the target taxa by fitting the MM equation to the individual-based species accumulation curve, and solving to estimate the number of sites required to sample 75%, 80%, 90% and 95% of the total estimated species richness for each taxon. We presented the median and mean ± standard error for all taxa for each percentage completeness.
Sites that were sampled repeatedly in November, January and March were used to assess temporal variation across the summer season (n = 5 sites). We assessed annual variation using sites sampled in the same month across different years (n = 2 sites). We assessed temporal variation by calculating the number of species sampled in the month or year and the percentage of unique species. We used anosim to compare species assemblages sampled across months and years.
RESULTS
Survey results
During 77 days of sampling by 11 people (847 ‘man-days’) we collected 49 961 individuals from 716 species belonging to the focal taxa. These figures can be broken down as follows: 40 284 individuals from 212 species sampled by plots, quadrats, random and litter samples, 2218 individuals from 221 species sampled along transects, 2893 individuals from 86 species sampled by baited butterfly traps, 2321 individuals from 260 species sampled using colour pan traps, 762 individuals from 78 species sampled by sweep netting and 1483 individuals from 139 species sampled by tree beating (Table 2).
| Taxa | Subgroup | No. of species | No. of individuals | Average number of individuals sampled per species |
|---|---|---|---|---|
| Lepidoptera | 99 | 1 808 | 18 | |
| Apoidea | 51 | 1 027 | 20 | |
| Orthoptera | 46 | 1 331 | 29 | |
| Blattodea | 27 | 4 349 | 161 | |
| Odonata | 12 | 67 | 6 | |
| Cicadellidae | 87 | 1 148 | 13 | |
| Isoptera | 8 | 322 | 40 | |
| Cetoniinae | 31 | 1 940 | 63 | |
| Scarabaeinae | 43 | 314 | 7 | |
| Diptera | Bombyliidae | 21 | 133 | 6 |
| Asilidae | 46 | 248 | ||
| Neuroptera | 26 | 149 | 6 | |
| Araneae | Oxyopidae | 33 | 714 | 14 |
| Thomisidae | 49 | 751 | ||
| Araneidae | 31 | 130 | ||
| Scorpionida | 6 | 140 | 23 | |
| Chilopoda | 17 | 568 | 33 | |
| Diplopoda | 26 | 14 519 | 558 | |
| Annelida | 7 | 261 | 37 | |
| Mollusca | 50 | 20 042 | 400 | |
| All invertebrates | 716 | 49 961 | 70 |
Assessment of assemblages sampled by different methods
As expected there was a clear separation of epigaeic and flying/plant-dwelling assemblages sampled across all methods (anosim: R = 0.71, P < 0.01) (Fig. 2a). The methods for sampling flying and plant-dwelling taxa largely complemented each other, with distinct assemblages being sampled by different methods (Fig. 2b). For epigaeic taxa, plots, quadrats and random methods sampled the same assemblages (anosim: plot vs. quadrat: R = 0.105, P = 0.002; quadrat vs. random: R = 0.122, P = 1.003; plot vs. random sampling: R = −0.043, P = 0.982) (Fig. 2c), indicating duplication rather than complementarity in methods. There was no significant difference in the Cicadellidae (anosim: R = 0.011, P = 0.231), Apoidea (R = 0.103, P = 0.001) or Diptera (R = 0.089, P = 0.023) species assemblages sampled by blue versus yellow pan traps, indicating duplication. However, blue pan traps sampled 21 unique species not sampled by yellow pan traps (Taxon (N Species)): Apoidea (11), Diptera (4), Cicadellidae (6), while yellow pan traps sampled 75 unique species (Apoidea (8), Diptera (18), Cicadellidae (49)).
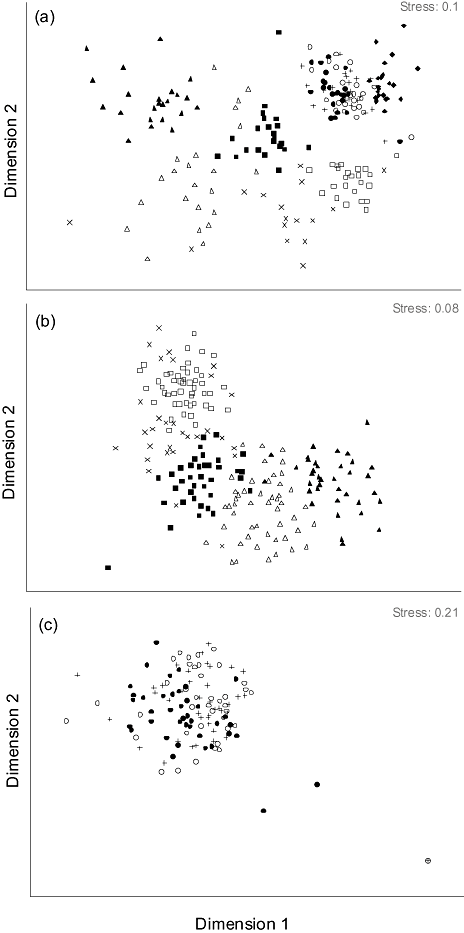
Assessment of invertebrate species assemblages sampled across all sampling events using the following methods: baited traps (▴), transect (Δ), pan traps ( ), sweep (×), tree beat (□), quadrat (●), plot (○), random (+) and leaf litter (◆). A two-dimensional multi-dimensional scaling plot based on Bray-Curtis similarity matrix from presence–absence species data across sites. Multi-dimensional scaling plot for (a) all invertebrates sampled using all methods, (b) invertebrates sampled using baited traps, transects, pan traps, sweeps and tree beats and (c) plots, quadrats and random sampling.
), sweep (×), tree beat (□), quadrat (●), plot (○), random (+) and leaf litter (◆). A two-dimensional multi-dimensional scaling plot based on Bray-Curtis similarity matrix from presence–absence species data across sites. Multi-dimensional scaling plot for (a) all invertebrates sampled using all methods, (b) invertebrates sampled using baited traps, transects, pan traps, sweeps and tree beats and (c) plots, quadrats and random sampling.
Effectiveness and efficiency of different sampling methods
In terms of effectiveness, total species density per site sampled across all taxa was the highest using quadrats (Sd = 19.6), followed by transects (Sd = 14.4). Combining plots and quadrats increased the species density sampled to 24.8 species (Table 3). Quadrats were the most effective method (using % success of a particular method for each taxon) for Blattodea, Diplopoda and Mollusca; leaf litter was also effective for Mollusca. Baited traps were effective for Lepidoptera and Cetoniinae, colour pan traps for Apoidea, and transects for Orthoptera and Neuroptera, but not for Odonata. None of the methods used was particularly effective for Diptera or Araneae. Doubling the sampling effort increased the Sd from transects by 32%, and from colour pan traps by 60%. There was no significant difference between Sd sampled by blue versus yellow pan traps for Apoidea (Wilcoxon signed rank test: Z = −0.593, P = 0.59), but yellow pan traps sampled significantly more Cicadellidae (Z = −3.998, P < 0.001) and Diptera (Z = −4.156, P < 0.001).
| Plant and flying taxa | Transect(50 × 10 m) | Baited traps(10) | Pan traps(blue, yellow) | Sweep netting(2 sets) | Tree beating(20 trees) | ||
|---|---|---|---|---|---|---|---|
| 2 replicates† | 4 replicates | 5 traps | 10 traps | ||||
| Lepidoptera | 3.8 ± 0.39 (40%) | 4.4 ± 0.64 (32%) | 4.2 ± 0.4 (62%) | ||||
| Apoidea | 1.2 ± 0.19 (5%)* | 1.2 ± 0.18 (12%) | 0.7 ± 0.12 (24%)* | 4.2 ± 0.29 (44%)* | 6.3 ± 0.64 (54%) | ||
| Orthoptera | 5.2 ± 0.48 (57%) | 7.9 ± 0.73 (75%) | |||||
| Odonata | 1.1 ± 0.1 (38%) | 1.1 ± 0.0 (38%) | |||||
| Cicadellidae | 3.7 ± 0.38 (38%)* | 6.6 ± 0.86 (44%) | 0.6 ± 0.11* | 0.4 ± 0.1* | |||
| Cetoniinae | 3.4 ± 0.35 (66%) | ||||||
| Diptera | 1.8 ± 0.14 (40%)* | 2.7 ± 0.27 (25%) | 0.1 ± 0.05* | 1.3 ± 0.16 (37%*) | 2.4 ± 0.2 (34%)* | ||
| Neuroptera | 1.3 ± 0.14 (40%) | 1.7 ± 0.33 (56%) | 0.3 ± 0.14 | ||||
| Araneae | 1.2 ± 0.14 | 7.0 ± 0.4 (38%) | |||||
| Total | 14.4 | 19 | 8.7 | 9.2 | 15.3 | 1.8 | 8.0 |
| Epigaeic taxa | Plot(2 × 20 × 20 m/1 h) | Quadrat(2 × 10 × 2 m) | Quadrat + plot | Random(2 × 1 h) | Leaf litter (5 L) | ||
|---|---|---|---|---|---|---|---|
| 4 reps | 8 reps | ||||||
| Blattodea | 2.0 ± 0.23 (43%)* | 4.3 ± 0.36 (59%)* | 4.9 ± 0.38 (65%) | 1.7 ± 0.25* | |||
| Scarabaeinae | 0.9 ± 0.19 | 2.1 ± 0.27 (26%) | 2.1 ± 0.42 (20%) | 1.7 ± 0.17 | |||
| Scorpionida | 0.5 ± 0.13* | 1.3 ± 0.12* | 1.5 ± 0.14 | 0.7 ± 0.1* | |||
| Chilopoda | 0.9 ± 0.16 (32%)* | 1.8 ± 0.15 (32%)* | 2.3 ± 0.18 (40%) | 0.6 ± 0.13* | |||
| Diplopoda | 2.8 ± 0.28 (69%) | 3.7 ± 0.34 (75%) | 4.3 ± 0.59 (79%) | 2.9 ± 0.31 | |||
| Mollusca | 5.9 ± 0.44 (47%) | 5.1 ± 0.39 (41%) | 7.0 ± 0.43 (56%) | 6.1 ± 0.39 (49%) | 7.2 ± 0.46 (58%) | 8.9 ± 1.43 (69%) | |
| Annelida | 0.7 ± 0.21 | 1.5 ± 0.22 (93%) | 1.5 ± 0.22 (93%) | 0.3 ± 0.21 | |||
| Total | 14.5 | 19.6 | 24.8 | 13.9 | 7.2 | 8.9 | |
- * Data are mean species density per site (observed mean species density as a percentage of the estimated species density calculated using the Michaelis–Menten equation). No % sampled was calculated for low values. Methods with significantly different species densities (P < 0.05).
- † † For some methods we increased the sampling effort from the minimum at all sites to additional sampling at some sites. Because sites used for additional sampling were different from those for minimum sampling, values can be lower with additional sampling.
In terms of efficiency, plots sampled the highest number of species per hour (7.17 S h−1) for all taxa combined, followed by transects (6.74 S h−1), sweep netting (5.77 S h−1), tree beating (4.42 S h−1), leaf litter collection (3.57 S h−1), quadrats (2.05 S h−1), pan traps (0.49 S h−1) and baited traps (0.22 S h−1). Methods that added the most species per unit effort (had the steepest curve) were transects for Orthoptera (Fig. 3a), and the following for Mollusca: leaf litter samples (Fig. 3e), quadrats (Fig. 3f) and random searching (Fig. 3h). Baited traps were also relatively effective for Lepidoptera and Cetoniinae (Fig. 3e).
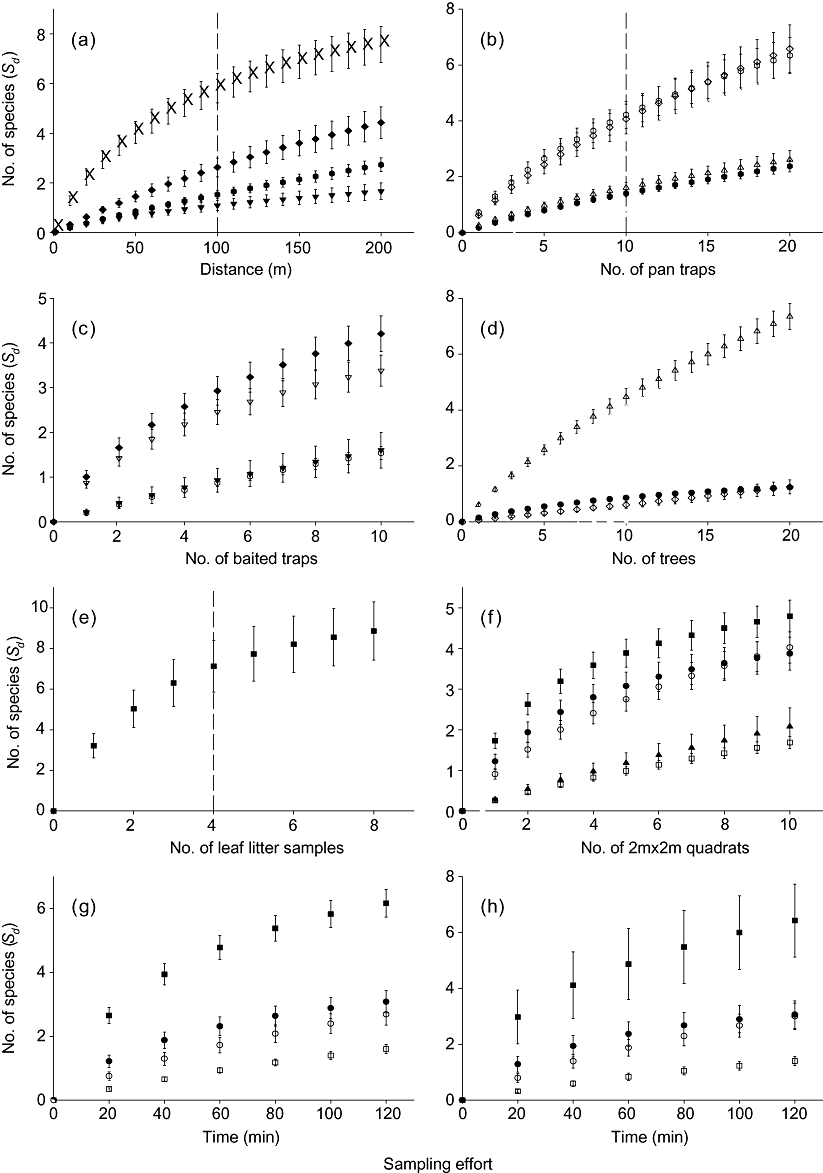
Effectiveness and efficiency of methods for different taxa as shown by species accumulation curves with increasing sampling effort on species density for different sampling methods for each taxon. (a) Transects, (b) pan traps, (c) baited traps, (d) tree beats, (e) leaf litter, (f) quadrats, (g) plots and (h) random sampling for (◆) Lepidoptera, (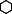 ) Apoidea, (×) Orthoptera, (○) Blattodea, (◊) Cicadellidae, (▿) Cetoniinae, (▴) Scarabaeinae, (
) Apoidea, (×) Orthoptera, (○) Blattodea, (◊) Cicadellidae, (▿) Cetoniinae, (▴) Scarabaeinae, ( ) Diptera, (▾) Neuroptera, (▵) Araneae, (□) Chilopoda, (●) Diplopoda and (
) Diptera, (▾) Neuroptera, (▵) Araneae, (□) Chilopoda, (●) Diplopoda and ( ) Mollusca. Species accumulation curves were produced by randomizing sampling order. Error bars represent standard error; horizontal dashed lines across the graphs represent the minimum sampling effort per site. NB: Insufficient numbers of species were recorded to generate meaningful curves for Odonata, Isoptera, Scorpionida and Annelida for each method.
) Mollusca. Species accumulation curves were produced by randomizing sampling order. Error bars represent standard error; horizontal dashed lines across the graphs represent the minimum sampling effort per site. NB: Insufficient numbers of species were recorded to generate meaningful curves for Odonata, Isoptera, Scorpionida and Annelida for each method.
The epigaeic taxa were generally more completely surveyed at a site (over 70% of species sampled) than the flying or plant-dwelling taxa (less than 50% sampled) (Table 3). The Orthoptera and Cetoniinae (flying) were more effectively sampled, with over 50% of the estimated species at a site collected, and Chilopoda and Scorpionida were less effectively sampled than other epigaeic taxa, with less than 50% collected.
Inventory completeness
We assessed inventory completeness by contrasting the observed species richness (from the minimum sampling effort across all sampling events) with the estimated species richness using different estimators (Table 4). Diptera, Scarabaeinae, Cicadellidae and Neuroptera were poorly sampled, with the minimum sampling effort capturing around half of the estimated total species richness. These groups also showed a wide range of estimates of species richness using the different estimators. Cetoniinae, Scorpionida, Blattodea, Diplopoda, Chilopoda and Mollusca and were sampled well, with more than 80% of the estimated species richness recorded.
| Taxon | Observed mean species density | Estimated mean species density using MM† | ||||
|---|---|---|---|---|---|---|
| Minimum‡ | Maximum | Minimum | % sampled§ | Maximum | % sampled | |
| Lepidoptera | 6.9 | 7.9 | 15.1 | 46 | 18.5 | 43 |
| Apoidea | 4.8 | 7.1 | 12.6 | 38 | 13.7 | 52 |
| Orthoptera | 5.2 | 7.9 | 9.1 | 57 | 10.5 | 75 |
| Blattodea | 5.6 | 4.7 | 8.9 | 63 | 7.4 | 64 |
| Odonata | 1.1 | 1.0 | 2.9 | 38 | 2.6 | 38 |
| Cicadellidae | 3.8 | 7.2 | 11 | 35 | 17.9 | 40 |
| Isoptera | 1.2 | 2.6 | 4.5 | 27 | 6.1 | 43 |
| Cetoniinae | 3.4 | – | 5.1 | 67 | – | – |
| Scarabaeinae | 2.1 | 2.5 | 7.6 | 28 | 12.9 | 19 |
| Diptera | 2.4 | 3.8 | 8.4 | 29 | 14.8 | 26 |
| Neuroptera | 1.7 | 1.9 | 6.7 | 25 | 6.2 | 31 |
| Araneae | 9.1 | 10.4 | 22.5 | 40 | 23.3 | 45 |
| Scorpionida | 1.4 | 1.7 | 4.9 | 29 | 5.4 | 31 |
| Chilopoda | 2.4 | 2.3 | 5.6 | 43 | 5.8 | 40 |
| Diplopoda | 4.8 | 5.0 | 6.2 | 77 | 6.2 | 81 |
| Mollusca | 8.9 | 10.9 | 12.5 | 71 | 13 | 84 |
| Annelida | 1.3 | 1.4 | 1.6 | 81 | 1.8 | 78 |
- † Estimated from Michaelis–Menten equation.
- ‡ Minimum = lowest sampling effort; Maximum = additional replicates included (see Table 3).
- § § Percentage sampled of the estimated species density. Data are from different sets of sites so estimates using maximum can be less than minimum in some cases.
We estimated the number of individuals required for a specific percentage of sampling completeness across all taxa by solving the logarithmic regression (not considering how species rich a taxon may be in an area). Sampling 80% of species present required 1704 individuals, 75% required 784 individuals and 70% completeness required 361 individuals. We incorporated the local richness by calculating a value per species (irrespective of taxon), and then for 70% completeness we required 15.4 individuals per species; 75% required 26.9 individuals per species and 80% required 47 individuals per species.
For the 17 taxa surveyed, the estimated number of sampling events to sample 75% of the estimated species richness varied from 17 to 204 events (median = 58) (Table 5). Attempting to survey 95% of the species substantially increased the number of sites required for those taxa, which were poorly sampled, with over 1000 sites being required for Odonata, Scarabaeinae, Diptera and Neuroptera (assuming our sampling methods and intensity). The median value for 95% completeness was 361 sites.
| Taxon | Observed richness | Estimated species richness† | Estimated N sites to reach completeness | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MM | %‡ | Jack | % | ICE | % | Chao | % | 75%§ | 80% | 90% | 95% | ||
| Lepidoptera | 76 | 96 | 79 | 114 | 66 | 103 | 74 | 106 | 72 | 58 | 77 | 172 | 361 |
| Apoidea | 41 | 48 | 85 | 56 | 73 | 51 | 81 | 48 | 86 | 39 | 51 | 116 | 248 |
| Orthoptera | 44 | 53 | 83 | 57 | 77 | 52 | 85 | 50 | 89 | 52 | 69 | 154 | 329 |
| Blattodea | 26 | 28 | 93 | 30 | 87 | 28 | 92 | 27 | 96 | 17 | 24 | 50 | 104 |
| Odonata | 10 | 18 | 56 | 14 | 71 | 16 | 62 | 11 | 89 | 189 | 253 | 578 | 1254 |
| Cicadellidae | 69 | 121 | 57 | 133 | 52 | 117 | 59 | 135 | 51 | 155 | 207 | 465 | 974 |
| Isoptera | 8 | 12 | 67 | 11 | 73 | 9 | 91 | 9 | 94 | 111 | 148 | 328 | 683 |
| Cetoniinae | 29 | 35 | 83 | 43 | 68 | 39 | 74 | 36 | 80 | 48 | 64 | 145 | 308 |
| Scarabaeinae | 27 | 48 | 56 | 55 | 49 | 54 | 50 | 57 | 48 | 162 | 217 | 488 | 1033 |
| Diptera | 51 | 93 | 55 | 100 | 51 | 97 | 53 | 95 | 53 | 170 | 227 | 511 | 1084 |
| Neuroptera | 23 | 46 | 50 | 50 | 46 | 61 | 38 | 49 | 47 | 204 | 272 | 612 | 1294 |
| Araneae | 97 | 126 | 77 | 155 | 63 | 144 | 67 | 138 | 70 | 67 | 90 | 202 | 428 |
| Scorpionida | 6 | 7 | 86 | 5 | 120 | 6 | 100 | 6 | 100 | 25 | 34 | 77 | 166 |
| Chilopoda | 17 | 18 | 94 | 22 | 78 | 19 | 91 | 19 | 92 | 23 | 31 | 70 | 146 |
| Diplopoda | 25 | 27 | 93 | 26 | 96 | 26 | 96 | 25 | 100 | 17 | 23 | 51 | 109 |
| Mollusca | 48 | 51 | 94 | 55 | 87 | 51 | 95 | 51 | 95 | 20 | 27 | 61 | 130 |
| Annelida | 7 | 9 | 78 | 11 | 55 | 10 | 59 | 8 | 80 | 111 | 148 | 326 | 662 |
| All taxa | 604 | 836 | 937 | 883 | 870 | 58 | 77 | 172 | 361 | ||||
- † MM, Michaelis–Menten equation; Jack, second-order Jackknife; ICE, incidence-based coverage estimator; Chao, Chao 2. All taxa = sum.
- ‡ ‡ Percentage observed of the estimated total species richness calculated using the minimum sampling effort.
- § Estimated number of sites required to sample 75%, 80%, 90% and 95% of the estimated species richness, calculated using individual-based species accumulation curves in the MM equation. All taxa = median.
Temporal effects on survey data
There were no significant differences among the species assemblages over the temporal periods (anosim: individual contrasts not presented for brevity). The mean species density and the mean number of unique species across all taxa were the highest in March and in the third sampling year, although there was some variation in the trend for different taxa (Table 6). Note that these results are based on contrasts across relatively few sites, and should be interpreted with caution.
| Taxon | January | March | November | Combined months | Year 1 | Year 2 | Year 3 | Combined years | |
|---|---|---|---|---|---|---|---|---|---|
| Lepidoptera | S d | 28 | 37 | 12 | 50 | 3 | 1 | 10 | 12 |
| Unique (%) | 18 | 38 | 6 | – | 8 | 8 | 67 | – | |
| Apoidea | S d | 16 | 14 | 12 | 24 | 1 | 4 | 10 | 13 |
| Unique (%)† | 17 | 17 | 4 | – | 7.7 | 15 | 62 | – | |
| Orthoptera | S d | 14 | 18 | 7 | 22 | 1 | 4 | 10 | 12 |
| Unique (%) | 14 | 32 | 5 | – | 8 | 17 | 47 | – | |
| Blattodea | S d | 11 | 14 | 18 | 16 | 11 | 7 | 12 | 16 |
| Unique (%) | 6 | 19 | 6 | – | 25 | 0 | 25 | – | |
| Cetoniinae | S d | 10 | 8 | 11 | 16 | 6 | 3 | 9 | 13 |
| Unique (%) | 13 | 6 | 25 | – | 23 | 0 | 46 | – | |
| Scarabaeinae | S d | 5 | 3 | 6 | 11 | 3 | 1 | 2 | 4 |
| Unique (%) | 27 | 9 | 46 | – | 50 | 0 | 25 | – | |
| Araneae | S d | 20 | 23 | 23 | 43 | 11 | 11 | 19 | 27 |
| Unique (%) | 16 | 26 | 21 | – | 19 | 11 | 37 | – | |
| Chilopoda | S d | 5 | 11 | 6 | 11 | 2 | 2 | 5 | 7 |
| Unique (%) | 0 | 6 | 0 | – | 14 | 14 | 43 | – | |
| Diplopoda | S d | 13 | 9 | 11 | 16 | 14 | 17 | 14 | 19 |
| Unique (%) | 3 | 0 | 19 | – | 0 | 11 | 0 | – | |
| Mollusca | S d | 22 | 19 | 19 | 25 | 23 | 23 | 29 | 32 |
| Unique (%) | 12 | 8 | 4 | – | 3 | 60 | 16 | – |
- † N = 5 sites sampled repeatedly across months and N = 2 sites in the same month across years. The number and percentage of unique species sampled in that period. Sd, species density.
DISCUSSION
Survey data are essential for conservation planning and management, for establishing a baseline for monitoring environmental change, for selecting surrogates for monitoring, for developing the methods for a monitoring programme (Rohr et al. 2007), as well as being useful for taxonomic, phylogenetic and biogeographic studies. In order for surveys to serve all of these purposes, sampling methods must be quantified (Rohr et al. 2007), which is a problem with much of the material collected by taxonomists. Specimens should be identified by taxon experts to species level, which is not always the case with quantified data produced in ecological or community characterization studies (Longino & Colwell 1997), and ideally, the survey should cover more than one taxon (Rohr et al. 2007). The amount of time and effort required to carry out an invertebrate survey that addresses even most of these requirements are considerable. The 847 ‘man-days’ spent on carrying out the field survey excluded the time required in the laboratory to sort mixed samples, and to label and capture almost 50 000 specimens into a database before they were sent to experts for identification. Our experience, as Rohr et al. (2007) suggested, was that this post-sampling time was higher than the field sampling period. However, if biodiversity is to be effectively conserved, it needs to be measured and monitored (Buckland et al. 2005), and as invertebrates are such a major component of biodiversity, they must be included in biodiversity conservation activities. As shown by this survey, the difficulties of carrying out multi-taxa surveys are not insurmountable. Even though for most of the taxa surveyed not all species were recorded, and the number of taxa covered was limited, we have provided an enormous amount of quantified data, including a list of species, their distribution within the survey area, the relative abundance of different species, and habitat and other ecological associations. This dataset can be used for a wide variety of analyses and applications. In addition, a critical assessment of the approach used for the survey is valuable for similar studies that may be carried out in the future.
A large constraint on multi-taxa surveys is the logistical cost associated with field work and expert time. While taxon experts may believe that developing an inventory is only achievable by personally carrying out the sampling using the most appropriate, often taxon-specific methods, this does increase costs and time required if several taxa are to be surveyed separately. In addition, taxon experts or museum collectors are usually focused on obtaining material and perhaps a species list for an area, rather than quantifying methods or counting individuals to estimate abundance. Having collectors with different objectives can make it difficult to ensure consistency in the methods used and the sites selected, thus limiting the use of data for comparative purposes. We have demonstrated that it is possible to effectively and efficiently sample a range of taxa that represent a wide variety of functional groups, body sizes and taxonomic diversity. Further, we were able to implement a range of methods that provided effort-based sampling with associated ecological data.
We managed to achieve a relatively high sampling completeness for epigaeic fauna. This is despite not using traditional methods such as pitfall trapping (Neville & Yen 2007). Studies that have critically assessed methods for epigaeic invertebrates found pitfalls to be less effective than active sampling for diplopods (Mesibov et al. 1995; Druce et al. 2004a; Snyder et al. 2006) and chilopods (Druce et al. 2004a). Pitfalls are also unlikely to be effective for sampling slow-moving taxa such as annelids and molluscs. Further, some taxa (e.g. Annelida) require careful preservation to allow identification. Active searching allows preliminary sorting in the field, which means that the impact on the fauna is reduced as a large number of duplicate specimens can be released once they have been recorded, and there is no by-catch inadvertently killed (New 1999). This is especially important for relatively long-lived taxa of conservation importance such as Scorpionida (Polis 1990) or Diplopoda (Hamer & Slotow 2002). Winkler sorting of litter samples is another method commonly used for collecting small epigaeic taxa, but again it is not particularly effective or efficient for the target taxa of our study (Krell et al. 2005).
Our results for epigaeic fauna indicated duplication of sampling methods, and we recommend selection of one of the approaches. While quadrat searching is more time-consuming, it did provide more species than random or plot searches. This demonstrated that constraining the area that is searched does not reduce the number of species collected, and is actually more effective than limiting time to search a large area.
Flying and plant-dwelling invertebrates were less successfully sampled than the epigaeic fauna. This may be related to the sampling methods used, but there may also be other explanations, such as their greater mobility within a limited sampling area, and the greater structural complexity of the habitats used by flying and plant-dwelling taxa. We highlight that there are a wide range of alternative methods (e.g. Malaise traps, suction methods (New 1998)) that could be added to the sampling methods in order to improve completeness. However, we believe that, unlike for epigaeic taxa, high completeness for flying and plant-dwelling taxa will always require a range of methods, and as such may not be achievable in broad surveys. For these a combination of both active and passive methods may be appropriate. Note that while the overall assemblages captured by blue and yellow pan traps were not different, they did capture unique species. We emphasize that it is not necessarily the overall completeness of the survey that is important, but the ability to capture as many unique species as possible, particularly if one accepts a low overall completeness for certain taxa. Despite the low completeness for Bombilyiidae, we were still able to make an ecological and conservation assessment of the fauna (Greathead et al. 2006).
Increasing the sampling effort did not greatly increase the sampling effectiveness or efficiency for most methods tested and for most taxa considered individually. Particularly for flying and plant-dwelling species, increasing the number of methods will probably be more effective than increasing the replicates of a method within a site. Sampling over different months and years will also contribute to the addition of new species. At a large scale (regional), the addition of new sites will probably also add more species than increasing sampling intensity. These suggestions are also supported by Boonzaier et al. (2007) who found that increasing spatial and temporal replicates increased turnover more than increasing sampling duration at a site for ants.
The number of individuals collected does influence the estimates of species richness (Buddle et al. 2005), and for taxa with low completeness, few individuals were collected. For those taxa where less than 10 individuals per species were recorded, the fauna was approximately half sampled, whereas once this proportion increased to more than 20 individuals, the sampling completeness increased substantially. We used the number of individuals and species sampled to calculate a ratio of the number of individuals required per species. Gotelli and Colwell (2001) warned that using this approach for determining required sampling intensity is acceptable for assessing relative inventory completeness for communities with more or less equivalent species richness and abundance patterns, but not for comparing species richness between sites. Obviously the values produced for our study should be tested in other savanna habitats in order to determine whether they are valid for different savanna areas or even different biomes, and these values cannot be used as an indication of the relative diversity of different sites.
Further, estimated species richness values need to be considered critically; for example, it is unlikely that only half of the Asilidae were sampled (it is unrealistic that there are 92 species of Asilidae in our study area). Buddle et al. (2005) indicated that they were not aware of any reviews that critically assessed the use of rarefaction curves in current ecological research.
Our approach is of course open to criticism, and our results have highlighted some major concerns. It will always be possible to sample a particular target taxon more effectively using additional or alternative methods, and by increasing the time or number of periods spent in the field. However, surveys are by definition a trade-off of breadth (spatial and temporal scale of information from a range of taxa) against the depth of resolution of information at a particular point in space and time for a specific taxon. We have verified that for a heterogeneous savanna habitat: (i) such a multi-taxon survey is possible; (ii) it will produce a large number of species and individuals with associated ecological information; (iii) methods can be refined and dropped to avoid duplication; and (iv) we can provide guidance as to the survey effort required for a predicted success in terms of number of sites sampled (about one site per 10 km2 for 75% completeness using a stratified approach) and number of individuals collected (about 800 individuals per higher taxon (very similar to the 800–1000 spider individuals recommended by Buddle et al. (2005)), or 27 individuals per species for 75% completeness). All this can be achieved with relatively limited resources. Most importantly, because of the involvement of taxon experts for identification of species, this survey provides a rich database for application to conservation planning (e.g. Hamer et al. 2006) and management, as well as for investigating potential taxonomic surrogates (Lovell et al. 2007).
ACKNOWLEDGEMENTS
We are grateful to the Earthwatch Institute for funding, as well as to all the Earthwatch volunteers who participated in the surveys. The taxonomists involved in the identification of specimens (see Table 1) are thanked for their collaboration. A number of students assisted with the field surveys and with processing of the material in the laboratory. Additional funding was provided by the National Research Foundation via a grant to MLH. Conservation Corporation, and particularly Kevin Pretorius, is thanked for accommodation and logistical support in Phinda. KwaZulu-Natal Wildlife and the Greater St. Lucia Wetland Park Authority are thanked for permission to work in Mkhuze and False Bay. We also thank KZN Wildlife staff for logistical support, as well as the University of KwaZulu-Natal, Natal Museum and Durban Natural Science Museum for the use of vehicles and equipment.




