The 2008 Okuda lecture: Management of hepatocellular carcinoma: From surveillance to molecular targeted therapy
Abstract
Hepatocellular carcinoma (HCC) is responsible for approximately 600 000–700 000 deaths worldwide. It is highly prevalent in the Asia-Pacific region and Africa, and is increasing in Western countries. Alpha fetoprotein (AFP) alone is insufficient for HCC screening. A combination with other tumor markers, such as PIVKA-II and AFP-L3, and periodical ultrasound surveillance is necessary. Sensitivity of AFP in depicting HCC is highest, followed by PIVKA-II and AFP-L3, but the order of the specificity is inverse, AFP-L3, PIVKA-II, and AFP. Sonazoid-enhanced ultrasound (US) is extremely useful to characterize hepatic tumors equal to or more than multidetector row computed tomography (MDCT). Sonazoid-enhanced US with defect re-perfusion imaging is a breakthrough technique in the treatment of HCC. Defect re-perfusion imaging will markedly change the therapeutic strategy for liver cancer. Gd-EOB-DTPA-magnetic resonance imaging is a newly developed imaging technique in the detection and diagnosis of HCC. It is the most sensitive tool in the differentiation of early HCC from dysplastic nodules. Regarding the treatment strategy, there has been no established systemic chemotherapy for advanced HCC, except for Sorafenib. Empirically, intrahepatic arterial infusion chemotherapy using implanted reservoir port is known to be effective in response rate and overall survival for advanced HCC with vascular invasion. Sorafenib in combination with transcatheter arterial chemoembolization or adjuvant use after ablation or resection will significantly prolong the life expectancy if ongoing clinical trials provide positive results. In conclusion, it is expected that readers will gain deeper insight into the latest progress and updated diagnosis and treatment of HCC described in this review.
Surveillance for early detection of HCC
Definition of the population at high-risk for HCC
Hepatocellular carcinoma (HCC) is responsible for approximately 600 000–700 000 deaths worldwide. It is highly prevalent in the Asia-Pacific region and Africa, and is increasing in Western countries.1 Persistent infections with hepatitis B virus (HBV) and hepatitis C virus (HCV) are the highest risk factors for hepatocarcinogenesis. The carcinogenesis risk for HBV-infected persons is about 200 times higher than for those non-infected, and the risk may be higher by approximately fivefold in patients with HCV-related cirrhosis compared with those with HBV-related cirrhosis. The characteristics of HCV-associated carcinogenesis are fibrosis stage 4 (F4), in which liver cirrhosis is complete in most cases, male gender and age 60 years or older. The yearly carcinogenesis rate of cirrhosis type C is 7–8% in Japan, which is higher than in Europe, Australia and North America (1–3% per year),2 this difference might be attributed to the higher mean age of carriers.
Liver cirrhosis induced by causes other than HBV and HCV is also a risk. Thus, HCC occurs in some cases of liver cirrhosis associated with nonalcoholic steatohepatitis (NASH), alcoholic liver disease, primary biliary cirrhosis (PBC), hemochromatosis, alpha-1 antitrypsin deficiency and autoimmune hepatitis (AIH). For patients with any of these disorders, the course of the disease should be followed with close attention to hepatocarcinogenesis. In addition, alcohol increases the risk of chronic hepatitis B- and C-associated liver carcinogenesis, and obesity increases the risk of HCV-related hepatocellular carcinoma (HCC). In summary, patients with chronic hepatitis B and C and non-viral liver cirrhosis are defined as high-risk populations for HCC in both Evidence-Based Practice Guidelines,3 the Consensus-Based Clinical Practice Manual4 proposed by the Japan Society of Hepatology (JSH), and the Practice Guideline published by the American Association of Study of the Liver (AASLD).5 Patients with liver cirrhosis from HBV or HCV are defined as a super high-risk population.3,4
Surveillance protocol for early detection of HCC
For HCC detection, sensitivity of ultrasonography is higher than serum alpha fetoprotein (AFP) measurement alone, but the specificities are not markedly different. For liver cirrhosis, a combination of the two methods has been reported to increase detection rate compared with detection by ultrasonography or AFP.3
There is not yet clear evidence to determine the optimal interval for screening, but HCCs detected in periodic screening by AFP, a protein induced by vitamin K absence or antagonist-II (PIVKA-II), AFP lectin fraction (AFP-L3) measurement, and ultrasonography are solitary and small in many cases, as compared with those detected in symptomatic patients. Thus, the Japanese Evidence-Based Clinical Practice Guidelines3 and Consensus-Based Clinical Practice Manual4 propose ultrasonography and tumor marker measurement every 3–4 months in the super high-risk population, and every 6 months in high-risk populations. Based on HCC doubling times, these intervals appear to be appropriate in Japan, which is different from Western Countries, where screening is done every 6–12 months.5 At present, all three tumor markers, including AFP, PIVKA-II, and AFP-L3, are covered under the Japanese national health insurance as HCC tumor markers. Measurement of two or more tumor markers increases the sensitivity, while minimizing the specificity reduction, for small liver cancer. For patients with a very nodular background liver parenchyma because of cirrhosis or obesity, and therefore difficult to evaluate ultrasonographically, periodic imaging screening by dynamic computed tomography (CT) (MDCT) or dynamic magnetic resonance imaging (MRI) every 6–12 months is proposed by JSH,4 which is identical to the protocol in the Japanese Evidence-Based Clinical Practice Guidelines.3
Result of early detection of HCC in Japan
In Japan, approximately 65% of the patients are detected at an early stage, for which curative treatment intervention is possible according to the Nationwide survey in 198 000 patients6 (Fig. 1). This can be attributed to the establishment of a nationwide surveillance system across Japan.
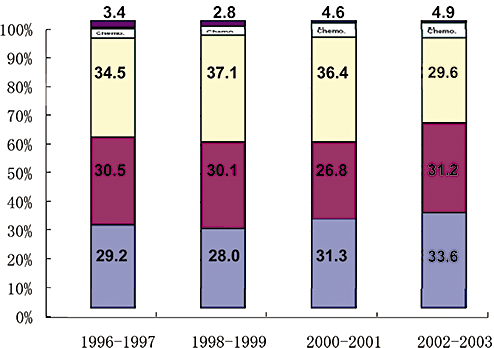
Treatment for newly diagnosed hepatocellular carcinoma (HCC) from 1996–2003 according to Nation-wide survey of Liver Cancer Study Group of Japan. 17th Nationwide survey clearly shows 64.8% of newly diagnosed HCCs receive potentially curative treatment such as operation or ablation. In other words, approximately 65% of HCCs are detected at early stage.  , others;
, others;  , Chemo.;
, Chemo.;  , transcatheter arterial chemoembolization (TACE);
, transcatheter arterial chemoembolization (TACE);  , Ablation;
, Ablation;  , Ope.
, Ope.
Markers of HCC tumor biology
Alpha fetoprotein
Alpha fetoprotein is a tumor marker for HCC used worldwide. In Japan, according to the 17th Nationwide Follow-up Survey of Primary HCC by the Liver Cancer Study Group of Japan (LCSGJ),6 most HCC patients were AFP-positive when the cutoff value was set at 15 ng/mL; however, AFP is positive in some patients with chronic hepatitis, particularly at the stage of liver cirrhosis, and in liver regeneration following necrosis. Therefore, AFP specificity is low depending on the cutoff value, and is considered inappropriate for screening HCC in the USA.7 Accordingly, to effectively use AFP in clinical practice, it is important to recognize that sensitivity and specificity vary depending on the cutoff value.
Lens-culinaris agglutinin-reactive fraction of alpha fetoprotein
Lens-culinaris agglutinin-reactive fraction of alpha fetoprotein (AFP-L3) fraction was developed as a tumor marker in Japan. When the cutoff value was set to 10%, the sensitivity was approximately 30% (the 17th nationwide Follow-up Survey of Primary HCC by LCSGJ). Hence its clinical usefulness as an HCC surveillance marker is not appreciated in Western countries;8 however, AFP-L3 is widely used, mainly in Japan, as a marker representing the degree of biological malignancy of HCC. Negative conversion of this marker after treatment is meaningful, although it is only approximately 50% after curative treatments.9 Conversely, the prognosis of cases remaining positive after treatment is poor, and the rate of distant metastases is high; the possibility of early metastasis within the liver and to other organs should be kept in mind, in such cases, which require careful follow up for early detection of recurrence or intervention (such as interferon [IFN] treatment).
Protein induced by vitamin K absence-II
The sensitivity of protein induced by vitamin K absence-II (PIVKA-II) was 59% for a cutoff value of 40 mAU/mL according to the 17th Nationwide Survey by LCSGJ. The specificity is > 95%, but the positivity rate for 3 cm or smaller HCC is low (∼40%). For HCCs larger than 5 cm, the positivity rate was 97%, indicating that this marker is superior to AFP. Further, the incidence of portal tumor thrombosis is high in PIVKA-II-positive cases (annual rate: 21%), and the risk ratio relative to negative cases is reportedly 5.65.10 Although PIVKA-II is routinely used for HCC surveillance in Japan, the 2003 Single Topical Conference of the American Association of Study of the Liver (AASLD) positioned it as a diagnostic method,11 rather than a screening method, because of its low sensitivity nature.
Other tumor markers
In addition to the above three tumor markers, glypican-312 and human telomerase reverse transcriptase (hTERT)13 are attracting attention as HCC markers. Glypican-3 is a cell membrane protein; its positivity rate in HCC patients and specificity were reported to be 40–50 and 95–100%, respectively, showing its usefulness as a tumor marker. Further, the positivity rate is particularly high in the early stage, and the sensitivity rises to more than 80% when used in combination with AFP. In the future, it seems likely that glypican-3 may be used for clinical practice, such as diagnosis and screening for HCC.
hTERT is a telomerase-containing protein that has attracted attention as a cancer marker since the late 1990s. Sensitivity at the time of blood mRNA measurement was 88%, but specificity was lower, 70%. It may be clinically applicable by setting an optimum cutoff value based on a receiver operator curve (ROC).
Newly introduced diagnostic techniques
Contrast-enhanced ultrasound with a new contrast agent, Sonazoid
Clinical significance of contrast-enhanced ultrasound
In the management of HCC, despite advances in diagnostic imaging techniques such as ultrasound (US), CT or MRI, there remain many limitations, such as screening, staging, evaluation of treatment response, treatment guidance, localization of local recurrence after radiofrequency ablation (RFA), and detection of recurrence. Among these problems, Levovist-enhanced US has made a contribution to differential diagnosis,14,15 evaluation of malignancy grade,16 evaluation of therapeutic response to transcatheter arterial chemoembolization (TACE),17–19 and needle insertion guidance.20,21 However, there are still limitations in the evaluation of the therapeutic response to RFA,22 screening or staging.
Sonazoid (GE HealthCare, Milwaukee, WI, USA) is a newly introduced second generation ultrasound contrast agent exclusively approved in Japan in 2007. The important characteristics of Sonazoid are that it facilitates real-time imaging in blood flow images at low acoustic power and stable Kupffer phase imaging, tolerable for multiple scanning from 10 to 120 min after its injection. Sonazoid is considered to be more effective and easier to use than Levovist in vascular imaging, and allows visualization, even using non-high-end equipment, and therefore, dependence on operator's skill/equipment is decreased, which may facilitate the widespread use of contrast-enhanced US. Sonazoid-enhanced US provides very stable post-vascular phase images for up to 60–120 min,23 which resulted in the invention of the breakthrough method, defect reperfusion imaging. Thus, sonazoid-enhanced US with defect reperfusion imaging is an innovative technology that should greatly change the daily clinical practice of HCC investigation.
Development of defect reperfusion imaging (dual phase fusion imaging)
We recently developed defect reperfusion imaging24–26 using the properties of very stable Kupffer images and real-time fine blood flow images obtained with Sonazoid for typical HCC, which is depicted by CT but not by B mode scanning. This method is a breakthrough for accurate localization and treatment guidance.25 Until recently, diagnosis in dynamic studies was usually based on enhancing patterns according to a time sequence or phase; however, by introducing the novel idea of dual phase imaging with the re-injection method, both Kupffer and arterial phase images are obtained at the same slice of the ultrasound plane, which is really an innovative technique. Namely, this method is performed as follows: re-injection of Sonazoid is performed into areas that show defects in the post-vascular phase.23–26 The introduction of this method has solved several limitations in the diagnosis and treatment of HCC, such as detection of small HCCs,27 evaluation of treatment response,28 or needle insertion guidance. Detection rate of small HCCs by Sonazoid-enhanced US is even more sensitive than that by MDCT (Fig. 2),27 and it seems likely that this novel technique will eventually be used worldwide.
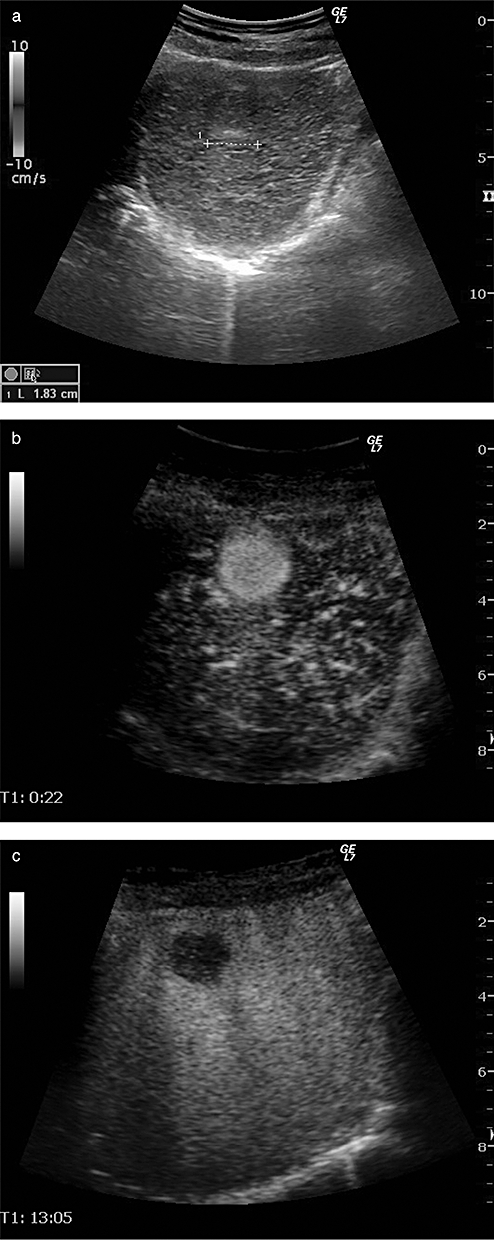
A case of hepatocellular carcinoma (HCC) demonstrated by Sonazoid-enhanced ultrasound. (a) B-mode image shows ill-defined iso-echoic nodule measuring 1.83 cm in size. (b) Sonazoid-enhance ultrasound (US) clearly demonstrates this nodule as a hypervascular tumor. (c) Kupffer phase image shows this nodule as a clear defect, suggesting typical HCC.
MRI using a new contrast agent, Gd-EOB-DTPA in the diagnosis of early HCC
Hepatocellular carcinoma is known to show multistep progression from the hyperplastic nodule to early HCC and finally to moderately/poorly differentiated HCC (Fig. 3). It is important to differentiate between premalignant nodules and early HCC. The imaging diagnosis of HCC by CT/MRI has been made by dynamic acquisition (hemodynamic diagnosis) using extracellular contrast medium, such as iodine contrast agent or gadolinium-diethylene-triamine-pentaacetic acid (GD-DTPA). HCC is supplied solely from arterial, not portal blood flow. Super paramagnetic iron oxide (SPIO) is specifically taken up by Kupffer cells and has been used as a liver-specific contrast agent for MRI since 1997; Kupffer cells are not present in overt HCC.
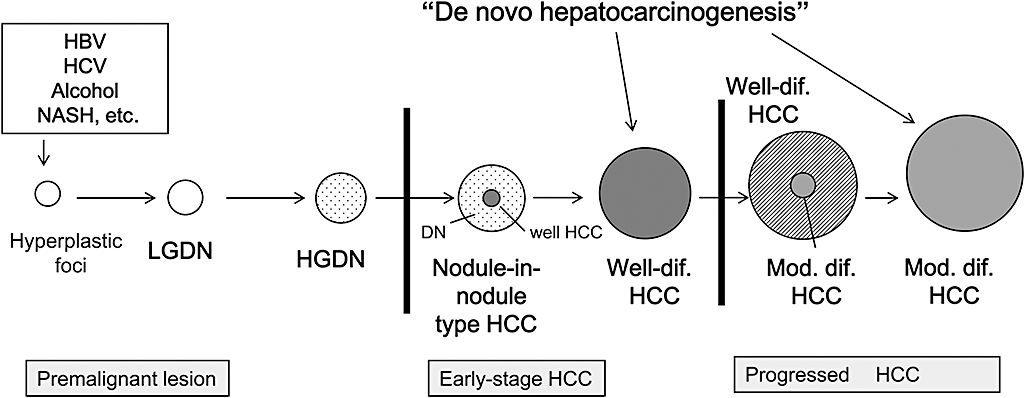
Schematic representation of multistep progression of human hepatocarcinogenesis. Differentiation between early-stage hepatocellular carcinoma (HCC) and premalignant lesion is extremely important. ○, Hyperplastic foci or low grade dysplastic nodule (LGDN); 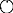 , High grade dysplastic nodule (HGDN);
, High grade dysplastic nodule (HGDN);  , Well-differentiated HCC (well HCC);
, Well-differentiated HCC (well HCC);  , Moderately differentiated HCC (classical overt HCC). HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis.
, Moderately differentiated HCC (classical overt HCC). HBV, hepatitis B virus; HCV, hepatitis C virus; NASH, nonalcoholic steatohepatitis.
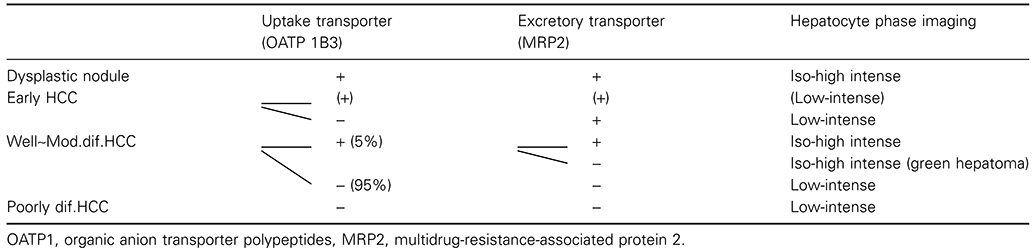
A newly introduced contrast agent, Gd-EthOxyBenzl-DTPA (Gd-EOB-DTPA), approved in 2008 in Japan, is a hepatocyte-specific MRI contrast medium with a different mechanism, using both dynamic and Kupffer cell imaging. This new contrast medium is useful to diagnose cases that would have been difficult using previous techniques such as dynamic MRI or SPIO-MRI. Gd-EOB-DTPA consists of the extracellular contrast medium, Gd-DTPA, and the lipid-soluble EOB group. Acquisition of both water and lipid solubility increases cell membrane permeability and the agent is therefore taken up by hepatocytes. Although the mechanism for hepatocellular uptake has not been fully clarified, it may involve organic anion transporting polypeptide (OATP1)29 (Fig. 4). Recently, it was reported that uptake of Gd-DTPA-EOB is regulated by OATP1B3 in humans.30 For excretion into bile, the active transport out of hepatocytes is by multidrug resistant protein (MRP2) system31 (Fig. 4). Active transport is indicated by the high biliary excretion rate (∼50%) of Gd-EOPB-DTPA. Imaging diagnosis of HCC can be made within 10–20 min after Gd-EOB-DTPA injection.
Pharmacokinetics of Gd-EOB-DTPA. Gd-EOB-DTPA is uptaken to the hepatocyte by organic anion transporter peptides (OATP1). Excretion to bile juice is believed to be regulated by multidrug resistance-associated protein (MRP)2.
Typical HCCs show high intensity of Gd-EOB-DTPA in the arterial-dominant phase and low intensity in the portal-dominant phase and thereafter. In the arterial-dominant phase, Gd-EOB-DTPA is not taken up by normal hepatocytes, and thus, HCC nodules are intensely stained in the arterial dominant phase. In the portal-dominant phase and thereafter, Gd-EOB-DTPA is gradually taken up by normal hepatocytes, increasing the clear contrast between normal liver parenchyma and HCC nodules (Fig. 5).29,32 After 20 min, the liver/tumor contrast is as high as or superior to that in CT during arterial portography (CTAP) except for approximately 5% of overt HCC cases, which show high or iso-intense on hepatocyte phase image (Table 1). Hepatocyte phase image of Gd-EOB-DTPA MRI is speculated to be regulated by the balance of OATP1B3 and MRP2 expression (Table 1).
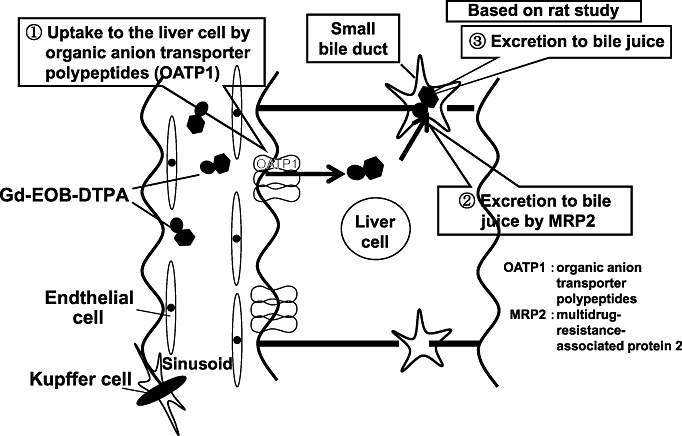
Typical findings of hepatocellular carcinoma (HCC) on Gd-EOB-DTPA magnetic resonance imaging (MRI). (a) Arterial enhancement (arrow) is evident or arterial phase. (b) Slight washout is seen on portal phase. (c) Clean defect is seen on hepatocyte specific phase 20 min later.
In well-differentiated early HCC, some nodules may not be completely shown as defective areas on CTAP, but Gd-EOB-DTPA uptake is apparently lower than that in the surrounding normal liver parenchyma, being imaged as a low-intensity nodule. Well-differentiated early HCCs having Kupffer cells with enhanced SPIO uptake and receiving portal blood flow on CTAP have been difficult to characterize by SPIO-MRI or CTAP. However, they can be imaged clearly as hypointense nodules using Gd-EOB-DTPA hepatocyte phase MRI in many early HCC cases due to differences in the biological characteristics. This indicates that this new contrast agent may lead to a breakthrough in the diagnosis of early HCC (Table 2) (Fig. 6),32,33 which has been clinically difficult and difficult even by pathological diagnosis in biopsy samples. It could be that this technique may be the most sensitive tool for detection of the phenotypic change of early hepatocarcinogenesis, much more sensitive than CTAP, computed tomography hepatic arteriography (CTHA), or SPIO-MRI (Fig. 7).
| Only resected specimens: 30 | Pathological findings | ||
|---|---|---|---|
| e-HCC | DN or RN | ||
| Signal intensity in hepato-biliary phase with Primovist | Low—slightly low(24) | 23 | 1 |
| Iso—high(6) | 1 | 5 | |
- AccuracyL 93% (23+5/30). DN, dysplastic nodule; e-HCC, early hepatocellular carcinoma; RN, regenerative nodule.
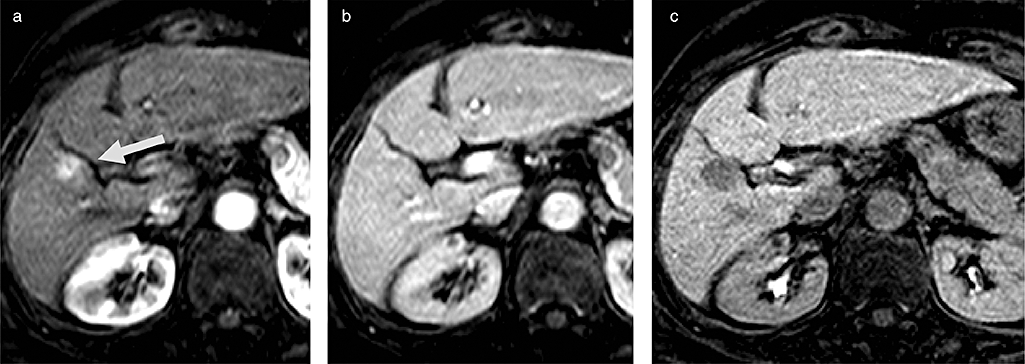
Early hepatocellular carcinoma (HCC), which was confirmed by Gd-EOB-DTPA magnetic resonance imaging (MRI). (a) Computed tomography hepatic arteriography (CTHA) does not show any hypervascularity. (b) CT during arterial portgraphy (CTAP) shows slight low dense mass on Segment 6. (c) Gd-EOB-DTPA magnetic resonance imaging (MRI) shows low intense mass at the hepatocyte phase, strongly suggestive of early HCC. (d) Pathological findings of resected specimen clearly shows vaguely nodular type HCC, suggesting early HCC. (e) Microscopical findings clearly show well-differentiated HCC with stromal invasion, which is a strong diagnostic clue of early HCC.
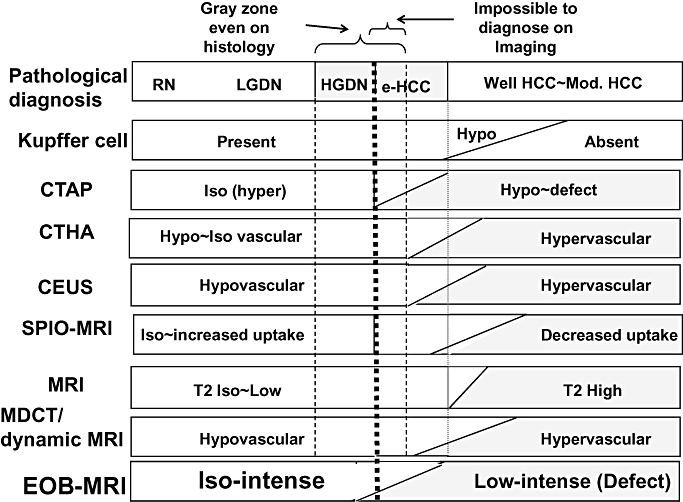
Gd-EOB-DTPA magnetic resonance imaging (MRI) is the most sensitive technique in the detection of initial phenotypic change of human hepatocarcinogenesis among various pre-existing imaging modalities. CEUS, contrast-enhanced ultrasound; CTAP, CT during arterial portgraphy; CTHA, computed tomography hepatic arteriography; EOB, EthOxyBenzl; HGDN, high grade dysplastic nodule; LGDN, low grade dysplastic nodule; MDCT, multidetector row CT; MRI, magnetic resonance imaging; SPIO, super paramagnetic iron oxide.
There are two reasons why pathological diagnosis of early HCC is sometimes difficult using biopsy: (i) possibility of sampling error; and (ii) stromal invasion, an important clue of pathological diagnosis of early HCCs,34 can occasionally not be found in the biopsy sample compared with the resected specimen. Recently, a consensus on pathological diagnosis of early HCC has been established between ‘East and West’.34 Diagnosis of early HCC by Gd-EOB-DTPA-MRI may be the most comparable tool with that by expert liver specialized pathologist compared with pre-existing imaging modalities according to multicenter trials (Table 2). Accuracy in diagnosing early HCC is as high as 93%, which is much better than CTAP (Table 2). If so, this will change the diagnostic algorithm by introducing Gd-EOB-DTPA MRI in hypervascular and hypovascular liver nodules4 (8, 9).
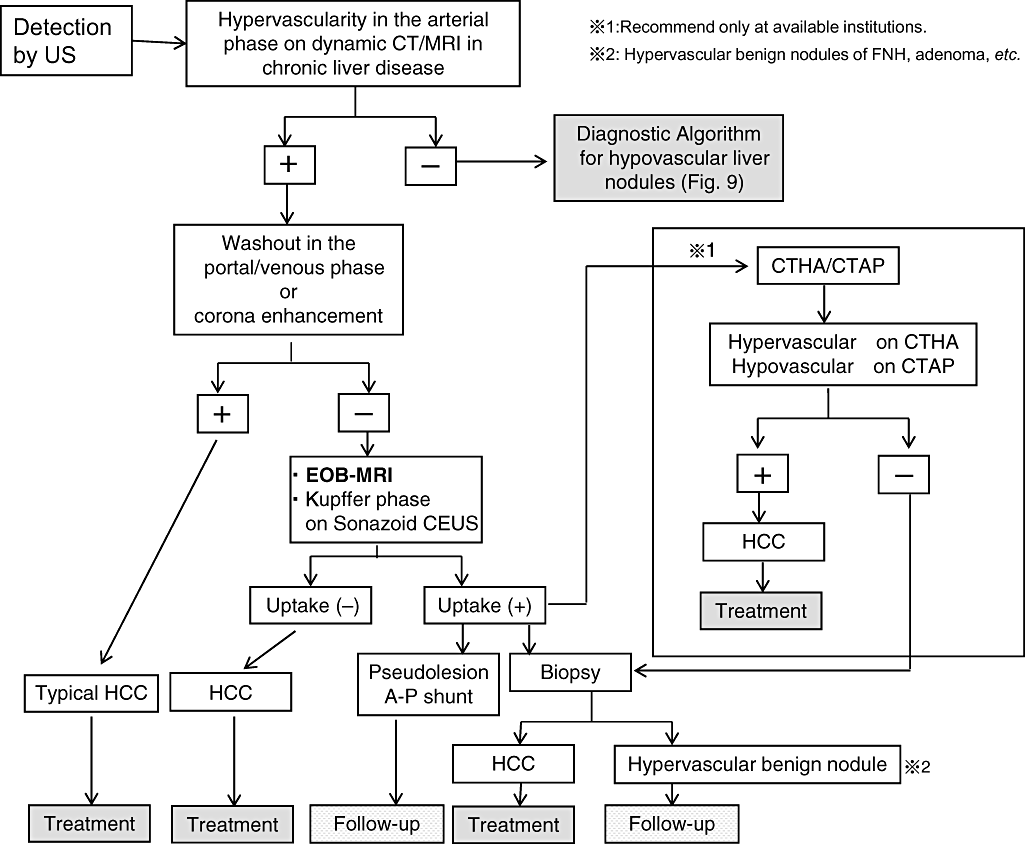
Diagnostic and treatment algorithm for hypervascular liver nodules according to clinical practice manual recommended by Japan Society of Hepatology (partially modified and cited from Narita et al. 200930). CEUS, contrast-enhanced ultrasound; CT, computed tomography; CTAP, CT during arterial portgraphy; CTHA, computed tomography hepatic arteriography; EOB, EthOxyBenzl; FNH, focal nodular hyperplasia; HCC, hepatocellular carcinoma; MRI, magnetic resonance imaging; US, ultrasound.
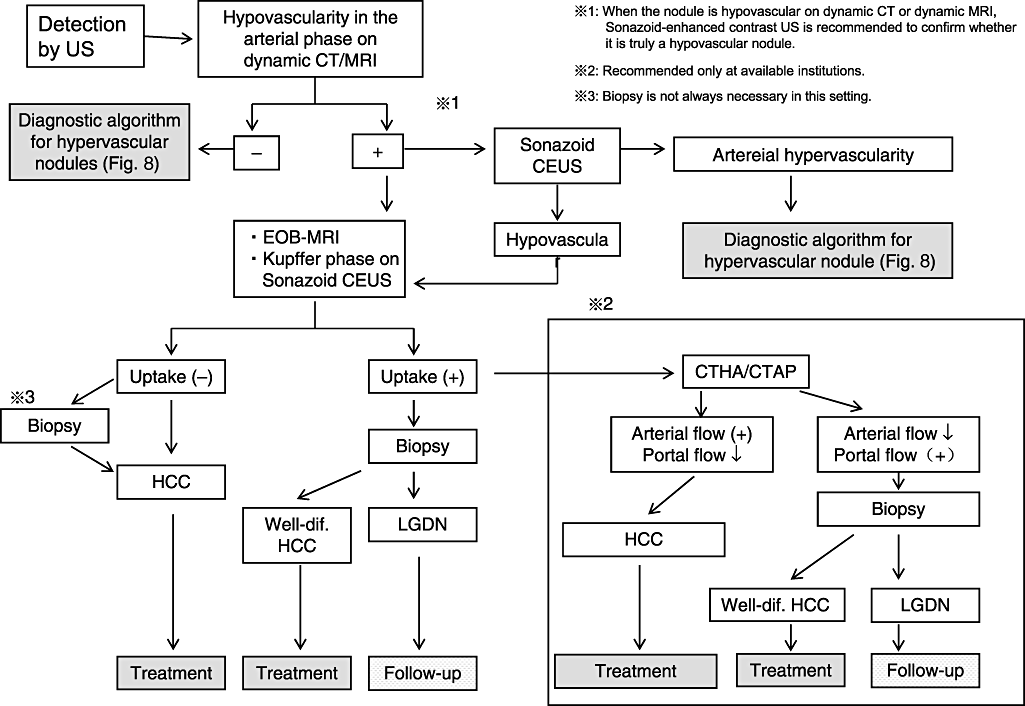
Diagnostic treatment algorithm for hypovascular liver nodules according to Japan Society of Hepatology (cited from Kudo et al. 200750). CEUS, contrast-enhanced ultrasound; CT, computed tomography; CTAP, CT during arterial portgraphy; CTHA, computed tomography hepatic arteriography; EOB, EthOxyBenzl; FNH, focal nodular hyperplasia; HCC, hepatocellular carcinoma; LGDN, low grade dysplastic nodule; MRI, magnetic resonance imaging; US, ultrasound.
Value of an integrated staging system
Various staging systems have been proposed for HCC and are used in the different regions, such as: (i) Okuda stage; (ii) Barcelona Clinic Liver Cancer (BCLC) stage;35,36 (iii) Cancer of Liver Italian Program (CLIP) score;37 (iv) Japan Integrated Staging (JIS) score;38,39 and (v) Tokyo Score.40 In Japan, the JIS score, using both the LCSGJ TNM41 and Child-Pugh stages, is considered to be the most useful for integrated staging of HCC. The CLIP score has several disadvantages: specification of the tumor-spreading degree is approximate, only AFP is used as a biological malignancy marker, and stratification ability is poor in advanced cases (many cases cluster to a score of 0–2).
The original JIS score used Child-Pugh staging, but the modified JIS score using liver damage instead is frequently used by liver surgeons.42 The modified JIS score may be useful in planning hepatectomy because LCSGJ liver damage is more strictly classified. Recently, new staging systems for predicting prognosis have been developed; for example, the BALAD score,43 which consists of the albumin level, bilirubin level, and three tumor markers (AFP, AFP-L3, PIVKA-II). The reported advantages of the BALAD score are that it does not require a tumor-spreading stage. The second method is the biological marker-combined JIS score,44 which is a combination of the original JIS score and three tumor markers (AFP, PIVKA-II, AFP-L3). This staging system seems to be superior to the original JIS score and BALAD score.44
Globally, CLIP scores and BCLC stage are used in Europe and North America as staging systems; however, they have different characteristics: the BCLC stage is basically a treatment-selection system for deciding on a therapeutic strategy, whereas CLIP and JIS scores are prognostic predictors for staging. The CLIP score and BCLC stage tend to predict the prognosis of only large HCCs, but the JIS score is most useful to predict the prognosis of many small liver cancers.
Attention needs to be paid to the fact that the BCLC stage corresponds to the Japanese treatment algorithm, but is not a prognostic prediction staging system. For countries incapable of detecting HCC early or in developing countries with insufficient screening systems and diagnostic instruments, the CLIP score may provide good stratification as a prognostic prediction system. In the future, the JIS score may be used worldwide when surveillance systems for early detection of HCC become more common.
For practical purposes, the following conditions are essential for comprehensive analysis or staging of all cases of liver cancer: the system should: (i) be simple; (ii) have no missing data; (iii) be able to be used by anyone anywhere; (iv) be easy to memorize; and (v) be superior for stratifying early, intermediate, advanced, and terminal cases. Considering these conditions, the JIS score or bm-JIS score may be the most appropriate among current systems for the overall stratification of liver cancer cases in Japan.
Hepatic arterial infusion chemotherapy for advanced HCC
Until sorafenib was introduced, there was no effective anticancer drug for advanced liver cancer. ‘Far advanced liver cancer represents stage IVa liver cancer accompanied by vascular invasion and stage IVb liver cancer accompanied by distant metastasis, for which low-dose fluorouracil platinum (FP) (5FU and cisplatinum)45 therapy, and hepatic arterial infusion of 5FU in combination with IFN treatment46 have been established as an effective treatment option in Japan. In fact, response rate (complete response + partial response [CR+PR]) reaches to 46% according to the Nationwide Survey by LCSGJ6 (Fig. 10). In addition, it is well established that overall survival of the responder is superior to that of non-responders or best supportive care groups. However, intra-arterial infusion is complex because establishment of a reservoir port for arterial infusion is necessary; therefore, this technique is not performed in Western counties.
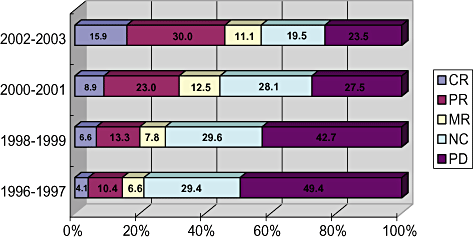
Response rate of hepatic arterial infusion chemotherapy (HAIC) from 1996 to 2003 reported by Nation-wide survey of Liver Cancer Study Group of Japan. Response rate during 2002–2003 reached 45.9%, which is very high. CR, complete response; MR, minor response; NC, no change; PD, progressive disease; PR, partial response. HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization.
Recently, maintenance of the blood IFN level using pegylated IFN (PEG-IFN), and its efficacy in combination with an oral 5FU prodrug, S-1, (PEG-IFN + S1 combination therapy),47 have been demonstrated to some extent. Further investigation, including a prospective randomized study, is necessary. Moreover, hepatic intra-arterial infusion chemotherapy is not recommended in the AASLD guidelines.5 Although the response rate is high, efficacy, especially survival benefit of intra-arterial infusion chemotherapy and that using an intractable delivery port system should be confirmed by further randomized studies.
New treatment option: Molecular targeted agent, Sorafenib
Molecular-targeted drugs are agents that exploit genetic differences between cancer and normal cells and specifically inhibit molecules involved in cancer growth and metastasis. The earliest successful agents have been Imatinib, Trastuzumab, and Gefitinib, all breakthrough agents developed from basic studies on tyrosine kinase or serine-threonine-mediated intracellular signal transduction.
Although HCC is the 3rd greatest cause of cancer death worldwide, the molecular mechanism(s) of its growth and progression have not been fully clarified. It is a hypervascular tumor, similar to renal cell carcinoma, but until recently, the efficacy of angiogenesis inhibitors alone has been limited. Sorafenib is a multikinase inhibitor that clearly prolongs the overall survival in patients with advanced HCC by 44%;48 it has been approved for advanced HCC in Western countries since 2007, and is regarded as standard of care treatment option for advanced HCC with vascular invasion or extrahepatic metastases.
Sorafenib, developed by Bayer HealthCare (Germany), is a low-molecular-weight compound discovered by screening inhibitors of Raf kinase, an important molecule in the mitogen activating protein (MAP) kinase cascade located downstream of growth factor receptors. Sorafenib exhibits strong inhibitory activity for not only wild type c-Raf, but also for V600E mutant b-Raf and other receptor tyrosine kinases involved in angiogenesis and cell growth, such as vascular endothelial growth factor receptor-2 (VEGFR-2), VEGFR-3, platelet-derived growth factor receptor (PDGFR), Fms-related tyrosine kinase-3 (Flt-3), and c-Kit.
The phase III study for HCC (SHARP trial)48 was performed as a randomized double-blind placebo-controlled multicenter study initiated in March 2005. The subjects had advanced HCC at ECOG PS 0–2 with Child-Pugh A liver function and no previous systemic chemotherapy. There were two study groups, Sorafenib (400 mg b.i.d.) and placebo treatment, and the primary end point was overall survival (OS). Secondary endpoints were time to progression (TTP).
Six hundred and two patients met the inclusion criteria, and 299 and 303 were randomly allocated to the Sorafenib and placebo groups, respectively. On interim analysis, the median OS was 10.7 months in the Sorafenib group and 7.9 months in the placebo group, showing 44% improvement (hazard ratio: 0.69, P-value = 0.0006). TTP was 5.5 months in the Sorafenib group and 2.8 months in the placebo group, showing 73% prolongation (hazard ratio: 0.587, P-value = 0.000007). Grade 3 and 4 adverse events for which a causal relationship with Sorafenib could not be ruled out were diarrhea and skin reaction.
In August 2007, it was reported that Sorafenib also prolonged overall and progression-free survival in a phase III study for HCC performed in the Asia Pacific region, involving 226 Chinese, Korean, and Taiwanese patients. Data demonstrated similar efficacy and safety of Sorafenib on HCC as in the SHARP study.49 In Japan, a phase I study has been completed, and a phase III study in HCC patients following TACE is currently underway. In addition, a phase III trial for HCC of acyclic Retionid, a vitamin A analog, after resection or RFA is also underway in Japan.
A global phase III trial of Sorafenib as adjuvant therapy after surgery or ablation is now ongoing (STORM trial) and a global phase II trial of Sorafenib as a maintenance therapy with a combination of TACE is also ongoing (SPACE trial). A phase I/II trial of a combination therapy of Sorafenib with hepatic arterial infusion chemotherapy (HAIC) is also ongoing in Japan (SILIUS trial). These results are awaited to confirm its usefulness in the daily clinical practice.
Treatment algorithm for HCC and impact of molecular targeted agents
Evidence-based treatment algorithm for HCC in Japan
Treatment algorithm in the west
The treatment algorithms in Europe and North America were published as the European Association For the Study of the Liver (EASL) consensus in 2001,35 and then as the AASLD Clinical Practice Guidelines in Hepatology in 2005.5 Both were prepared based on BCLC staging. The BCLC staging classification consists of stages 0 to D. Palliative treatment only is specified for stage D, while stage 0 is defined as a very-early stage, specifying 2 cm or smaller solitary liver cancers with carcinoma in situ, which corresponds to early HCC in Japan. These are solitary, and resection is desirable when portal pressure and bilirubin levels are normal. When portal hypertension is present, other potentially curative treatments, such as liver transplantation and local treatment, are recommended. For solitary or ≤ 3 HCC, ≤ 3 cm lesions with mild portal hypertension, liver transplantation or local ablation is recommended. These are very strict criteria, and only stages 0 and A are indicated for radical treatments, that is, resection, local ablation, and liver transplantation. The intermediate stage (Stage B) specifies multinodular lesions, and the advanced stage (Stage C) specifies cases with vessel invasion or extrahepatic spread. For Stage B patients, TACE is recommended and for Stage C patients Sorafenib is recommended as a standard of care treatment.
A consensus-based treatment algorithm for HCC proposed by the Japan Society of Hepatology
A Japanese expert panel established a consensus-based treatment algorithm based on therapeutic policies widely used in Japan.50 Since Sorafenib is proved as a standard of care treatment for advanced HCC with major vascular invasion or extrahepatic spread,50 a modified version of this consensus-based algorithm has been proposed.51
The original algorithm first divides cases based on the presence or absence of extrahepatic lesions, liver function, vascular invasion, number of tumors, and tumor size. It also divides treatment options into curative treatments (resection or local ablation), TACE, arterial infusion chemotherapy, liver transplantation, and palliative treatment. The algorithm essentially follows the evidence-based treatment algorithm,3 but treatments widely performed in Japan were included by consensus, even though evidence is not always present.
Resection or local ablation is performed for three or fewer nodules of ≤ 3 cm with no extrahepatic lesion, good liver function, and no vascular invasion. In this group, local ablation or resection is potentially curative and a good prognosis can be expected. Although the number of nodules is three or fewer, when the tumor exceeds 3 cm, resection or TACE is selected. Additional local ablation following transarterial treatment (Lipiodol TACE or HAIC) may increase curability. IFN therapy after curative therapy has proved to be useful for improving patient survival;52 therefore, it is recommended to treat patients with HCV who can tolerate IFN therapy. In the future, Sorafenib may become a first choice of treatment for adjuvant therapy if positive results are obtained by ongoing global clinical trial (STORM trial) (Fig. 11).
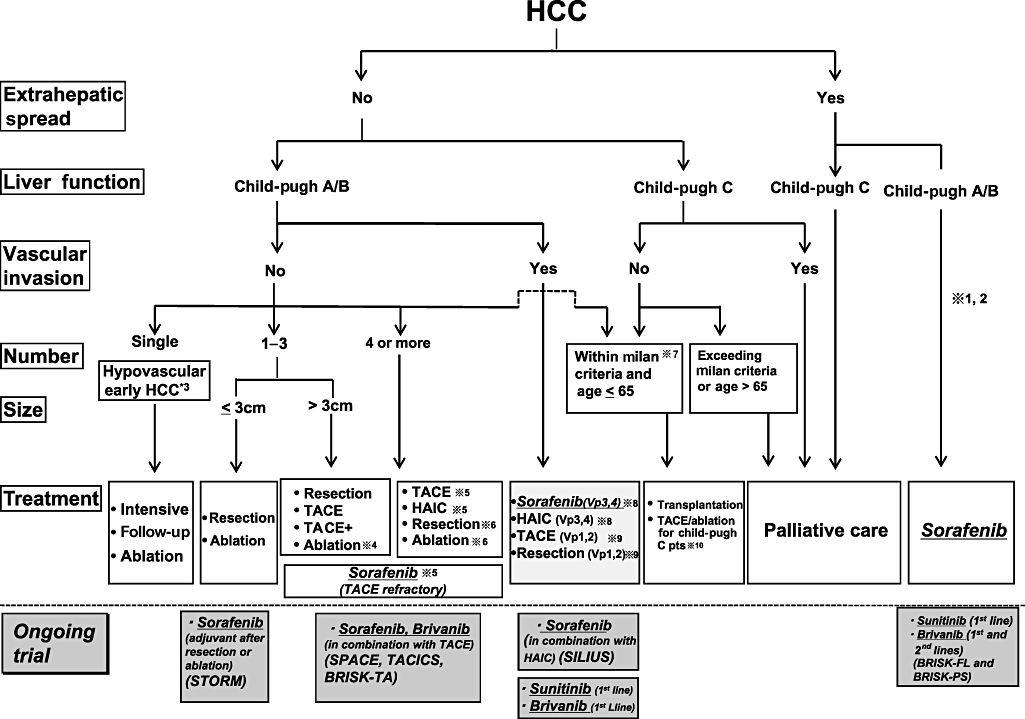
Consensus-based treatment algorithm for hepatocellular carcinoma (HCC) proposed by the Japan Society of Hepatology modified and updated in 2009 from its original version in 2007. Sorafenib is a standard of care for advanced HCC with extrahepatic spread and/or vascular invasion in major branches. Ongoing clinical trials include Sorafenib treatment after resection or ablation (STORM trial), combination therapy of transcatheter arterial chemoembolization (TACE) + Sorafenib (SPACE trial, TACTICS trial) and TACE + Brivanib (BRISK-TA), combination therapy of Sorafenib + Hepatic arterial infusion chemotherapy (HAIC) (SILIUS trial), and finally head-to-head trial between Sorafenib and Sunitinib/Brivanib for advanced HCC.  1: Treatment should be performed as if extrahepatic spread is negative, when extrahepatic spread is not regarded as a prognostic factor.
1: Treatment should be performed as if extrahepatic spread is negative, when extrahepatic spread is not regarded as a prognostic factor.  2: Sorafenib is the first choice of treatment in this setting as a standard of care.
2: Sorafenib is the first choice of treatment in this setting as a standard of care.  3: Intensive follow-up observation is recommended for hypovascular nodules by the Japanese Evidence-Based Clinical Practice Guidelines. However, local ablation therapy is frequently performed in the following cases: (i) when the nodule is diagnosed pathologically as early HCC; (ii) when the nodules show decreased uptake on Gd-EOB-MRI; or (iii) when the nodules show decreased portal flow by computed tomography during arterial portgraphy (CTAP), since these nodules are known to frequently progress to the typical advanced HCC.
3: Intensive follow-up observation is recommended for hypovascular nodules by the Japanese Evidence-Based Clinical Practice Guidelines. However, local ablation therapy is frequently performed in the following cases: (i) when the nodule is diagnosed pathologically as early HCC; (ii) when the nodules show decreased uptake on Gd-EOB-MRI; or (iii) when the nodules show decreased portal flow by computed tomography during arterial portgraphy (CTAP), since these nodules are known to frequently progress to the typical advanced HCC.  4: Even for HCC nodules exceeding 3 cm in diameter, combination therapy of TACE and ablation is frequently performed when resection is not indicated.
4: Even for HCC nodules exceeding 3 cm in diameter, combination therapy of TACE and ablation is frequently performed when resection is not indicated.  5: TACE is the first choice of treatment in this setting. HAIC using an implanted port is also recommended for TACE refractory patients. The regimen for this treatment is usually low-dose fluorouracil platinum (FP) (5FU+CDDP) or intra-arterial 5FU infusion combined with systemic interferon therapy. Sorafenib is also a treatment of choice for TACE refractory patients with Child Pugh A liver function.
5: TACE is the first choice of treatment in this setting. HAIC using an implanted port is also recommended for TACE refractory patients. The regimen for this treatment is usually low-dose fluorouracil platinum (FP) (5FU+CDDP) or intra-arterial 5FU infusion combined with systemic interferon therapy. Sorafenib is also a treatment of choice for TACE refractory patients with Child Pugh A liver function.  6: Resection is sometimes performed even when number of nodules exceeds four. Furthermore, ablation is sometimes performed in combination with TACE.
6: Resection is sometimes performed even when number of nodules exceeds four. Furthermore, ablation is sometimes performed in combination with TACE.  7: Milan criteria: Tumor size ≤ 3 cm and tumor numbers ≤ 3; or solitary tumor ≤ 5 cm. Even when liver function is good (Child-Pugh A/B), transplantation is sometimes considered for relatively younger patients with frequently or early recurring HCC after curative treatments.
7: Milan criteria: Tumor size ≤ 3 cm and tumor numbers ≤ 3; or solitary tumor ≤ 5 cm. Even when liver function is good (Child-Pugh A/B), transplantation is sometimes considered for relatively younger patients with frequently or early recurring HCC after curative treatments.  8: HAIC or Sorafenib is recommended for HCC patients with Vp3 (portal invasion at the 1st portal branch) or Vp4 (portal invasion at the main portal branch).
8: HAIC or Sorafenib is recommended for HCC patients with Vp3 (portal invasion at the 1st portal branch) or Vp4 (portal invasion at the main portal branch).  9: Resection and TACE is frequently performed when portal invasion is minimal, such as Vp1(portal invasion at the 3rd or more peripheral portal branch) or Vp2 (portal invasion at the 2nd portal branch).
9: Resection and TACE is frequently performed when portal invasion is minimal, such as Vp1(portal invasion at the 3rd or more peripheral portal branch) or Vp2 (portal invasion at the 2nd portal branch).  10: Local ablation therapy or subsegmental TACE is performed even for Child-Pugh C patients when transplantation is not indicated when there is no hepatic encephalopathy, no uncontrollable ascites, and a low bilirubin level (< 3.0 mg/dL). However, it is regarded as an experimental treatment since there is no evidence of its survival benefit in Child-Pugh C patients. A prospective study is necessary to clarify this issue. Even in Child-Pugh A/B patients, transplantation is sometimes performed for relatively younger patients with frequently or early recurring HCC after curative treatments.
10: Local ablation therapy or subsegmental TACE is performed even for Child-Pugh C patients when transplantation is not indicated when there is no hepatic encephalopathy, no uncontrollable ascites, and a low bilirubin level (< 3.0 mg/dL). However, it is regarded as an experimental treatment since there is no evidence of its survival benefit in Child-Pugh C patients. A prospective study is necessary to clarify this issue. Even in Child-Pugh A/B patients, transplantation is sometimes performed for relatively younger patients with frequently or early recurring HCC after curative treatments.
For patients with four or more lesions, TACE or HAIC is recommended. Local ablation in combination with TACE or HAIC may be more beneficial for ≤ 5–6 lesions. Sorafenib may be useful as a maintenance therapy between several procedures of TACE in order to reduce the numbers of TACE, thus avoiding the impaired liver function caused by repeated TACE. As a result, it may be beneficial to improve patient survival, but there is not yet solid evidence to support this concept. The positive results of several clinical trials (SPACE trial, TACTIS trial, Brisk-TA trial) (Fig. 11) in this setting awaited before this strategy is introduced in the clinical settings.
For patients with an extrahepatic lesions and good liver functional reserve, Sorafenib is currently the standard of care.
Establishment of an original Japanese treatment algorithm was necessary because the situation in Japan, including the availability of transplantation, is different from that in Western counties. The algorithm established by the Japan Society of Hepatology is not necessarily based on scientific evidence; indeed, consensus-based algorithm was combined with an evidence-based algorithm and opinions of JSH experts. Since it is also difficult to state whether the European or North American algorithm is strictly based on evidence, the JSH consensus-based treatment algorithm may be valid; thus, a treatment algorithm based on large scale of specialists' consensus and treatment strategy performed in real practice in Japan is important. However, this algorithm should be carefully revised through prospective trials for issues lacking evidence.
Ongoing clinical trials with molecular targeted agents
In addition to STORM, SPACE trials and TACTICS trials using Sorafenib in combinations with TACE (see earlier), the SILIUS trial to compare Sorafenib in combination with HAIC is under investigation in Japan. Furthermore, head-to-head trials of Sunitinib versus Sorafenib and Brivanib versus Sorafenib (BRISK-FL trial) for advanced HCC are ongoing globally. Finally, second line trials of Brivanib for Sorafenib failure have been initiated as a global clinical trial (BRISK-PS trial). In addition, Brivanib in combination with TACE (BRISK-TA trial) is also ongoing. The results of all of these trials are eagerly awaited for their hope to provide better outcomes at different stages of HCC (Fig. 11). If positive results are obtained in these trails, the life expectancy at each stage could be much prolonged, at least as calculated theoretically by using hazard ratios incorporated from the SHARP trial. Subanalysis data presented at the ASCO 2008 clearly showed that in HCC patients without vascular invasion or extrahepatic spread hazard ratio of the prolongation of life expectancy is 0.52 and median survival time (MST) is 1.5 times better than placebo arms. If it can be incorporated in earlier stage HCC patients, Sorafenib will prolong the life expectancy approximately 1.5–2.0 times compared with the standard of care group in early and intermediate stage patients (Fig. 12). This could be translated that Sorafenib use in earlier stage in combination with standard of care treatment (resection, ablation, or TACE) will prolong HCC patients' life expectancy (1.5–5.0 years) (Fig. 12).
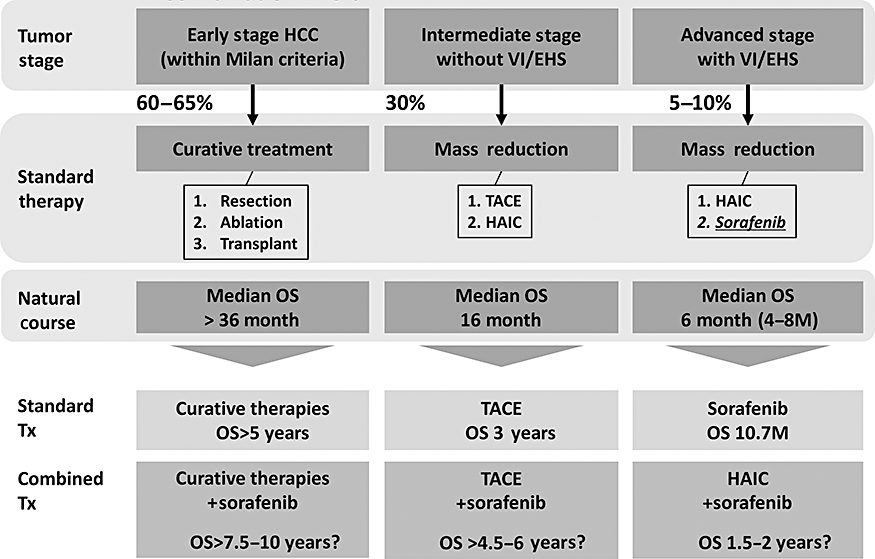
Outcome of standard treatment modality and expected future outcome by combination therapy with Sorafenib or other molecular targeted agents (MTAs). Prolonged life expectancy was calculated as 1.5 to 2.0 times better than the placebo arm by calculating hazard ratio (0.52) and mean survival time (MST) (14.5 vs. 10.2 months) by SHARP Subanalysis study, presented at the American Society of Clinical Oncology Meeting in 2008. For early stage hepatocellular carcinoma (HCC) without vascular invasion (VI) and/or extrahepatic spread (EHS), outcome is expected to be prolonged from MST of 5.0 years to 7.5 to 10.0 years by adjuvant use of Sorafenib after resection or ablation. For intermediate stage HCC without VI or EHS, outcome is expected to be prolonged from 3.0 years to 4.5–6.0 years when combination therapy with transcatheter arterial chemoembolization (TACE) is performed. Similarly, for advanced stage HCC with VI and/or EHS, outcome is expected to be prolonged from 10 months to 1.5–2.0 years when hepatic arterial infusion chemotherapy is combined with Sorafenib. HAIC, hepatic arterial infusion chemotherapy.
Conclusion
In this review, recent progress of the management of HCC, including issues from surveillance to molecular-targeted therapy for HCC, has been reviewed. It is strongly expected that this article will enhance the most up-to-date knowledge on HCC for the readers of the Journal of Gastroenterology and Hepatology.