The Simmet Lecture: New Horizons on an Old Landscape – Oxidative Stress, DNA Damage and Apoptosis in the Male Germ Line
Contents
Our ability to diagnose and treat male infertility is gradually improving in concert with advances in our understanding of the molecular mechanisms underpinning defective sperm function. In this context, one of the factors to emerge as a major causative agent in male infertility is oxidative stress. Spermatozoa are particularly susceptible to such stress because they are exceptionally rich in vulnerable substrates such as polyunsaturated fatty acids, proteins and DNA. The lack of sperm cytoplasm also provides these cells with little capacity to protect themselves from oxidative attack or to effect any repair, should damage occur. Similarly, sperm chromatin is in a quasi-crystalline state and has very little capacity to respond to any DNA damage induced by oxidative attack. When the latter does occur, it appears to be initiated by reactive oxygen species (ROS) generated by the sperm mitochondria. These free radicals attack the lipids present in the sperm mitochondria generating electrophilic aldehydes, which bind to components of the mitochondrial electron transport chain stimulating yet more ROS production. The oxidative stress created via this self-propagating mechanism initiates an apoptotic cascade as a result of which the spermatozoa loose their capacity for fertilization and suffer damage to their DNA. Phosphatidylserine externalization is a late event in sperm apoptosis and may facilitate the silent phagocytosis of moribund cells in the female reproductive tract, that is, the phagocytosis of senescent spermatozoa without the accompanying generation of an inflammatory response. Encouragingly, the involvement of oxidative stress in the aetiology of male infertility has opened up new opportunities for therapeutic interventions involving the judicious administration of nucleophiles and other forms of antioxidants.
Introduction
In the beginning, our approach to diagnostic andrology was entirely descriptive and based upon the fundamental tenet that fertility could be defined in terms of the number of motile, morphologically normal spermatozoa present in the ejaculate. With the passage of time, we have come to appreciate that fertility is not primarily a ‘numbers game’; rather, it is the quality of the spermatozoa that is all important. There is probably no better demonstration of the veracity of this maxim than the unexpected fertility of men receiving steroid treatment in the context of contraceptive development. In such men, azoospermia can be reached in approximately 60% of treated subjects depending on the regime used, but the remainder exhibit severe oligozoospermia. Under these conditions, pregnancies have been repeatedly reported in men exhibiting sperm counts of <5 million/ml, which in an infertility clinic, would have been regarded as severely pathological (Barfield et al. 1979; Wallace et al. 1992). The difference between the infertility patient and the male treated with exogenous steroids is the difference between a quantitative and a qualitative decrease in sperm numbers. In donors treated with steroids, spermatogenesis is qualitatively normal, but quantitatively suppressed because of a diminished level of gonadotrophin support. By contrast, the endocrine profile of male patients attending infertility clinics is generally normal, only 1.7% possessing a clinically important endocrinopathy (Sigman and Jarow 1997). However, in such cases, the response of the testes to gonadotrophic stimulation is generally impaired via mechanisms that reduce the quality of spermatogenesis, resulting in generation of defective spermatozoa. It is for this reason that most infertile males possess relatively normal sperm counts, and sperm number per se is a relatively weak criterion for the prediction of fertility (Guzick et al. 2001). It is also for this reason that the pooling of successive ejaculates from oligozoospermic patients never generates a positive therapeutic outcome; whereas the number of spermatozoa available for insemination is clearly increased, the quality of the gametes remains unchanged, and it is the latter that defines fertility (te Velde et al. 1989; Tur-Kaspa et al. 1990).
The same argument applies to sperm morphology. This is a better criterion for predicting fertility than sperm count (Guzick et al. 2001). However, fundamental questions remain about what constitutes a ‘normal’ spermatozoon and the extent to which sperm morphology directly reflects the functionality of a given cell or indirectly reflects the quality of the spermatogenetic process that generated it. Although progress has been made in standardizing sperm morphology assessments in man and animals, the subjective nature of such assessments is a major impediment to their clinical application. Attempts to achieve a higher level of standardization by automating such analyses have not been widely adopted largely because the existing technology lacks repeatability and cannot discriminate those elements of sperm morphology that define fertility – although improvements in this area, such as the ability to measure the dimensions of the sperm head and tail, are emerging (Thurston et al. 1999; Marco-Jiménez et al. 2010; Hoogewijs et al. 2011). It should also be recognized that automated sperm morphology assessments are significantly influenced by methods used to prepare, fix and stain the spermatozoa, which have to be independently optimized for every species (Maree et al. 2010).
To some extent, such deficiencies in the morphological assessment of spermatozoa may be overcome by using stains to highlight particularly important elements of sperm morphology, for example aneuploidy (Carrell and Emery 2008) or retention of excess residual cytoplasm (Gomez et al. 1996). The development of fluorescent stains has led naturally to the use of flow cytometry protocols to analyse specific attributes of sperm structure and quality, including those reflecting sperm viability, acrosomal status, capacitation, mitochondrial membrane potential, reactive oxygen species generation (ROS), lipid peroxidation, apoptotic markers and DNA damage (Martínez-Pastor et al. 2010).
Sperm motility is another component of the descriptive semen profile, which is clearly correlated with fertility and can be accurately measured in a wide variety of species using CASA (Computer Aided Semen Analysis) systems. Many such systems are available but the most widely used product is the Hamilton Thorne Sperm Analyzer (Hamilton Thorne Inc., Beverly, MA, USA). With the aid of CASA, the trajectories of spermatozoa can be objectively assessed and correlations sought between particular aspects of sperm movement and fertility. The anticipated positive correlations between movement and fertility have been observed, first with human spermatozoa (Marshburn et al. 1992; Hirano et al. 2001) and then extending to large domestic species such as the stallion (Love 2011), bull (Kathiravan et al. 2011) and boar (Broekhuijse et al. 2012). These correlations are often weak, however, and in the ram are not statistically significant, at least when applied to frozen thawed spermatozoa (O’ Meara et al. 2008).
One of the key issues to be addressed when considering the relationship between sperm movement and fertility is that the motility of mammalian spermatozoa is not a single constant entity, but is constantly changing in response to the physiological demands of the cell. Thus, in vaginal inseminators such as the human, a particular kind of forward progressive movement, featuring a moderate amplitude, symmetrical flagellar wave form is needed to penetrate cervical mucus. Reflecting this, infertile human patients have been identified in which the only detectable defect is an inability to penetrate cervical mucus coupled with an inadequate flagellar wave amplitude, as reflected in ALH (Amplitude of Lateral Head displacement) (Aitken et al. 1985, 1986; Feneux et al. 1985). Wave amplitude is important for mucus penetration, because it determines the propulsive force that can be generated by the spermatozoa as they enter the mucus matrix. A similar moderate amplitude, flagellar beat pattern, is probably required to penetrate cervical mucus in all species utilizing an intra-vaginal mode of insemination (Cox et al. 2006). The attributes of sperm movement required to penetrate the utero-tubal junction in intra-uterine inseminators may well be similar but has been difficult to establish experimentally.
During the later stages of sperm transport, motility is suppressed in such a way that these cells accumulate as a sperm reservoir in the isthmic region of the Fallopian tubes for 18–20 h (Hunter and Wilmut 1984) and then bind to the oviductal epithelium in a state of relative quiescence (Suarez 2008). Then, in response to uncharacterized signals associated with ovulation, the spermatozoa are induced to activate and complete the process of capacitation. As a consequence of the latter, the spermatozoa are able to break away from the oviductal epithelium in a hyperactivated state. This specific form of motility, characterized by high-frequency high-amplitude, asymmetric flagellar waves facilitates the release of the spermatozoa away from the oviductal epithlium and subsequent penetration of the vestments surrounding the oocyte, including the cumulus mass and zona pellucida (Suarez, 2008). The measurement of hyperactivated movement can be achieved by CASA (Sukcharoen et al. 1995) although problems encountered with the reliable induction of hyperactivation in vitro and the intermittent nature of this phenomenon in some species make it a difficult diagnostic criterion to apply.
Biologically, this highly specialized sequence of changes in the movement characteristics of mammalian spermatozoa during their transport through the female reproductive tract and fertilization of the oocyte highlights a major conundrum in the diagnosis of male fertility. It is an inevitable feature of this process that the analysis of sperm function involves taking of measurements from recently ejaculated spermatozoa that will ultimately predict how these cells will behave during their subsequent ascent of the female reproductive tract and interaction with the cumulus-oocyte complex. It is not unlike trying to predict the lifetime career achievements of adults, by watching how they behaved as children in the school playground.
The answer to this problem must lie in an increased awareness of the molecular mechanisms regulating normal sperm function and an understanding of how these cells become disrupted in cases of infertility. In this context, one of the most important advances in recent years has been the discovery that one of the major players in defective sperm function is oxidative stress.
Oxidative Stress
The idea that oxidative stress plays a major role in the aetiology of male infertility can be traced back to a landmark paper by John MacLeod, which demonstrated that human spermatozoa incubated under high oxygen tensions exhibited a loss of motility that could be negated by the addition of catalase to the incubation medium (MacLeod 1943). The clear implication of this research was that hydrogen peroxide generated as a by-product of sperm metabolism has a suppressive effect on oxygen consumption and motility. Further support for this notion was secured by Tosic and Walton in a publication that appeared in Nature in 1946, identifying hydrogen peroxide as the molecule responsible for suppressing the metabolism of bull spermatozoa (Tosic and Walton 1946). These authors subsequently confirmed the generation of hydrogen peroxide by boar, ram and bull spermatozoa and experimentally demonstrated the toxicity of this compound in vitro (Tosic and Walton 1950). The particularly toxic action of hydrogen peroxide was further emphasized years later when an in vitro system was used to create a complex mixture of ROS that were extremely effective in suppressing the motility of human spermatozoa. The inhibitory action of ROS in this situation could be completely reversed by catalase (a hydrogen peroxide scavenger), whereas superoxide dismutase (a superoxide scavenger) had no effect, clearly emphasizing the importance of the former in the disruption of sperm function (Aitken et al. 1993).
Mammalian spermatozoa were therefore the first cell type in which the cellular generation of ROS was confirmed, predating the discovery of this activity in leucocytes by more than a decade. Since Tosic and Waltons’ original report, the cellular production of ROS has been confirmed in the spermatozoa of a wide variety of mammals including man, mouse, hamster, rat, rabbit and horse (Aitken and Curry 2011). In many ways, this potential for ROS production is unexpected, given the susceptibility of mammalian spermatozoa to oxidative stress. These cells are well known to possess particularly high concentrations of polyunsaturated fatty acids, particularly docosahexaenoic acid (22:6), which are very vulnerable to attack by reactive oxygen metabolites resulting in the initiation of lipid peroxidation cascades. As a consequence of such peroxidative damage, the sperm plasma membrane loses its fluidity and, as a result, the spermatozoa lose their functional integrity. In addition, ROS are capable of attacking the DNA in the sperm nucleus and mitochondria generating high levels of potentially mutagenic oxidized base adducts such as 8-hydroxy, 2-deoxyguanosine (8OHdG) (De Iuliis et al. 2009b). Indeed, most of the DNA damage in spermatozoa appears to be oxidatively induced, at least as far as human spermatozoa are concerned (De Iuliis et al. 2009b). Overall, peroxidative damage appears to be responsible for all of the functional defects we see in defective spermatozoa including impaired sperm motility, a reduced capacity for sperm–oocyte fusion and substantial losses of DNA integrity (Aitken and Clarkson 1987; Aitken 1999; Aitken and Curry 2011).
Mitochondria as mediators of oxidative stress
The ROS responsible for the disruption of mammalian sperm function appear to come from the mitochondria. Definitive evidence that spermatozoa generate superoxide anion has been secured, by virtue of the ability of these cells to orchestrate the superoxide dependent conversion of dihydroethidium (DHE) to 2-hydroxyethidium (De Iuliis et al. 2006). Originally, we thought that this superoxide anion came from a non-mitochondrial source because of its resistance to reagents that collapse the mitochondrial membrane potential, namely rotenone and CCCP (carbonyl cyanide m-chlorophenylhydrazone) (De Iuliis et al. 2006). However, we subsequently learned that the measurement of cellular superoxide generation with DHE is, in fact, highly correlated with the generation of superoxide from the sperm mitochondria, as measured with MitoSox red (MSR) (Koppers et al. 2008). Indeed, the correlation between the spontaneous MSR and DHE signals generated by human spermatozoa are so tight that we have concluded that mitochondria represent the major source of ROS in the spermatozoa of most, if not all, mammalian species (Chapman et al. 1985; Koppers et al. 2008; Storey 2008). However, the mechanisms responsible for mitochondrial ROS generation in spermatozoa are unusual in that they do not depend on the existence of mitochondrial membrane potential. Rather, mitochondrial ROS generation increases when the membrane potential is collapsed pharmacologically (Koppers et al. 2008). In the light of these data, it is obviously critical to understand what cellular factors normally serve as triggers for free radical generation by sperm mitochondria.
The answer to this question came from an unexpected source. In another research project, we have been studying the impact of various quinones on the motility of human spermatozoa as an approach to male contraception (Hughes et al. 2009). In the course of these studies, we found that simple quinones such as 1,4 benzoquinone were capable of activating ROS generation by human spermatozoa. Further investigation of this phenomenon revealed that a wide variety of synthetic electrophiles (N-ethylmaleimide, iodoacetamide, ethyl vinyl ketone) could elicit the generation of ROS by populations of human spermatozoa (Aitken and Whiting, unpublished observations). Even more importantly, naturally occurring electrophiles such as acrolein or 4-hydroxynonenal (4HNE) could elicit ROS generation by human sperm mitochondria by binding to flavoprotein constituents of the mitochondrial electron transport chain. The ability of electrophiles to stimulate ROS generation by sperm mitochondria is not just an in vitro phenomenon because the spontaneous generation of mitochondrial ROS by human spermatozoa is highly correlated (R2 = 0.85) with the 4HNE content of these cells (Aitken and Whiting, unpublished observations). The relevance of these findings lies on the fact that 4HNE is a major breakdown product of lipid peroxide formation. As a result, we can propose a self-perpetuating system for mitochondrial free radical generation, which is initiated by the induction of lipid peroxidation cascades that culminate in the generation of small molecular mass electrophilic aldehydes such as 4HNE (Fig. 1). The latter then bind to key elements of the electron transport chain, impeding the flow of electrons from NADH to water and resulting in the generation of superoxide anion as a consequence of electron leakage to oxygen. Experimentally, we can recapitulate the oxidative stress we see in the spermatozoa of infertile patients by adding a known inhibitor of the mitochondrial electron transport chain, such as rotenone (an inhibitor of electron transfer from FeSN-2 cluster to ubiquinone) to the sperm suspension. Under these circumstances, rotenone can stimulate a significant time-dependent increase in the percentage of MSR-positive cells as well as a corresponding increase in lipid peroxide formation in the sperm midpiece, where the mitochondria are located (Koppers et al. 2008).
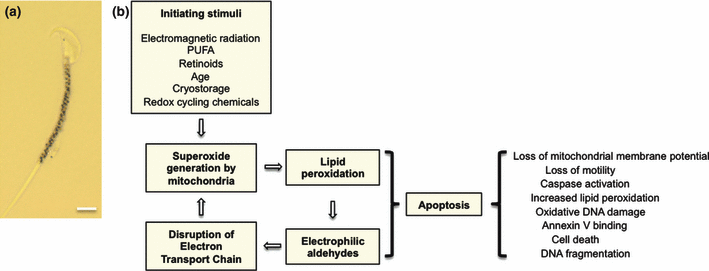
Mitochondria as the regulators of sperm function and fate. (a) mitochondrial gyres stained with MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) in the midpiece of a mouse spermatozoon. Scale bar = 3.5 μm. (b) scheme for the generation of oxidative stress in mammalian spermatozoa. According to this model, mitochondrial ROS generation can be triggered by a range of metabolic and environmental factors. Once initiated, the electrophilic aldehydes generated from the breakdown of lipid peroxides perpetuate the generation of ROS by interfering with the flow of electrons along the mitochondrial electron transport chain. This process accentuates the generation of ROS and results in the initiation of an intrinsic apoptotic cascade as a consequence of which the spermatozoa lose their motility and damage their DNA. Phosphatidylserine exposure late in the apoptotic process ensures the efficient, ‘silent’ removal of senescent, moribund spermatozoa by the maternal immune system. It is possible that premature activation of this process may result in low level leucocytic infiltration into the male tract
The idea that ROS generation by human spermatozoa is attributed to the ability of electrophilic aldehydes to form adducts with flavoprotein components of the mitochondrial electron transport chain is also consistent with the observation that the flavoprotein inhibitor DPI (diphenylene iodonium) can suppress spontaneous ROS generation by mouse, human and stallion spermatozoa (Ecroyd et al. 2003; Aitken et al. 2004; Sabeur and Ball 2006). In addition, the strong relationship that exists between spontaneous ROS generation and the free polyunsaturated fatty acid (PUFA) content of human spermatozoa is also consistent with the idea that the products of lipid peroxidation are instrumental in stimulating mitochondrial ROS generation (Koppers et al. 2010) because the PUFA would provide ample substrate for lipid peroxide formation. The idea that lipid peroxide formation triggers mitochondrial ROS in a self-perpetuating cycle is also consistent with the observation that exposure of human spermatozoa to ROS such as hydrogen peroxide triggers yet more ROS generation (du Plessis et al. 2010). It is also in keeping with the positive effect that exposure to activated, free radical-generating leucocytes has on mitochondrial ROS generation by spermatozoa (Zorn et al. 2010) and the high levels of ROS generation by spermatozoa in association with the oxidative stresses associated with increasing age (Weir and Robaire 2007; Cocuzza et al. 2008; Suresh et al. 2010).
Initiation of the Peroxidative Process
If mitochondrial ROS generation is triggered by aldehydes such as 4HNE, then factors responsible for the initiation of lipid peroxidation in the first instance need to be resolved. The initiating factors are probably many and various and include transition metals such as iron, copper, lead or cadmium (Jones et al. 1979; Aitken et al. 1989; Kiziler et al. 2007) as well as long chain hydrophobic molecules such as retinoids or cis-unsaturated fatty acids (Aitken et al. 2006), which can intercalate into the mitochondrial membranes and perturb electron flow. In addition, treatments such as cryostorage (Peris et al. 2007; Thomson et al. 2009; Kim et al. 2010), deficiencies in antioxidant protection (Aitken and Curry 2011) exposure to redox cycling compounds such as quinones (De Iuliis et al. 2006) or catechol estrogens (Bennetts et al. 2008) as well as certain physical factors such as the mechanical shearing forces associated with centrifugation (Agarwal et al. 1994) or electromagnetic radiation of various kinds, including heat, visible or ultraviolet C light (De Iuliis et al. 2009a; Lavi et al. 2010; Torres et al. 2010) may also trigger ROS and lipid peroxidation directly.
Although most of these factors have been analysed for their direct effects on spermatozoa, it is also possible that reagents that impede the differentiation of spermatozoa during spermiogenesis or their maturation in the epididymis can also have an indirect effect on the spontaneous ability of these cells to generate ROS. Thus, repeated studies have shown that the ROS-generating capacity of spermatozoa is correlated with their morphology (Aitken and Curry 2011; Chen et al. 2012). Of all the elements of sperm morphology that have been assessed, the retention of excess residual cytoplasm in the midpiece has shown a particularly strong relationship with the ROS-generating capacity of the spermatozoa (Aitken et al. 1994; Gomez et al. 1996). One possible reason for this association is that the retention of excess residual cytoplasm is correlated with the presence of relatively high levels of cytoplasmic enzymes, including glucose-6-phosphate dehydrogenase (G6PDH) (Gomez et al. 1996). The latter is a key enzyme in the hexose monophosphate shunt and would be expected in increase the availability of NADPH in these morphologically abnormal cells. The presence of NADPH is, in turn, associated with the generation of ROS via a flavoprotein-mediated process that could involve the NADPH oxidases in spermatozoa including, possibly NADPH oxidases that are located in the mitochondria (Aitken et al. 1997; Baker and Aitken 2004; Urner and Sakkas 2005; Graham et al. 2010).
Apoptosis
The induction of mitochondrial ROS generation is the first step in the activation of an intrinsic apoptotic cascade that is associated with the induction of a series of changes in the cell that (i) prevent the spermatozoa from participating in the process of fertilization and (ii) prepare the gamete for phagocytosis by the maternal immune system. One of the early changes precipitated by this process is the induction of lipid peroxidation in the mitochondrial membranes and a loss of mitochondrial membrane potential (Koppers et al. 2008, 2011). This loss of mitochondrial membrane potential is associated with a loss of sperm motility; however, the power of this association is highly species dependent because there is considerable inter-species variation in the extent to which the spermatozoa are dependent on oxidative phosphorylation for ATP generation, with equine, bovine and porcine spermatozoa being particularly susceptible to changes in mitochondrial function (Storey 2008; Paoli et al. 2011). In addition to the loss of motility, apoptosis also induces an increase in oxidative DNA damage in mammalian spermatozoa (Koppers et al. 2011). Indeed, most DNA damage in the male germ line appears to be oxidatively induced in the first instance (De Iuliis et al. 2009b). Secondarily, the formation of oxidative base adducts weakens the DNA by labilizing the glycosyl bond that attaches the base to the ribose unit, leading to the loss of the affected base and the generation of an abasic site. Abasic sites are well known to have a strong destabilizing effect on the DNA backbone locally, which can result in strand breaks. As a result of this cascade of events, DNA strand breaks detectable with, say, the TUNEL assay, occur very late in the apoptotic process and often represent a perimortem change, at least in human spermatozoa (Mitchell et al. 2011).
In considering the role of apoptosis in sperm cell biology, it should be recognized that their physical architecture and, particularly, their high level of compartmentalization, shape the features of this process in the male gamete. Thus, even though caspases may become activated and phosphatidylserine may be exteriorized, endonuclease-mediated DNA cleavage is prevented because nucleases activated in the cytoplasm such as CAD (caspase activated DNase) or released from the mitochondria, including AIF (apoptosis inducing factor) and Endo G (endonuclease G), remain resolutely locked in the midpiece of the cells after apoptosis has been activated in mature spermatozoa (Koppers et al. 2011). In addition, even if nucleases were to reach the sperm nucleus, they could not penetrate this structure because sperm DNA is so tightly compacted that it is in an almost crystalline state. The only product generated during the intrinsic apoptotic cascade that can induce DNA damage in the sperm nucleus is the hydrogen peroxide released from the sperm mitochondria, which, because of its small size and lack of charge, can readily move from the midpiece to the sperm head and penetrate the nucleus. This is presumably why a majority of the DNA damage in mature human spermatozoa is oxidative in nature, irrespective of whether apoptosis is involved or not. Perimortem, as the physical structure of the spermatozoon starts to collapse, nucleases released by the dying cell or possibly delivered by the reproductive tract may result in the final destruction of the DNA prior to phagocytosis (Sotolongo et al. 2005; Dominguez and Ward 2009). DNA strand breakage and cell death are thus tightly correlated in the male germ line. In situations where live cells are carrying DNA damage (which is the critical factor, clinically), it is usually in the form of DNA adducts or abasic sites that can be detected with assays such as SCSA (sperm chromatin structure assay) or COMET.
The induction of DNA damage as a result of oxidative stress underpins paternal effects on embryonic growth and development as well as the health and wellbeing of the offspring (Aitken et al. 2009). For example, DNA damage in spermatozoa has been correlated with a wide range of pathologies in man and animals, ranging from miscarriage to cancers and neurological defects (autism, spontaneous schizophrenia, bipolar disease and epilepsy) in children (Aitken et al. 2009). A recent variant on this theme has been accumulating evidence that epigenetic changes can also be transmitted through the male germ line that have a major impact on the health trajectory of the offspring (Trasler 2009). A recent example of this phenomenon is evidence of metabolic disease in the female offspring of male rats made obese on a high-fat diet (Ng et al. 2010). Whether there is a functional link between oxidative stress in the germ line and epigenetic defects in the offspring is currently unknown, although the fact that obesity is linked with oxidative stress in spermatozoa is certainly suggestive (Bakos et al. 2011).
Finally, one of the late changes in sperm biochemistry that occurs during the apoptotic cascade is exteriorization of phosphatidylserine as evidenced by Annexin V binding to viable spermatozoa (Koppers et al. 2011). This change may be related to the ultimate phagocytosis of the spermatozoa in the female reproductive tract. Following insemination in mammals, there is a massive leucocytic infiltration into the female tract, to remove dead and senescent spermatozoa. It is important that this process is ‘silent’ in the sense that there is no full-blown phlogistic response to the presence of these potentially antigenic cells. Phosphatidylserine externalization plays a major role in informing the infiltrating leucocytes that they should not mount an inflammatory response against a phagocytized cell (Rossi and Aitken 1997). It is also possible that the apoptotic cascade initiated by mitochondrial ROS generation in spermatozoa serves to induce the leucocytic infiltration that leads to this phagocytosis. Moreover, it may be no coincidence that male infertility is frequently associated with a modest infiltration of leucocytes into the male reproductive tract (Henkel et al. 2005; Pelliccione et al. 2009; Tremellen and Tunc 2010; Zorn et al. 2010). Under these circumstances, defective spermatozoa may be prematurely undergoing senescence in the excurrent duct system of the male reproductive tract that they were supposed to undergo in the female tract. The only spermatozoa avoiding this fate will be the small minority that successfully engage the process of fertilization.
Implications for treatment
If oxidative stress is such an important cause of male infertility, then there is every possibility that antioxidants will be part of the cure (Gharagozloo and Aitken 2011). Although most of this research has been conducted in the context of human infertility, much progress has also been made in large domestic animals. For example, for many years, catalase has been used as an additive to the cryostorage media used for bovine spermatozoa (Shannon and Curson 1982; Fernández-Santos et al. 2009). More recent studies have demonstrated that the presence of antioxidant additives such as ascorbate, carnitine, cysteine, inositol, manganese or vitamin V12 during cryostorage improves bovine sperm motility, while methionine, carnitine and inositol improves DNA integrity (Cheema et al. 2009; Sariözkan et al. 2009; Bucak et al. 2010; Hu et al. 2010, 2011). Similar data are also available for stallion and boar spermatozoa, emphasizing the universality of oxidative stress as the agent provocateur of sperm senescence in mammalian spermatozoa (Bathgate 2011; Contri et al. 2011; da Silva et al. 2011).
Conclusions
We stand on the shoulders of giants. Just as Dr Ludwig Simmet was a pioneer in the field of assisted conception as applied to domestic animals, Thaddeus Mann was incontrovertibly the grandfather of semen biochemistry who, in addition to his authoritative textbooks on the subject (Mann 1954; Mann and Lutwak-Mann 1981), pioneered the idea that lipid peroxidation is a major contributor to defective sperm function. He also highlighted the vulnerability of these cells to oxidative stress because of their high PUFA content and their lack of endogenous antioxidant protection (Jones et al. 1979). In the 1980s, Bayard Storey and colleagues emphasized the importance of sperm mitochondria as sources of ROS in mammalian spermatozoa and the significant differences between species in the way in which their redox balance is maintained (Storey 2008). We now have a much deeper understanding of the molecular mechanisms responsible for activating ROS generation by the mitochondria and can see this activity as part of a carefully regulated programme of cellular redox regulation controlling sperm function. When ROS generation is low, it drives the process of sperm capacitation by enhancing the tyrosine phosphorylation cascade characteristic of this process (de Lamirande et al. 1997; Aitken et al. 1998; de Lamirande and O’Flaherty 2008). However, the constant generation of ROS eventually overwhelms the limited antioxidant defence characteristic of spermatozoa and triggers a self-perpetuating apoptotic cascade that results in motility loss and DNA damage (Aitken 2011). Ultimately, this process culminates in a regulated cell death that prepares the spermatozoa for phagocytosis by leucocytes in the female reproductive tract. If electrophilic aldehydes generated as a consequence of oxidative stress are instrumental in triggering mitochondrial ROS generation and apoptosis then, antioxidants, particularly nucleophiles, should be of some value in counteracting this highway to cellular senescence in the development of optimized assisted conception procedures for domestic animals. However, any application of antioxidants in this context would have to be considered carefully, because it would be important not to suppress ROS generation so completely or irreversibly that capacitation could not occur.
Acknowledgements
I gratefully acknowledge the funding from the ARC, NHMRC and Priority Research Centre in Reproductive Science, University of Newcastle that made this research possible. I am also grateful to Sarah Whiting and Sarah Lambourne for technical assistance and Jody Powell for managing our donor panel.
Conflicts of interest
None of the authors have any conflicts of interest to declare.
Author contribution
The author conceived and prepared this review.




