The Membrane of the Mammalian Spermatozoa: Much More Than an Inert Envelope
Contents
Sperm plasma membrane is a very important structure that functions to protect sperm against extracellular injuries and to respond to physiological challenges. It plays a crucial role during sperm capacitation, in sperm–egg interaction and, finally, in fertilization. Concerning sperm technology, possibly the most important factors causing damage in mammalian spermatozoa membranes are initiated by the osmotic stress generated by dehydration of the cells during freezing and thawing. These changes are rapidly derived to the plasma and organelle membranes that gradually experiment loss of membrane architecture, causing unbalanced production of reactive oxygen species and increased lipid peroxidation. Other procedures such as sperm sorting or liquid storage of sperm also induce harmful changes in the integrity of the membrane. The specific composition of lipids of the sperm membranes may provide clues for understanding the mechanisms behind the differences found in the response to stress in different species. In the present review, we deal with the composition, architecture and organization of the sperm plasma membrane, emphasizing the factors that can affect membrane integrity. The intracellular signalling pathways related with membrane reorganization during capacitation and acrosome reaction are also reviewed.
The Composition of the Plasma Membrane
The lipid composition of the plasma membrane of mammalian sperm is relatively different from somatic cells. Early analysis of these lipids developed in the late nineteenth Century in mammals and birds sperm showed the presence of neutral fatty acids, phospholipids and glycolipids. Further analysis has shown that glycerophospholipids (GPLs) are the most representative fraction of sperm membrane and, among them, phosphatidylcholine and phosphatidylethanolamine are predominant, with no significant differences between species (Parks and Lynch 1992). GPLs are characterized by a glycerol backbone with the hydroxyls at C1 and C2 esterified to fatty acids, and the C3 hydroxyl of glycerol is esterified to phosphate. However, a fairly high proportion of these phospholipids are composed of plasmalogens, which contain one fatty acid esterified with glycerol and an unsaturated ester with a long carbon chain (C-20; C-24). The hydrolysis of plasmalogen yields a fatty aldehyde and a fatty acid (Lenzi et al. 2000). Plasmalogens more than likely possess the ability to eliminate free radicals (Fuchs et al. 2007), as will be discussed later.
The main difference in the composition of the phospholipids in the biological membranes is represented by the fatty acids of which are composed and, more importantly, by the degree of saturation of these fatty acids. Something similar happens in sperm membranes. The fatty acid most often found in the phospholipids of pig sperm membranes is the C22:6 n-3 (Cerolini et al. 2000), in rooster is the C22:4 n-6 (Bongalhardo et al. 2002), C22:6 n-3 in bull (Schiller et al. 2003), C22:6 n-3 in humans (Khosrowbeygi and Zarghami 2007) and C22:5 n-6 in stallion (Parks and Lynch 1992; Garcia et al. 2011). In view of these reports, it is clear that sperm cells show surprisingly high levels of unsaturated acyl chains, particularly docosahexanoyl (22:6) and docosapentaenoyl residues (22:5). These highly unsaturated acyl chains confer great fluidity to the sperm membrane and, therefore, greater elasticity to resist changes in volume. However, this makes spermatozoa particularly vulnerable to attack by reactive oxygen species (ROS), thus are more susceptible to experiment lipid peroxidation (LPO) (Wathes et al. 2007). In a previous study, we evaluated individual differences in fatty acids composition of the membrane of stallion spermatozoa, and we found significant correlations of this particular lipid composition with sperm quality. In general terms, higher saturation of the phospholipids of the sperm cell membrane was correlated with poor sperm quality. This was especially relevant to C16:0 (palmitic), C18:0 (stearic) and C20:0 (arachidic). Conversely, the proportion of highly polyunsaturated fatty acids (HUFAs) was correlated positively with the proportion of spermatozoa showing intact membranes (Garcia et al. 2011). This was especially evident for C22:5 n-6 (docosapentanoic). This finding may be related to the ability of this fatty acid to provide fluidity to the sperm membrane. While long chain, saturated fatty acids increase the rigidity of the membrane, the polyunsaturation of the fatty acids gives a more physiological, flexible and fluid membrane (Gadella et al. 2008; Garcia et al. 2011).
As mentioned earlier, the majority of natural GPLs is characterized by ester linkages, by which the fatty acyl residues are connected with the glycerol backbone. However, in many cells and tissues, there are also GPLs with alkyl and alkenyl linkages. Plasmalogens (ether-linked phospholipids) are one particularly important lipid class and it was estimated that the percentage of plasmalogens is approximately 18% of the total GPL mass in human cells (Nagan and Zoeller 2001; Fuchs et al. 2007). Spermatozoa are unique among mammalian cells in that they contain even higher levels of plasmalogens (Gorgas et al. 2006; Fuchs et al. 2007; Oresti et al. 2011). In a previous study from our laboratory in stallion spermatozoa, we obtained similar observations, with plasmalogens representing on average 25% of the phospholipids of the membranes of stallion spermatozoa (Garcia et al. 2011). The considerable plasmalogen content is also a common feature of spermatozoa from human (Lessig et al. 2004), boar (Lessig et al. 2004), rat (Oresti et al. 2011) and ruminants such as ram (Hinkovska et al. 1986), cattle (Fuchs et al. 2007, 2009), roe deer (Fuchs et al. 2007, 2009) and others (Fuchs et al. 2009). The exception to this seems to be represented by the feloideae species, including cats, whose phospholipid composition of sperm is clearly dominated by diacyl phospholipids and contains only marginal amounts of plasmalogens (Fuchs et al. 2009).
The role of these compounds in the sperm membrane is still unknown, but ether-lipids were suggested to contribute to both the formation of the macro- and microdomains required for the compartmentalization of the highly polarized sperm membrane (Gorgas et al. 2006). Indeed, in ejaculated rat spermatozoa, the distribution of proportion of plasmalogens within the head and the tail showed dissimilarities, being much higher in the head (Oresti et al. 2011). In a recent study in horses, we found that the percentage of plasmalogens in the phospholipids of the membranes in freshly ejaculated spermatozoa was good forecasts for both good and bad potential freezers. The percentage of C18 plasmalogens predicts potential freezeability the best and was negatively correlated with LPO post-thaw. On the contrary, the percentage of C16 plasmalogens and total plasmalogens were better diagnostic markers for low freezability (Macias et al. 2011). To the best of our knowledge, there are no additional studies addressing the ability of plasmalogens to predict sperm freezeability in other species; therefore, it clearly seems premature to generalize this theory to other mammalian species. The mechanisms underlying such differences in horses are still largely unknown, but could be reflecting the antioxidant properties of plasmalogens (Nagan and Zoeller 2001; Fuchs et al. 2007), hypothesis that is reinforced by the negative correlation of C18 plasmalogens with LPO post-thaw found in our study (Macias et al. 2011).
Overall, even knowing that differences may occur among species, the reports described above are emphasizing that sperm functions are dependent, at least in part, on their plasmalogens composition, pointing once again to the dissimilar importance of specific components of the sperm plasma membrane for its ability to response to both external injuries and physiological challenges. Moreover, approaches such as antioxidant supplementation and/or nutritional strategies to modulate the lipid compositions of sperm membranes may help to increase the quality of semen in a number of species in term of resistance to the reproductive technologies such as sperm freezing, sperm selection and sperm sorting (Hossain et al. 2011; Macias et al. 2011; Peña et al. 2011).
In addition to phospholipids, cholesterol is one of the most important components of the plasma membrane, being the most abundant lipid per se. Ninety per cent of cellular cholesterol is found in the plasma membrane and represents a percentage ranging between 25% and 50% of total lipids, depending on cell type (Moce et al. 2010a). Cholesterol has many effects on membrane properties and plays different roles such as stabilizing the membrane (Aksoy et al. 2010), control the size and area fraction of phase transition of the domains (Thompson et al. 1995), modulate the activity of some transmembrane proteins (Tannert et al. 2007) and facilitate interactions between cells mediated by changes in phase transition of phospholipid bilayers (Travis and Kopf 2002). Changes in either sperm cholesterol or phospholipid levels might serve to alter the membrane cholesterol : phospholipid molar ratio, which has been implicated in the regulation of capacitation (Travis and Kopf 2002; Moce et al. 2010a; Saez et al. 2011), as will be described ahead. Indeed, the sperm of species that have a high ratio of cholesterol to phospholipids are more resistant to cold shock than those with a low ratio (Moce et al. 2010a; Oliveira et al. 2010), indicating that this ratio is critically defining the physical features and resistance of sperm membranes. However, cholesterol is not the only sterol present in the sperm membrane. On the contrary, a particularity of the sperm cells in their sterol composition is the high proportion of desmosterol (Saez et al. 2011) that was first reported in hamster spermatozoa (Bleau and VandenHeuvel 1974). The physiological significance of this high proportion of desmosterol in mammalian sperm cells is still unknown, although it has been proposed that could be involved in the regulation of sperm movement or, like cholesterol, could be involved in the regulation of sperm capacitation (Saez et al. 2011).
The Architecture of the Plasma Membrane
Cell membranes are organized in lipid bilayers composed primarily of polar lipids associated with numerous proteins. As stated earlier, GLPs and sphingolipids are usually the predominant polar lipids in biological membranes. These molecules possess a highly polar headgroup in addition to (two) non-polar hydrophobic tails. The hydrophobic tails consist of the hydrocarbon chain portions of fatty acids and/or the base sphingosine (Erwin 2004). The polar headgroups of the lipids can have various chemical structures. Often they contain phosphate to which a polar or charged alcohol derivative is attached. In other cases, they contain carbohydrates. Sterols differ from other polar lipids in having a simple, small hydroxyl group as their polar portion. This hydroxyl group is attached to four fused aliphatic hydrocarbon rings. In addition, they contain a branched hydrocarbon chain that is attached to the end of the rings opposite the hydroxyl (Erwin 2004). In bilayers, lipids orient such that their polar groups are in contact with the aqueous environment and their hydrocarbon segments face each other. As a result, the polar groups in each half of the bilayer, namely monolayer or leaflet, face away from one another. A gradient of decreasing polarity is encountered moving from the aqueous solution to the bilayer centre (Erwin 2004).
Although the bilayer nature of the cell membrane was described in the mid-1920s, it was not until 1972 that was proposed the fluid mosaic model of membrane structure by Jonathan Singer and Garth L. Nicolson (Singer 1972; Singer and Nicolson 1972). This model describes the membranes as a fluid mosaic in which proteins are inserted into a lipid bilayer. While phospholipids provide the basic structural organization of membranes, membrane proteins carry out the specific functions of the different membranes of the cell. These proteins are divided into two general classes, based on the nature of their association with the membrane: transmembraneous proteins, which are embedded directly within the lipid bilayer; and non-transmembraneous, which are not inserted into the lipid bilayer but are associated indirectly with the polar surface of the bilayer (Singer and Nicolson 1972). The latter play a crucial role in cell to cell recognition (i.e. in fertilization) and in the organization of cell shape and motility, while the transmembraneous proteins, such as ionic channels or receptors, are often involved in the regulation of signalling processes allowing the cell to respond to external signals, or involved in the selective transport of molecules across the membrane, among many others functions. In addition to their specific roles, proteins can have a significant influence on membrane structure. They are likely to be responsible for the distortion of natural membranes into more complex shapes than those observed for pure lipid bilayers (Erwin 2004).
In the mosaic fluid model, it is assumed a more or less randomly, non-homogeneous lipid distribution. In the model, phospholipids present lateral diffusion movements, changes of position from the outward to the inward monolayer, so-called flip-flop, and movements of flexion and rotation of the hydrocarbon chains (Singer and Nicolson 1972). Among these, the flip-flop, or transbilayer lipid motion, is considered the most relevant in terms of cell membrane regulation. The flip-flop can be enzyme-assisted by flippases, floppases and scramblases (Contreras et al. 2010). Flippases and floppases are ATP-dependent transbilayer lipid translocators. Accordingly to an extensively used nomenclature, although not universal, they catalyse lipid transfer towards the inward monolayer (flippase) or towards the outward monolayer (floppases). Both enzymes are required to equalize the number of lipids at both sides of the membrane, thus flippases and floppases usually tends to maintain the membrane more symmetric. However, in some cases they operate the other way around, thus generating lipid asymmetry in a process not well defined yet (Contreras et al. 2010). The other enzymes that assist the translocation of lipids within membrane monolayers are the scramblases, which constitute a group of homologous, ATP-independent, bidirectional lipid translocators. They are mediating a calcium-dependent transbilayer movement of phospholipids, tending to eliminate their asymmetric distribution across the membranes (Basse et al. 1996; Contreras et al. 2010). Finally, the flip-flop movements can be also induced by external agents, like insertion of foreign molecules (detergents) in one of the membrane leaflets, or be the result of the enzymatic generation of lipids (Contreras et al. 2010).
In human, boar and stallion, spermatozoa have been reported scramblase activity during capacitation (Fig. 1) (Gadella and Harrison 2000; de Vries et al. 2003; Thomas et al. 2006). Indeed, it has been reported that phospholipid scramblase type 2 (PLSCR2) is an isoform that is specifically expressed in the testis (Wiedmer et al. 2000), reinforcing the idea that the dynamics of the sperm plasma membrane is essential in sperm homeostasis. The most obvious change associated with scramblase activity during capacitation, as seen in boar sperm, consists in a rapid and drastic variation in the arrangement of the phospholipids of the lipid bilayer (Gadella and Harrison 2000; Harrison and Gadella 2005). The loss of membrane asymmetry has two main characteristics: (i) is triggered by the influx of bicarbonate in the sperm cell and, therefore, on the activation of the cyclic AMP-dependent protein kinase pathway (see below); and (ii) the redistribution of phospholipids occurs very rapidly, within minutes, indicating that is an early event that likely precedes many other capacitating modifications in sperm (Gadella and Harrison 2000; Harrison and Gadella 2005). Finally, it is worth mentioning that bicarbonate-induced reversible scrambling takes place in boar spermatozoa in the complete absence of extracellular calcium (Gadella and Harrison 2000; Harrison and Gadella 2005), although in somatic cells, the action of the scramblases has been described as calcium-dependent (Contreras et al. 2010). This apparent discrepancy could be related, at least partially, with the fact that most of the studies undertaken to show the scramblase activity are based in the labelling of external phosphatidylserine with annexin V, process that is strictly calcium-dependent. The fact that annexin V needs calcium to bind aminophospholipids does not necessarily imply to categorize the phosphatidylserine externalization as a calcium-dependent process. To completely elucidate this matter are likely required additional experiments performed with calcium-independent aminophospholipids probes.
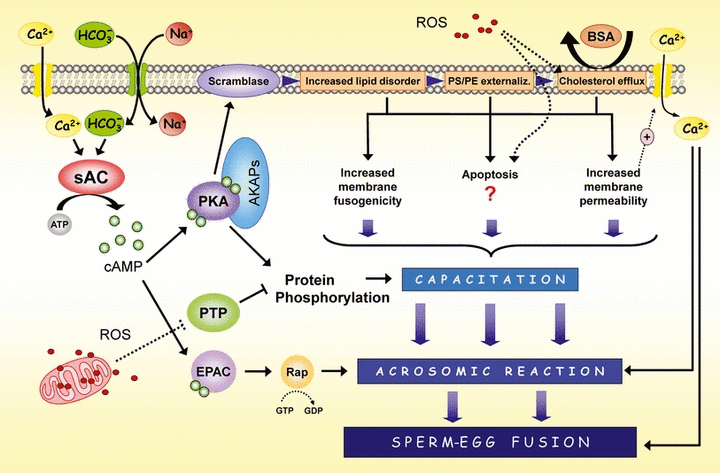
Main intracellular pathways activated during sperm capacitation. In the figure are represented the central molecular events that occur during sperm capacitation. First, the entry of bicarbonate (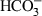 ) into the cell causes pH alkalinization and, concomitantly with calcium (Ca2+) in some species, stimulates the activity of the sAC, which increases intracellular cAMP levels. cAMP induces the activation of PKA that directly or indirectly is mediating most of the changes that occur during sperm capacitation, including phosphorylation of multiple substrates and the externalization of membrane lipids. Other molecular targets of cAMP, such as EPAC, are also activated in the sperm but theirs function seem to be restricted mainly to the regulation of the acrosome reaction and membrane fusion. At lower physiological rates, ROS play a role in capacitacion, hyperactivation and sperm-oocyte fusion. ROS have two main sources in sperm: the mitochondrion (intracellular) and the contaminant leucocytes of semen (extracellular). Independently of their origin they show a significant role in many sperm physiological processes, such as protein phosphorylation and cholesterol efflux. The effect on protein phosphorylation could involve protein phosphatases, which are highly susceptible to inactivation by oxidation thus increasing protein phosphorylation. The formation of oxysterols prior to albumin-mediated removal of cholesterol from the membrane is likely required. This removal increases the membrane fluidity and permeability. On the other hand, when levels of ROS overwhelm the antioxidant defence system can appear deleterious effects such as lipid peroxidation and apoptotic changes. Finally, ion movements are very important in sperm function, first during the activation of the sAC and later during the establishment of the acrosome reaction. sAC: soluble adenylyl cyclase; cAMP: cyclic adenosine monophosphate; ROS: reactive oxygen species; PKA: Protein kinase A; AKAP: A-kinase anchor proteins; EPAC: Exchange protein directly activated by cAMP; PTP: Protein tyrosine phosphatases; PS: Phosphatidylserine; PE: Phosphatidylethanolamine
) into the cell causes pH alkalinization and, concomitantly with calcium (Ca2+) in some species, stimulates the activity of the sAC, which increases intracellular cAMP levels. cAMP induces the activation of PKA that directly or indirectly is mediating most of the changes that occur during sperm capacitation, including phosphorylation of multiple substrates and the externalization of membrane lipids. Other molecular targets of cAMP, such as EPAC, are also activated in the sperm but theirs function seem to be restricted mainly to the regulation of the acrosome reaction and membrane fusion. At lower physiological rates, ROS play a role in capacitacion, hyperactivation and sperm-oocyte fusion. ROS have two main sources in sperm: the mitochondrion (intracellular) and the contaminant leucocytes of semen (extracellular). Independently of their origin they show a significant role in many sperm physiological processes, such as protein phosphorylation and cholesterol efflux. The effect on protein phosphorylation could involve protein phosphatases, which are highly susceptible to inactivation by oxidation thus increasing protein phosphorylation. The formation of oxysterols prior to albumin-mediated removal of cholesterol from the membrane is likely required. This removal increases the membrane fluidity and permeability. On the other hand, when levels of ROS overwhelm the antioxidant defence system can appear deleterious effects such as lipid peroxidation and apoptotic changes. Finally, ion movements are very important in sperm function, first during the activation of the sAC and later during the establishment of the acrosome reaction. sAC: soluble adenylyl cyclase; cAMP: cyclic adenosine monophosphate; ROS: reactive oxygen species; PKA: Protein kinase A; AKAP: A-kinase anchor proteins; EPAC: Exchange protein directly activated by cAMP; PTP: Protein tyrosine phosphatases; PS: Phosphatidylserine; PE: Phosphatidylethanolamine
The Organization of the Plasma Membrane
It is classically known that lateral inhomogeneities in lipid composition and physical properties exist in biological membranes. The plasma membrane components are ordered in discrete domains which are classified as macrodomains and microdomains or lipid rafts (Mukherjee and Maxfield 2004). These membrane domains are proposed to play important roles in the regulation of processes such as signal transduction and membrane trafficking (Peterson and Russell 1985; Bearer and Friend 1990). Similarly to somatic cells, sperm surface membrane can be also organized in large, non-diffusible lipid domains (Peterson and Russell 1985; Martinez and Morros 1996; Travis and Kopf 2002). This regionalization affects the distribution of both lipids and proteins and could be facilitated by the usual high percentage of plasmalogens in sperm membranes (Martinez and Morros 1996). In sperm, these domains are dynamic regions that change their organization and composition along the cell maturation and in certain physiological conditions (Bearer and Friend 1990), such as during sperm capacitation (Martinez and Morros 1996; Travis and Kopf 2002; Gadella et al. 2008). Indeed, among the numerous processes associated with sperm capacitation, there is a remarkable remodelling of the lipid and protein architecture of the sperm plasma membrane (Nixon et al. 2011). While the mechanism(s) governing this dynamic reorganization remain elusive, emerging evidence has raised the prospect that it may be coordinated, at least in part, by specialized membrane lipid rafts (Gadella et al. 2008; Nixon et al. 2011). Capacitation causes a lateral aggregation of microdomains at the apical area. Such lipid aggregation creates lateral heterogeneity of sperm membrane proteins that are involved in zona recognition, so that zona binding can take place (Gadella 2010). Furthermore, in the same area where the aggregation of membrane rafts and zona binding proteins takes place, it is also detected a characteristic redistribution of SNARE proteins at the intracellular side during in vitro capacitation. These proteins are involved in the formation of multimolecular SNARE fusion protein complexes which establish interactions with the acrosomal membrane (Mayorga et al. 2007; Gadella et al. 2008). Upon the appropriate stimulus (typically zona-triggered calcium influx), these complexes experiment conformational changes that lead to the fusion of the plasma and acrosome membranes at specific sites, thus inducing the acrosome reaction in a well-regulated manner (Mayorga et al. 2007; Gadella et al. 2008). The final outcome of the process is that, upon cholesterol removal, membrane rafts more than likely represent platforms for the organization of proteins involved in sperm–oocyte interactions and the acrosome reaction (Gadella et al. 2008; Nixon et al. 2011).
Signalling Evoked by the Plasma Membrane
Several studies by different laboratories using in vitro models support the idea that the progression of capacitation requires transmembrane signalling and imbricated intracellular signal transduction pathways, which must to be activated in a coordinated manner. The development of in vitro capacitation protocols for sperm of several different species has shown the critical importance of three media constituents, namely calcium (Ca2+), bicarbonate (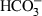 ) and a protein that can function as a cholesterol acceptor, such as serum albumin. Work by several laboratories shows that capacitation is likely regulated by a signal transduction pathway involving cross-talk between cAMP, cyclic AMP-dependent protein kinase (PKA) and tyrosine kinases. This signalling leads to the phosphorylation on serine, threonine and, remarkably, tyrosine residues of several proteins during the progression of the capacitation processes (Fig. 1) (Travis and Kopf 2002).
) and a protein that can function as a cholesterol acceptor, such as serum albumin. Work by several laboratories shows that capacitation is likely regulated by a signal transduction pathway involving cross-talk between cAMP, cyclic AMP-dependent protein kinase (PKA) and tyrosine kinases. This signalling leads to the phosphorylation on serine, threonine and, remarkably, tyrosine residues of several proteins during the progression of the capacitation processes (Fig. 1) (Travis and Kopf 2002).
The mechanism by which bicarbonate enters the sperm is still poorly understood. In mouse sperm, it has been described a cotransporter Na+/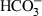 that would mediate this entry at least partially (Fig. 1) (Demarco et al. 2003). Furthermore, in sperm of this species, the entry of bicarbonate also seems to require the participation of a sperm-specific Na+/H+ exchanger (sNHE) because the sperm from sNHE-null mice are immobile and fail to develop other capacitating-related changes, including increases in tyrosine phosphorylation (Wang et al. 2003, 2007). After entering, bicarbonate causes rapid effects in the sperm cells including intracellular pH alkalinization, which, besides other effects, activates a number of ionic channels in the membrane (Navarro et al. 2008; Ren and Xia 2010). However, most of the bicarbonate-induced effects in sperm are mediated through stimulation of the activity of soluble adenylyl cyclase (sAC), the major adenylyl cyclase isoform in spermatozoa (Hanoune and Defer 2001; Litvin et al. 2003). This is an unusual mechanism of activation: although there is clear evidence that intracellular calcium can regulate the activity of specific adenylyl cyclase, the effects of bicarbonate on adenylyl cyclase activity have been demonstrated in only a small number of cells or tissues (Travis and Kopf 2002). It is worth mentioning, however, that, in addition to the sAC regulated by ions, in human and mouse sperm have been also described adenylyl cyclase isoforms associated with the membrane (mAC), which appear to be regulated by G proteins but whose way of activation and function have not yet been fully revealed in sperm (Baxendale and Fraser 2003; Fraser et al. 2003).
that would mediate this entry at least partially (Fig. 1) (Demarco et al. 2003). Furthermore, in sperm of this species, the entry of bicarbonate also seems to require the participation of a sperm-specific Na+/H+ exchanger (sNHE) because the sperm from sNHE-null mice are immobile and fail to develop other capacitating-related changes, including increases in tyrosine phosphorylation (Wang et al. 2003, 2007). After entering, bicarbonate causes rapid effects in the sperm cells including intracellular pH alkalinization, which, besides other effects, activates a number of ionic channels in the membrane (Navarro et al. 2008; Ren and Xia 2010). However, most of the bicarbonate-induced effects in sperm are mediated through stimulation of the activity of soluble adenylyl cyclase (sAC), the major adenylyl cyclase isoform in spermatozoa (Hanoune and Defer 2001; Litvin et al. 2003). This is an unusual mechanism of activation: although there is clear evidence that intracellular calcium can regulate the activity of specific adenylyl cyclase, the effects of bicarbonate on adenylyl cyclase activity have been demonstrated in only a small number of cells or tissues (Travis and Kopf 2002). It is worth mentioning, however, that, in addition to the sAC regulated by ions, in human and mouse sperm have been also described adenylyl cyclase isoforms associated with the membrane (mAC), which appear to be regulated by G proteins but whose way of activation and function have not yet been fully revealed in sperm (Baxendale and Fraser 2003; Fraser et al. 2003).
Independently of the kind of adenylyl cyclase involved, the resultant increased level of cyclic AMP in the cell activates the PKA (Harrison and Gadella 2005) which regulates the phosphorylation status of various downstream proteins, including other kinases and phosphatases (Furuya et al. 1992). In turn, these proteins phosphorylate/dephosphorylate a number of additional signalling components, such as adaptor and scaffold proteins (AKAP) or additional kinases and phosphatases that amplify the signal and, overall, confer specificity to the whole process (Fig. 1) (Urner and Sakkas 2003; Gonzalez-Fernandez et al. 2009). One remarkable aspect of this signalling cascade is that the activation by bicarbonate of some PKA-mediated signals is extremely rapid (seconds), whereas much more time (hours) is required to develop others, indicating that the timing and intensity of the PKA-elicited signals are extremely important to correctly accomplish the biological changes (Harrison and Gadella 2005).
PKA was thought to be the single effector of cAMP in sperm signalling, but recently it has been discovered a role in sperm for a family of proteins, namely Epacs, that are also directly activated by cAMP (Bos 2006). Epac comprise a family of cAMP-regulated guanine nucleotide exchange factors (cAMP-GEFs), coupling cAMP production to the activation of Rap, a small molecular weight GTPase of the Ras family (Bos 2006). In somatic cells, the role of Epac has been extensively documented, being involved in a wide range of functions such as the regulation of ion channel function, regulation of intracellular calcium, exocytosis/secretion, cell adhesion and cell junctions, among others (Bos 2006). However, scarcely literature about Epac can be found in germinal cells. Epac 1 has been recently identified in ejaculated human and stallion sperm (Branham et al. 2006; McPartlin et al. 2011) and epididymal mouse sperm (Amano et al. 2007), while Epac 2 has been detected in mouse spermatogenic cells (Aivatiadou et al. 2009) and stallion ejaculated sperm (McPartlin et al. 2011). Using AMPc analogues that are cell permeant and selective activators of Epac, it has been observed that these drugs are able to induce an increase in the AR (Fig. 1) (Branham et al. 2006; McPartlin et al. 2011) or modulate the membrane potential on mammalian spermatozoa (McPartlin et al. 2011).
Calcium has long been regarded as essential to sperm function, and the possibility that calcium might act in parallel with bicarbonate through stimulation of adenylyl cyclase(s) has been demonstrated (Fig. 1) (Litvin et al. 2003). However, while a role for external calcium as well as bicarbonate in capacitation-dependent tyrosine phosphorylation has been demonstrated in mouse (Visconti et al. 1995) and human sperm (Lawson et al. 2008), in the boar bicarbonate is able to induce changes in plasma membrane architecture, increases in motility and increases in tyrosine phosphorylation in the complete absence of external calcium (Harrison et al. 1996; Green and Watson 2001; Garcia et al. 2005; Harrison and Gadella 2005). Indeed, in this species, it has been recently demonstrated that the addition of a calcium chelator, such as ethylenediaminetetraacetic acid, to the thawing solution improves the motility and viability of frozen/thawed boar spermatozoa and their fertility ability in vitro (Okazaki et al. 2011), probably because the treatment with a calcium chelator prevented the deleterious effect of an excessive rise of calcium within the spermatozoa during thaw that can be caused by a damage in the plasma membrane and/or ionic channels (i.e. altered permeability) associated with sperm cryopreservation. Nevertheless, although some of the earlier changes associated with sperm capacitation could be calcium-independent at least in some species (for example, an increase in membrane fluidity), the last stages in the process of acquiring fertilizing ability by spermatozoa, such as zona binding, hyperactivated motility and acrosome reaction, are strictly calcium-dependent in all mammalian species (Fig. 1). In addition, other processes such as chemoattraction of sperm cells to oocyte during ovulation, although not completely understood are thought to be also mediated by calcium (Navarro et al. 2008; Ren and Xia 2010).
Similarly to somatic cells, the calcium involved in the regulation of fertilization in sperm cells can entry from extracellular space and/or can be mobilized from intracellular stores (Ren and Xia 2010). Unlike most cells, mature sperm do not contain an endoplasmic reticulum, which is typically a major source for calcium release, and the principal piece of sperm does not contain intracellular organelles. Therefore, potential areas for calcium stores in sperm include the acrosome in the head, a redundant nuclear envelope (RNE) that colocalizes with the IP3 receptor in the neck region, and mitochondria packed in the midpiece (Ren and Xia 2010). How extracellular calcium enters sperm is also poorly understood. A large number of plasma membrane, calcium-permeable ion channels have been found in sperm. These include high-voltage-activated and low-voltage-activated (T-type) calcium-selective channels (CaVs), TRP channels, cyclic nucleotidegated (CNG) channels and CatSper channels (Navarro et al. 2008; Ren and Xia 2010; Kirichok and Lishko 2011). The predominant currents are a calcium-selective current requiring the CatSper channels, and a rectifying potassium current with properties most similar to mSlo3 channel. Intracellular alkalinization activates both channels and induces hyperactivated motility (Navarro et al. 2008). In addition to bicarbonate, the entry of calcium in sperm can be also facilitated by cyclic nucleotides, effect that is also mediated by the CatSper channels (Kirichok and Lishko 2011), although originally the cyclic nucleotide-induced calcium-entry into mammalian spermatozoa was assigned to the CNG channels (Wiesner et al. 1998). Finally, albumin, the main protein of the oviductal fluid and an important component of the in vitro capacitation media, has also been shown to cause CatSper-dependent calcium influx into mouse spermatozoa (Ren and Xia 2010). Therefore, the bicarbonate-induced hyperpolarization of the sperm plasma membrane causes the opening of voltage-dependent calcium channels. Similarly, the cyclic nucleotides and albumin can also induce calcium influx into mammalian spermatozoa, indicating that the capacitation process involves diverse phenomena that are tightly regulated and interlinked.
On the other hand, as stated before, removal of cholesterol in adult, ejaculated spermatozoa likely accounts for the changes in membrane fluidity observed during capacitation and the subsequent decrease in the membrane cholesterol/phospholipid ratios (Fig. 1) (Travis and Kopf 2002). Indeed, the modification of the sperm cholesterol content starts much earlier because it is already detected during epididymal maturation. These earlier changes are likely preparing the sperm cells to fulfil the last steps of the fertilization process in the female genital tract (Saez et al. 2011). During epididymal maturation has been reported a significant decrease in cholesterol of approximately 50% in ram, rat, hamster and mouse (Saez et al. 2011). This cholesterol loss is usually accompanied by a decrease in the cholesterol/phospholipids ratio, an indicator of membrane fluidity, thus suggesting that sperm cells increase their membrane fluidity when descending the epididymis, probably to confer the spermatozoon the ability to undergo the fusion steps in the female genital tract (Saez et al. 2011). In goat, however, the sperm cholesterol content increases during epididymal maturation, whereas in boar, no significant changes were detected (Saez et al. 2011).
In an excellent review, Travis and Kopf wondered how changes in the sterol content of the membrane regulate transmembrane signalling that can lead to capacitation and proposed two hypotheses (Travis and Kopf 2002). In the first one, they suggest that cholesterol could alter the bulk properties of biological membranes. For example, cholesterol can increase the orientation order of the membrane lipid hydrocarbon chains, restricting the ability of membrane proteins to undergo conformational changes by rendering their surrounding membrane less fluid. High concentrations of cholesterol can thereby inhibit capacitation indirectly by diminishing the conformational freedom and hence the biological activity of sperm surface proteins. In the second hypothesis, they suggest that cholesterol might directly affect specific membrane proteins that function in transmembrane signalling. Either or both of these effects of cholesterol could modulate ion transporters and effector enzymes like sAC (Travis and Kopf 2002). Additionally, albumin-mediated cholesterol efflux of the non-raft sperm surface is required to allow microdomain clustering in the apical ridge area, which, as stated before, is associated with the redistribution, localization and association of membrane proteins involved in zona recognition and acrosome reaction (Mayorga et al. 2007; Gadella et al. 2008; Gadella 2010).
It is worth mentioning that the mechanism by which cholesterol is depleted from the capacitating sperm surface still remains elusive. Albumin has a hydrophobic pocket normally involved in fatty acid transport. However, cholesterol fits in the plasma membrane bilayer extremely well but is preferentially and specifically depleted. Interestingly, fresh sperm incubated in capacitation media did give rise to the formation of oxysterols under conditions that are routinely used for in vitro fertilization. Under in vitro capacitation conditions, in which the medium is enriched in bovine serum albumin, a complete depletion of formed oxysterols into the albumin-enriched medium was observed (Brouwers et al. 2011). Therefore, these results clearly points to a relation between cholesterol oxidation and albumin-mediated cholesterol depletion. If this situation is confirmed, it also will account for the crucial role of mild oxidation for sperm activation (Fig. 1) (Reviewed in Aitken and Curry 2011).
Factors Affecting the Integrity of the Sperm Membrane
The reproductive technologies such as sperm cryopreservation, sperm selection and sperm sorting are, by far, the most aggressive factors affecting the integrity of the sperm membrane (Moce et al. 2010a; Peña et al. 2011). Regarding cryopreservation, a two-factors hypothesis describing sperm cryodamage has been commonly accepted among human and veterinary cryospermatologists (Mazur 1970, 1984; Peña et al. 2011). This hypothesis states that cells cooled too rapidly are killed by intracellular ice formation, and cells cooled too slowly may be killed by long exposure to concentrated solutions resulting from progressive conversion of water to ice. To maximize sperm survival, freezing protocols are designed to minimize these insults through the addition of cryoprotectors and the use of appropriate freezing and thawing rates, and are expected to maximize sperm survival (Peña et al. 2011). However, freeze-fracture techniques, freeze-substitution electron microscopy and ice formation measurement by differential scanning calorimetry were recently used to demonstrate that there is no intracellular ice crystal formation in human and stallion spermatozoa with the freezing rates currently in use (Morris 2006; Morris et al. 2007). Murine studies have also demonstrated that intracellular freezing only occurs if cells at extremely high cooling rates (Mazur and Koshimoto 2002), far of those used during sperm freezing. Therefore, the major source of sperm damage during cryopreservation almost certainly comes from osmotic stress, especially during thawing (Peña et al. 2011).
But how does osmotic stress cause sperm damage? The justification is related to the extracellular ice crystal formation, which is indirectly causing that spermatozoa are exposed to an increasingly hypertonic solution (Katkov et al. 1998; Koshimoto et al. 2000). The hypertonic extracellular environment in which sperm are suspended leads to intracellular water loss that, in turn, is dependent upon the rate of cooling (Mazur and Koshimoto 2002). Thus, cells lose water and shrink until intracellular and extracellular solute concentrations equilibrate (Mazur 1984). During thawing, spermatozoa are exposed to a hypotonic environment that results in an increase in sperm volume because of water influx into the cytoplasm, which once again continues until the intracellular and extracellular solute concentrations are equilibrated (Peña et al. 2011). Cellular damage potentially occurs owing to this double osmotic stress: the hyperosmotic stress during freezing is followed by the hypoosmotic insult at thawing. Experiments in our laboratory (Garcia et al. 2012) and others (Pommer et al. 2002) have demonstrated that this osmotic roller coaster is more detrimental for stallion spermatozoa than continuous exposure to a given hyperosmolar solution. Similar results were found in other species such as rhesus monkey (Rutllant et al. 2003), mouse (Si et al. 2009) and bull sperm (Guthrie et al. 2002), among others. Furthermore, the addition of permeating cryopretectans also creates an anisosmolar environment for the spermatozoa, causing the cells to shrink or swell. Removing these cryoprotectants can be especially damaging if the cells experience cell volume increases sufficiently large to induce membrane damage or cell lysis (Morris et al. 2007; Garcia et al. 2012). As a result, the most obvious injury after exposure to anisosmolar solutions is plasma membrane damage because of cell shrinkage and swelling.
However, the susceptibility to the plasma membrane damage induced by osmotic stress is fairly variable among species. As indicated before, the composition of fatty acids in the sperm membrane should be considered as a major factor explaining sperm quality in general, and ability to freeze in particular (Garcia et al. 2011; Macias et al. 2011). This composition largely conditions the sperm membrane fluidity, which is directly related to lipid composition of the sperm plasmalemma (Moce et al. 2010a). Thus, the species in which the ratio of cholesterol to phospholipids is higher are more resistant to cold shock than those with a low ratio (Moce et al. 2010a). For example, ram sperm are more sensitive to thermal shock than sperm from bull, rabbit, monkey or man, which can be explained by the fact that the latter display a higher ratio of cholesterol to phospholipid (Holt and North 1984). The addition of cholesterol to sperm cell membranes using cyclodextrins has proven effective in improving the process. The addition of cholesterol improves the viability, inhibits premature acrosome reaction and in some species increases fertility after thawing in ejaculates of boar (Tomas et al. 2011), bull (Moraes et al. 2010), horse (Spizziri et al. 2010), ram (Moce et al. 2010b) and rabbit (Serin et al. 2011). Although a high ratio of cholesterol to phospholipids stabilizes sperm and accounts for an enhanced cryosurvival, the other side of the coin is that the addition of cholesterol to sperm membranes by treatment with cholesterol-loaded cyclodextrins sometimes does not improve fertility rates of thawed spermatozoa (Spizziri et al. 2010), probably because of a membrane overstabilization that is not always completely reversible.
In addition, during the process of sperm cryopreservation rapid temperature changes occur that can affect the organization of plasma membrane lipids (Oldenhof et al. 2010). At the molecular level, these changes affect to lipid–protein interactions and to the asymmetry and composition of phospholipids, both implicated in the loss of the selective permeability of the membranes (Mazur 1984). Finally, recent research in our laboratory demonstrated a greater effect of osmotic-induced stress on mitochondria than on plasma membrane damage, with mitochondrial dysfunction appearing earlier than plasma membrane damage (Garcia et al. 2012). Obviously this is indicating that mitochondria and their membranes are also highly susceptible of damage during cryopreservation because of osmotic stress, but also is indicating that the oxidative damage experienced by sperm during cryopreservation could also be attributed to an osmotic mechanism that increase mitochondria membrane permeability and dysfunction (Peña et al. 2011). In support of this, recent research showed that incubation of spermatozoa in anisosmolar media resulted in an increase in ROS generation and LPO (Burnaugh et al. 2010; McCarthy et al. 2010; Garcia et al. 2012). These results clearly indicated that osmotic stress caused oxidative stress in spermatozoa, which supports the hypothesis that cryopreservation-induced osmotic stress may also lead to oxidative cell damage (Burnaugh et al. 2010).
Nevertheless, the picture seems to be much more complex because there are species in which the freezing-thawing process does not concomitantly cause oxidative stress. The most representative of this species is pig, in which there are several conflicting results that, in fact, seems to indicate that there was no ROS increase during freezing-thawing (Awda et al. 2009). Furthermore, the mitochondria not always contribute to a ROS unbalance by their excessive ROS production, but sometimes is rather more important the loss of their scavenger ability. One example of this comes from studies in boar spermatozoa, where it seems that the overall increase in ROS after thawing is associated with a decrease in the activity of mitochondrial-linked mechanisms involving the elimination and destruction of ROS (Flores et al. 2009). In rat sperm mitochondria, it has been demonstrated a selenium-associated polypeptide, presumably a glutathione peroxidase, which plays a significant role in the peroxyl scavenging mechanism and in maintaining sperm motility (Cataldo et al. 1996). Therefore, these results would be suggesting that an increase in ROS production during freezing-thawing process also would come from damage in mitochondrial activity, but not resulting in an excessive ROS production but rather in an alteration on the ROS scavenging ability of the mitochondria. Anyway, in all possible scenarios, it seems evident that preserving mitochondrial activity is one of the most important points for sperm resistance to cryopreservation. In support of this are recent reports showing that the dialysis of boar semen prior to freezing-thawing markedly improved the post-thaw sperm mitochondrial function, and it was associated with enhanced sperm motility and higher ATP level and quality of post-thaw semen (Fraser et al. 2007).
In many species, LPO has been claimed to be a major factor causing differences in sperm quality, especially when spermatozoa are stored or processed for later use (Alvarez and Storey 1992; Agarwal and Said 2005; Almeida and Ball 2005; Aitken et al. 2007; Peña et al. 2011). As indicated before, the particular susceptibility of the sperm plasma membrane of some individuals or species to peroxidative damage is attributed to their high content of HUFAs and, further, to the innate deficiency of spermatozoa regarding the availability of cytoplasmic protective enzymes, because they had lost most of them with the bulk cytoplasm loss during spermiogenesis (Wathes et al. 2007). These unsaturated fatty acids confer the plasma membrane the fluidity that the spermatozoon needs to participate in the membrane fusion events associated with fertilization (Gadella et al. 2008). However, these molecules are also vulnerable to attacks by ROS, free radicals that generate peroxyl (ROO˙) and alkoxyl (RO˙) radicals that to become stable as molecules both subtracting a hydrogen atom from an adjacent carbon atom in a neighbouring lipid, and thus generating the correspondent acid or alcohol. The subtraction of a hydrogen atom from an adjacent lipid creates a carbon-centred radical that combines with dioxygen to create another lipid peroxide and so on, perpetuating the propagation of peroxidative damage throughout the various cell membranes (Bielski et al. 1983). Such peroxidation damage would disrupt the fusogenity of the sperm membrane and its ability to support key membrane-bound enzymes such as ATPases (Storey 2008). Moreover, alterations in the fluidity of the sperm membranes could alter the activation of signal transduction pathways critical for sperm function (Storey 2008). However, ROS are not always deleterious for sperm biology. In fact, it has been described that mild oxidation is a necessary event for sperm activation, playing a crucial role in these processes (Fig. 1) (Aitken and Curry 2011). In this regard, as was indicated before, the membranes of capacitated sperm are virtually devoid of oxysterols, and thus of a fraction of the original cholesterol. Remarkably, the same treatments to frozen/thawed sperm did not result in the formation of oxysterols or in the albumin-mediated depletion of cholesterol (Brouwers et al. 2011). The elucidation of the causes underlying such differences will likely account for a better understanding of sperm membrane physiology and ultimately for better cryopreservation procedures.
Concluding Remarks and Future Directions
The most important factors causing major damage to the spermatozoa, particularly in their membranes (plasma and organelle membranes), are considered to be the osmotic stress generated by dehydration of the extender and the cells during freezing and thawing (Peña et al. 2011). In addition, a number of other events that occur during cooling, such as phase transitions in the plasmalemma or oxidative damage, also contribute to sperm death or to shorten their lifespan (Peña et al. 2011). Therefore, cryopreservation and other biotechnologies such as sex sorting cause differential damage to the spermatozoa of all mammalian species, of either lethal or sublethal nature (Macias et al. 2011). Obviously, sperm death is the major factor explaining the lower fertility of spermatozoa, but nonlethal modifications of the surviving cells also account for their reduced lifespan and research effort must be devoted to investigate this, including sublethal alterations of the membrane.
The most obvious components of the membrane potentially affected by subtle membrane defects are the transmembraneous proteins, such as ionic channels, receptors and others. For example, it has been described that a number of GLUT transporters (but not all) are differently distributed after cryopreservation in boar and dog spermatozoa. The final significance of these changes and why some transporters relocated while other did not is still unknown (Bucci et al. 2011). Not only transmembraneous proteins but a number of other signalling-transduction components is predicted to be affected by subtle membrane injuries, either because they rely in the membrane (although they are not constitutively membrane proteins) or because require some components of the membrane to exert theirs functions. Those could include proteins that are anchored in the membrane (i.e. Src kinases or cytoskeleton components) and proteins that need some components of the membrane to be activated (i.e. PKC or PLC). In support of this, recent reports have shown that a number of cytoskeletal proteins can be altered by the osmotic stress during the cryopreservation process (Felipe-Perez et al. 2011; Peña et al. 2011). The exact role of the cytoskeleton in osmoregulation remains speculative, but it could function as a cell-volume sensor, or it may offer mechanical protection against excessive swelling or shrinkage (Peña et al. 2011).
These are only examples of potential changes that can underlie subtle modifications of the mammalian sperm membrane. In somatic cells, more than 200 cellular components change with osmolality as a cellular adaptive mechanism (Burg et al. 2007). These changes include ion and water channels, transporters, heat shock proteins, other stress proteins, transcription factors and coactivators, kinases, phosphatases, other signalling proteins, enzymes, hormones, receptors, growth related proteins, second messengers and organic osmolites. Therefore, a huge amount of research work awaits ahead us.
Acknowledgements
This work was supported by Junta de Extremadura-FEDER, Merida, Spain (grants PCE1002 and GR10010) and by MICINN-FEDER, Madrid, Spain (grants BFU2011-30261, AGL2010-20758, and INIA RZ2008-00018-00-00). IMA was granted a fellowship by Junta de Extremadura-FEDER (Programa de Reincorporación de Doctores). AMM and BM-G were each granted a fellowship by the MICINN-FEDER. The authors thank Mrs. Mercedes Gomez Blazquez for her excellent technical assistance.
Conflict of interest
None of the authors have any conflict of interest to declare. There has been no financial support for this work that could have influenced its outcome.
Author contributions
JAT conceived and wrote the paper. IMA discussed and wrote the paper. BM-G, AM-M and CO-F performed most of the experiments from our lab cited in this manuscript. GMS and FJP revised the manuscript and give conceptual advice. All authors discussed the bibliography and commented on the manuscript at all stages.




