Azathioprine and allopurinol: A two-edged interaction
Introduction
The thiopurines, azathioprine and 6-mercaptopurine (6-MP), are immunosuppressive drugs used in a number of clinical settings, such as following transplantation and for the management of inflammatory conditions like inflammatory bowel disease (IBD). Allopurinol, a xanthine oxidase inhibitor used in the treatment of gout, is not infrequently coincidentally co-prescribed with the thiopurines. A recent safety report from the New South Wales Department of Health (NSW, Australia) highlights the potential risk with the co-administration of these two drugs.1 This commentary reviews the interaction of these drugs and highlights important precautions that should be taken by clinicians when using them together.
Safety notice
On May 7th, 2009, the New South Wales Department of Health issued a Safety Notice regarding the interaction between allopurinol and azathioprine.1 This notice followed the death of a patient who had been admitted to a hospital on existing azathioprine therapy. The patient was commenced on allopurinol by a consulting team. The interaction in this patient resulted in the patient's death, most likely as a consequence of bone marrow suppression. This Safety Notice was released to all NSW Department of Health services and described some of the contributing factors that led to the adverse outcome. The drug interaction was unfortunately not flagged by the hospital computer prescribing application as the allopurinol was prescribed as an inpatient while the azathioprine had been prescribed as an outpatient. The unintentional interaction was missed by both medical and clinical pharmacy staff. The case report recommended that medical teams review patients' medications and should always assess the patients' pre-existing therapy when commencing new drugs. The Safety Notice also recognized that intentional co-prescription of azathioprine and allopurinol may be indicated but the azathioprine dose must be reduced to 25%–33% of the normal dose with careful hematological monitoring thereafter. The Medical Advisor of the Clinical Quality, Safety & Governance Branch of NSW Department of Health recognized the importance of knowledge dissemination through collaborative publication in raising awareness of this drug interaction among peers. This editorial highlights the pharmacogenetic variations in the thiopurine metabolic pathways, the use of low-dose allopurinol to modify thiopurine metabolite levels, and safe intentional co-prescription of the two drugs with the aim of improving drug efficacy in thiopurine non-responders.
Thiopurines
Azathioprine and 6-MP are both inactive pro-drugs. Azathioprine is converted to 6-MP, which is then further metabolized via three different routes (Fig. 1). The balance of enzyme activities in the metabolic pathway determines the rate of production of each metabolite. The 6-thioguanine nucleotides (6-TGN) are responsible for the majority of the immune suppressant activity of the thiopurines but are also associated with bone marrow suppression at high concentrations. The 6-methylmercaptopurine nucleotides (6-MMP) are associated with hepatotoxicity in high concentration,2,3 although other metabolites probably also contribute to hepatotoxicity.
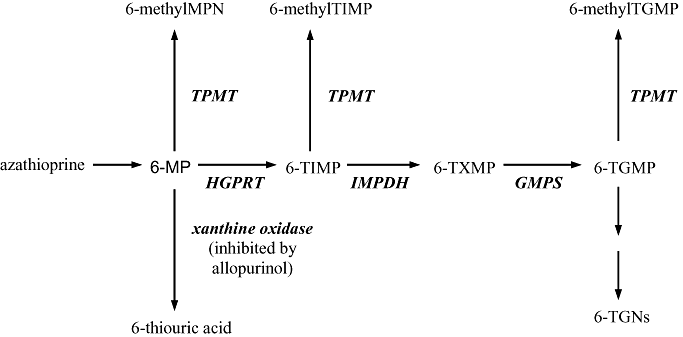
Metabolism of azathioprine and 6-mercaptopurine. 6-MP, 6-mercaptopurine; GMPS, guanosine monophosphate synthetase; HGPRT, hypoxanthine guanine phosphoribosyltransferase; IMPDH, inosine monophosphate dehydrogenase; TG, thioguanine; TGMP, thioguanine monophosphate; TGNs, thioguanine nucleotides; TIMP, thioinosine monophosphate; TXMP thioxanthosine monophosphate.
As illustrated in Fig. 1, inhibition of xanthine oxidase increases 6-TGN concentrations by preferential metabolism along this pathway, resulting in greater immune suppression but also greater risk of leukopenia. Our knowledge of the thiopurine pathway as shown suggests that inhibition of xanthine oxidase (XO) should also increase production of 6-MMP. Clinical studies have convincingly demonstrated that the opposite effect occurs, namely a dramatic reduction in 6-MMP concentrations. The mechanism for this reduction is not known, but it does not appear to be due to TMPT inhibition.4,5
Allopurinol and azathioprine combination therapy
Over fifteen years ago, prior to the development of assays to measure thiopurine metabolites, combination azathioprine-allopurinol therapy was proven efficacious in reducing episodes of rejection after renal transplantation when a low dose of 25 mg allopurinol on alternate days was added to routine triple immunosuppression with azathioprine, prednisolone and ciclosporin.6
In the era of thiopurine metabolite monitoring, the addition of allopurinol to thiopurine treatment in patients with IBD who are overproducing 6-MMP has been shown to reverse metabolic shunting such that 6-MMP concentrations decline and production of the efficacious metabolite 6-TGN is increased.7 An initial study reporting this metabolic interaction described fifteen IBD ‘shunters’ overproducing 6-MMP in whom a low dose of 100 mg allopurinol was added in conjunction with a simultaneous decrease in the thiopurine dose to 25–50% of the initial dose.5 After the addition of allopurinol, mean 6-TGN concentrations increased from 186 to 385 pmol/8 × 108 RBC (P < 0.001), whereas mean 6-MMP concentrations decreased from 10 380 to 1732 pmol/8 × 108 RBC (P < 0.001), thereby indicating a favorable metabolic switch. Clinical data were later reported on an expanded cohort of 20 IBD patients in whom the same metabolic switch was noted with allopurinol co-therapy.4 Concomitant allopurinol lead to reduced disease activity scores in both Crohn's disease (P = 0.001) and ulcerative colitis (P = 0.13), a reduction in mean prednisolone dose after three months of combination therapy (P < 0.001), and a reversal of the liver function test abnormalities that otherwise occur with preferential 6-MMP production (ALT—P = 0.01; AST—P = 0.12). Mean white cell counts dropped from 8.8 to 6.0 × 108/L, and while leukopenia occurred in five patients, it was reversible in all cases with further thiopurine dose reduction, and no infections occurred.
Long-term follow-up of this cohort out to three years has shown that these efficacy and safety results are maintained.8 Three further small, retrospective, single-centre studies, including one in a pediatric cohort, have replicated these results, although one viral infectious complication was reported in a total 28 patients.9–11 A summation of the current published retrospective studies shows that combination azathioprine-allopurinol therapy safely and effectively optimizes thiopurine metabolism and achieves the resultant clinical benefits. Prospective studies are underway to further validate the role of combination therapy in otherwise thiopurine-resistant IBD patients.
Implications for the Asia-Pacific region
There may be particular implications of thiopurine and xanthine oxidase metabolism for the Asia-Pacific region.12 Data from several recent studies demonstrates ethnic differences in thiopurine metabolism. TMPT polymorphisms have been defined in all ethnic groups studied. Significant variations in the frequency of SNPs are related to ethnicity. The most common SNP in Caucasians is TPMT*3A, which is characterized by a G to A transition at position 460 with a substitution of alanine for tyrosine at amino acid 154 (A154Y) and a transition of A to G at nucleotide 719 resulting in a change of tyrosine to cysteine at position 240 (Y240C). The other alleles are TMPT*2, TMPT*3B and TMPT*3C. The frequency of the TMPT alleles in different ethnic groups was defined in individuals of British Caucasian, British South West Asian and Chinese extraction.13 Whereas 10% of the Caucasians had a variant genotype, these were seen in 2% of the South West Asians and 4.7% of the Chinese. Variations in the TPMT*2 allele were seen in the Caucasians, but not in the other two groups. TPMT*3A was the only mutant allele found in the South West Asians, but was not seen in the Chinese. The Chinese individuals only had the TPMT*3C allele.
Efrati and co-authors14 examined TMPT risk alleles within groups in an Israeli population: Druze individuals differed greatly from Jewish and Muslim groups. Allelic frequencies of TMPT varied between the three groups. Furthermore, two variants (TMPT*2 and TMPT*3B), were not found in any of the subpopulations. Further studies have also examined variations in TMPT enzyme activity. For example, Cooper et al.15 defined TMPT enzyme activity in 1000 adults from various ethnic groups. Women, especially those of South-East Asian origin, had lower activity than men.
Variations in XO activity are also described. Kudo et al.16 defined variations in a group of 96 Japanese. Several polymorphisms were defined, which correlated with individual differences in XO enzyme activity. Ethnic variations in the frequencies of these polymorphisms may occur.
To date, there are no data regarding ethnic differences in shunting of thiopurine metabolism or in differential responses to the combination of allopurinol with thiopurines. Variations in the frequencies of polymorphisms in TMPT and XO likely contribute and need to be considered in dosing of allopurinol and thiopurines.
Conclusion
As emphasized in the Safety Notice, the interactions between allopurinol and azathioprine can lead to serious adverse outcomes. However, the careful combination of these drugs, along with close monitoring of metabolites and blood counts can be efficacious and lead to much improved health outcomes. While there are no published guidelines in this area, we would advise weekly blood counts for at least four weeks when this combination is commenced and three monthly blood tests in the long term. Individual and ethnic variations in the key enzymes involved in the metabolism of these drugs are important to consider in prescribing and monitoring.
Practice points
- •
Always check if a patient is on azathioprine before prescribing allopurinol
- •
Always check if a patient is on allopurinol before prescribing azathioprine
- •
Educate the patient and remind their General Practitioner about the interaction when prescribing azathioprine
- •
Dose reduce azathioprine to 1/4 of the original dose when co-prescribing allopurinol and monitor the blood count frequently
- •
Only co-prescribe azathioprine and allopurinol to improve azathioprine efficacy if thiopurine metabolites can be monitored.
- •
Monitor blood count at least weekly for the first four weeks of co-prescription.




