Development of Kawasaki syndrome in autoimmune neutropenia after treatment with granulocyte colony-stimulating factor
Kawasaki syndrome (KS) is an acute febrile illness with systemic vasculitis, which may cause coronary artery abnormalities (CAA).1 Laboratory findings show an increased white blood cell (WBC) count, a shift to the left with segmented neutrophils and increased C-reactive protein (CRP) levels in the acute phase of the disease. Pathological histology also shows infiltrating cells that initially mainly consist of neutrophils, followed in time by macrophages.2 Primary autoimmune neutropenia occurs frequently in newborns with an incidence of approximately 1/100 000, and is usually diagnosed at the age of 5–15 months.3,4 Although there is significant neutropenia at the time of disease onset (500–1000 neutrophils/µL), the clinical course tends to resolve by the age of 2 or 3 years in 95% of patients.4 Patients with autoimmune neutropenia who have severe infections sometimes require not only antibiotics but also additional treatment with corticosteroids, i.v. immunoglobulin (IVIG), and granulocyte colony-stimulating factor (G-CSF).3
Here, we describe an 8-month-old boy with autoimmune neutropenia, who developed Kawasaki syndrome soon after being treated with G-CSF. This may provide an interesting viewpoint about potential onset mechanisms of KS.
Case report
A previously healthy 5-month-old boy had high fever and lymphadenopathy, and was treated successfully with antibiotics. After treatment his lymphadenopathy soon improved but neutropenia with absolute neutrophil counts (ANC) <500/µL was noted (90–400/µL), and the condition lasted for months. He was diagnosed with autoimmune neutropenia because of the presence of anti-neutrophil antibody. At the age of 8 months he had high fever and complained of earache. Laboratory findings were as follows: WBC, 4500/mm3; ANC, 626/mm3; CRP, 5.4 mg/dL. He was diagnosed as having acute otitis media, and was admitted to the neighborhood hospital for initial treatment with the antibiotic flomoxef sodium. On the third day of illness he still had high fever, and developed right cervical lymphadenopathy. Computed tomography showed a ring-enhancing lesion, suggestive of a cervical abscess. WBC was 3560/mm3, ANC, 819/mm3; and CRP 11.9 mg/dL. The antibiotic was changed from flomoxef sodium to meropenem trihydrate and clindamycin, and G-CSF administration (5 µg/kg per day) was initiated as an additional treatment, but his clinical symptoms (i.e. high fever and lymphadenopathy) did not improve. On the fifth day of illness, 3 days after initiation of G-CSF, he suddenly developed skin rash, peripheral edema, injected lips, and conjunctival injection, and was then diagnosed as having KS. We administered IVIG (2 g/kg) for 1 day and oral aspirin (30 mg/kg) and stopped administration of clindamycin and G-CSF. Although the clinical symptoms partially subsided, he continued to have high fever, and WBC was 8430/mm3, ANC was 6643/mm3, and CRP was 22.9 mg/dL. He was then treated with IVIG (2 g/kg) for 1 day additionally on the seventh day, but he had poor clinical resolution of symptoms, and was then referred to Kagoshima University Hospital.
At referral, echocardiography demonstrated mild dilatation of the origin of the left and right coronary arteries; we administered a 2 day course of plasma exchange (PE) with the consent of the parents on the 10th day. During the course of PE therapy, his clinical symptoms gradually subsided, and, similarly, inflammatory markers decreased after PE therapy (total exchange volume, 1625 mL): WBC decreased from 8620 to 4300/mm3, ANC decreased from 4095 to 860/mm3, and CRP decreased from 11.9 to 3.5 mg/dL. There were no complications during PE therapy. Aspirin was reduced to 5 mg/kg per day on the 13th day when the serum CRP level decreased. He was discharged on the 25th day. At discharge, echocardiography showed mild dilatation of the left coronary artery and his WBC was 4750/mm3, and ANC 736/mm3 (Fig. 1). Sequential follow up with catheter angiography after 4 months from KS onset showed complete regression of the left coronary artery dilatation.
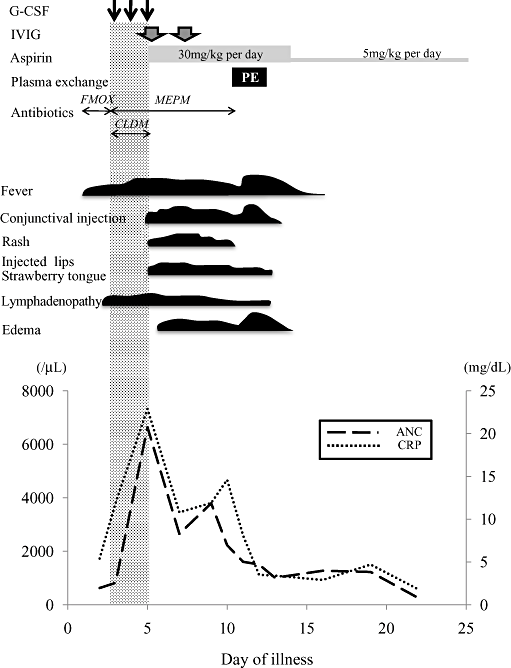
Clinical course and laboratory data. ANC, absolute neutrophil count; CLDM, clindamycin; CRP, C-reactive protein; FMOX, flomoxef sodium; G-CSF, granulocyte colony-stimulating factor; IVIG, i.v. immunoglobulin; MEPM, meropenem trihydrate.
Discussion
We encountered a patient with autoimmune neutropenia who developed KS clinical symptoms rapidly after receiving G-CSF treatment. Furthermore, he needed to undergo PE therapy for resistance to additional IVIG treatment. Functionally activated neutrophils are known to increase in number, and transient infiltration of neutrophils was identified in the early stage of KS.2 Elevated neutrophil counts are associated with the development of coronary artery lesions, but early neutropenia within 10 days of illness is reported to be associated with CAA formation. The significance of neutrophils in KS has not been fully elucidated.5 Inflammation associated with KS initially involves elevation of the levels of various cytokines such as interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α), and G-CSF may also play an important role in the acute phase of KS.6,7
The G-CSF treatment has also been associated with flares in patients with Felty's syndrome or other autoimmune diseases such as systemic lupus erythematosus.8 Autoimmune neutropenia is likely to have a relatively benign course, but G-CSF treatment is indicated in some children in whom severe infections occur. G-CSF has been identified as a glycoprotein that stimulates the production and functional activation of neutrophils, and modulates the function and activity of matured neutrophils including production of chemokines, phagocytosis and cell surface receptor expression.9 Activated neutrophils have been reported to induce organ damage by tissue infiltration and release of pro-inflammatory cytokines.9 G-CSF treatment also induces excessive migration or activation of neutrophils and induces monocyte or macrophage production, which would promote vascular permeability.9 KS patients, however, who present with only fever and cervical lymphadenopathy at admission have been reported to have an increased risk of additional IVIG treatment and of developing CAA.10 Therefore it is possible that the present case may have been due to the natural course of the KS itself, but G-CSF treatment may have potentially contributed to the severity of KS inflammation. In light of these considerations, we suggest that G-CSF treatment might be involved in the onset mechanism of KS clinical symptoms.
The present case raises concerns about the potential complication of G-CSF treatment in children with autoimmune neutropenia. Pediatricians should be aware of this risk and ensure careful clinical observation when using G-CSF treatment in children with autoimmune neutropenia complicated with severe or recurrent infections.




