Effect of Embryo Density on In vitro Developmental Characteristics of Bovine Preimplantative Embryos with Respect to Micro and Macroenvironments
Contents
To overcome developmental problems as a consequence of single embryo culture, the Well of the Well (WOW) culture system has been developed. In this study, we aimed to examine the effect of embryo densities with respect to both microenvironment and macroenvironment on developmental rates and embryo quality to get a deeper insight into developmentally important mechanisms. WOW diameter and depth significantly affected developmental rates (p < 0.05). WOWs with diameter of 500 μm reached significantly higher blastocyst rates (32.5 vs 21.1% vs 20.3%) compared to embryos cultured in WOWs of 300 μm diameter or plain cultured controls. Embryos cultured in WOWs with 700 μm depth reached significant higher developmental rates compared with embryos cultured in WOWs of 300 μm depth and control embryos (30.6 vs 22.6% vs 20.3%). Correlation of the embryo per WOW volume with developmental rates was higher (r2 = 0.92, p = 0.0004) than correlation of WOW diameter or WOW depth with developmental rates. However, the embryo per WOW volume did not affect differential cell counts. An embryo per culture dish volume of 1 : 30 μl was identified to be optimal when the embryo per WOW volume was 1 : 0.27 μl increasing developmental rates up to the level of mass embryo production. Giving the opportunity to track each embryo over the complete culture period while keeping high developmental rates with normal mitotic dynamics, the results of this work will provide benefit for the single culture of embryos in human assisted reproduction, mammalian embryos with high economic interest as well as for scientific purpose.
Introduction
The pre-implantation mammalian embryo can regulate cell cleavage and differentiation without being in contact with the maternal reproductive tract (Schultz and Heyner 1993). In vivo, the embryo is exposed to numerous factors, which are absent in vitro (for review: O’Neill 2008). These factors or metabolites mediate the maternal-embryonic dialogue (Paria and Dey 1990; Hill 2001) and absence or great distance from them could be one explanation for impaired in vitro development and viability of in vitro produced embryos in the bovine (Gopichandran and Leese 2006) and porcine (Stocks et al. 2005) indicated by low developmental rates, altered gene expression (Wrenzycki et al. 1998, 2001; Niemann and Wrenzycki 2000) as well as increased apoptosis (Brison and Schultz 1997). However, in vitro production of mammalian embryos has been shown to be relatively successful when embryos are kept in relatively large groups during the whole culture period (Holm et al. 1999; Stocks et al. 2005). Apart from the large-scale production of embryos for experimental purposes, most commercial and human embryo culture systems, however, require culturing individually or in small groups. The reason for this need is the small number of available oocytes (OPU technology, single slaughtered valuable oocyte donors, human IVF) as well as only single embryo culture that allows tracking individual embryos throughout the whole culture period (Moessner and Dodson 1995).
Therefore, several techniques for single in vitro production (IVP) systems have been developed (Lane and Gardner 1992;Kato and Tsunoda 1994;Blondin and Sirard 1995;Hazeleger et al. 1995; Carolan et al. 1996; O’Doherty et al. 1997; Wheeler et al. 2007). Nevertheless, embryo development in this situation is handicapped as culture of zygotes individually in large volumes results in inferior development to the blastocyst stage and a reduced cell number compared with those cultured in groups (Paria and Dey 1990; O’Doherty et al. 1997). Decreasing embryo densities reduce the rate of compaction (Stoddart et al. 1996), cavitation (Wiley et al. 1986; Paria and Dey 1990), zona hatching, inner cell mass (ICM) and tropectoderm (TE), cell number (Stoddart et al. 1996) and implantation rate in the mouse (Lane and Gardner 1992). In bovine embryo production group culture increases blastocyst formation, cell numbers and the production of interferon-tau (O’Doherty et al. 1997) too (Larson and Kubisch 1999; Khurana and Niemann 2000). Taken together, one of the most important factors seems to be embryo density during in vitro culture (Kato and Tsunoda 1994; Vajta et al. 2000; Wheeler et al. 2007), which determines the interaction of embryonic factors in the microenvironment.
To overcome these developmental problems, the Well of the Well (WOW) culture system has been developed previously (Vajta et al. 2000). Small Wells (semi-isolated microenvironments) at the bottom of a conventional four well dish provide a microenvironment for single embryos which has been used to culture bovine (Vajta et al. 2000), porcine (Taka et al. 2005; Kamiya et al. 2006) and even human (Vajta et al. 2008) embryos, previously. Although it is widely accepted that the beneficial effect of the WOW system is because of an increase in the concentration of auto- and paracrine substances in the embryo surrounding medium (Vajta et al. 2000; Taka et al. 2005), no studies have been performed to optimize the development of bovine embryos cultured in the WOW system by exploring the most suitable embryo per culture dish volume (macroenvironment) as well as embryo per WOW volume (microenvironment), which are functions of embryo number, WOW depth and WOW diameter so far. However, the WOW system has also been used to culture bovine embryos missing a zona pellucida derived from embryo bisection (Tagawa et al. 2008) or hand-made cloning (Booth et al. 2001; Feltrin et al. 2006; Shah et al. 2008) and one study dealing with hand-made cloned bovine embryos has already compared two different calibres of WOWs (Feltrin et al. 2006) so far. Change of the WOW volume directly affects the microenvironmental embryo density (embryo per WOW volume), whereas macroenvironmental embryo density (embryo per culture dish volume) is independent of the WOW volume.
It has been postulated that the WOW system accomplishes the controversial needs of large (nutrition and dilution of metabolized toxic products) and small volumes (accumulation of autocrine and paracrine factors) (Vajta et al. 2000); however, studies dealing with different embryo per volume ratios (embryo densities) performed so far have only taken plain embryo culture or embryo culture in microsystems (Wheeler et al. 2007; Melin et al. 2009) into account. These studies and others have been performed to identify optimal macroenvironmental embryo densities (Ferry et al. 1994; Fujita et al. 2006) and embryo distance (Gopichandran and Leese 2006) and the effect of the microenvironmental embryo density has only been explored in the context of microfluidic culture platforms (Wheeler et al. 2007; Melin et al. 2009). Moreover, the interdependent effects of different microenvironmental and macroenvironmental embryo densities have not been analysed and thereby not been understood so far.
Therefore, in this study, we aimed to examine the effect of embryo densities with respect to both microenvironment and macroenvironment on developmental rates and embryo quality in terms of different cell counts to get a deeper insight into developmentally important mechanisms. We aimed to compare the developmental characteristics of bovine embryos in different microenvironmental embryo densities (embryo per WOW volume) and to analyse the interdependency to the macroenvironmental embryo density by altering also the embryo number per culure dish volume.
Material and Methods
Preparation of the well in well culture dish
Into the bottoms of the wells of a common 5-well culture dish (Fig. 1a, Fa. Minitüb, Germany), we prepared WOWs by using industrial borer (ULTRA HSSE/Co Bohrer, DIN 1899, Nr. 186, Fa Baer Ultra Präzisionswerkzeuge GmbH, Weinheim) of different diameters (300–900 μm) according to the requirements of the experiments. A total of 16 small holes were produced in a 4 × 4 cluster in each well (see Fig. 1a). Holes were like cylinders with a slightly rounded bottom (see Fig. 1c). Bored WOWs were cleaned by rinsing with TCM 199 (Sigma, Taufkirchen, Germany) supplemented with 4.43 mm hepes, 33.9 mm NaCHO3, 2 mm pyruvate, 2.92 mm calcium lactate, 55 μg/ml gentamycine and 12% heat inactivated oestrus cow serum. Air bubbles inside of the holes were flushed out with a mouth-pipette out of glass under a stereomicroscope. Again, wells were washed three times with CR1aa culture medium, which was already equilibrated at 39°C and 5% CO2 in humidified air. Finally, all WOWs were overlaid with 500 μl CR1aa medium (Rosenkrans and First 1994). Thus, each embryo was fixed in its own microenvironment (Fig. 1b), but all embryos were together harboured in one culture dish of medium defined as macroenvironment in this work (Fig. 1a). The microenvironmental embryo density [n/μl] was defined as embryos per WOW volume and the macroenvironmental embryo density [n/μl] was defined as embryos per culture dish volume.
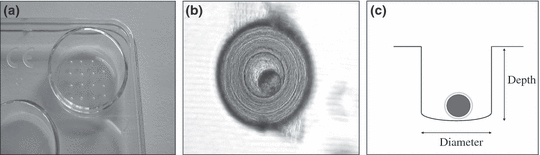
The Well in Well system: a total of 16 small holes were produced in a 4 × 4 cluster into the bottom of one well of a common culture dish (a). Subsequently, each embryo was fixed single in its own WOW (b) creating an individual microenvironment. WOWs are like cylinders with a subconcave bottom presented as cross section (c)
Preparation of IVF zygotes
Bovine ovaries were obtained from a local slaughterhouse and brought into lab in 30°C saline within 3 h. Cumulus oocyte complexes (COCs) were aspirated from small follicles (2–8 mm) and COCs with a homogenous, evenly granulated ooplasm, surrounded by at least three layers of compact cumulus cells, were transferred to modified tissue culture medium (TCM199, Sigma, Taufkirchen, Germany) supplemented with 4.43 mm hepes, 33.9 mm NaCHO3, 2 mm pyruvate, 2.92 mm calciumlactate, 55 μg/ml gentamycine and 12% heat inactivated oestrus cow serum. After three times of washing, COCs were cultured in groups of 50 in 400 μl modified TCM supplemented with 10 μg/ml FSH (FSH-p, Sheering, Kenilworth, NJ, USA) for 24 h at 39°C in a humidified atmosphere with 5% CO2 in air. Fertilization was performed in Fert-TALP (Parrish et al. 1988) supplemented with 20 μm penicillinamine, 10 μm hypotaurin, 2 μm epinephrine, 6 mg/ml BSA, 50 μg/ml gentamycine and 1 μg/ml heparin. Final concentration of sperms in fertilization droplets was adjusted to 2 × 106 sperms/ml. 18 h after coculture, the embryos were washed three times from sperms and cumulus cells and were placed transferred to in vitro culture.
In vitro culture of IVF embryos
Embryo culture was performed in humidified atmosphere with 5% CO2 in air at 39°C for up to 8 days in CR-1aa culture medium (Rosenkrans and First 1994). Embryos were randomly allocated to Well of Wells of different volumes according to the experimental design. As a control, we cultured simultaneously 16 embryos plain per group in an identical culture well, but without WOWs in an identical total volume of culture media.
Assessment of developmental competence
Developmental competence was noted 48 h after placing the embryos into culture by counting the percentage of cleaved embryos and at Day 8 by counting the number of embryos which reached blastocyst stage.
Differential cell number count of TE and ICM
Differential cell counts were based on the immunological methods originally described by Handyside and Hunter (1984). Briefly, the zona pellucida was removed by treatment with acid tyrode (pH 2.5). Zona free blastocysts were then incubated in an 1 : 10 dilution of rabbit antibody (Sigma, D-9656) in PBS for 30 min at 29°C. After washing in PBS + 10% FCS, blastocysts were incubated in a solution of complement (guinea pig complement, Sigma S-1639, final dilution 1 : 10), propidium iodide (Sigma, 10 μg/ml), FCS (8%) in PBS for 30 min at 39°C. Finally, the embryos were fixed in ice cold absolute ethanol with 19 μg/ml bisbenzimide stain (Hoechst 33258, Sigma B2883) for 20 h at −20°C. Then, the embryos were transferred to a drop of glycerol on a glass slide and covered with a cover slip. Cell counting was performed using a fluorescent microscope (Leika DM-IRB) with ICM cells appearing blue and TE cells appearing pink.
Experimental design
Experiment 1: Effect of WOW diameter and depth on in vitro development of bovine zygotes
IVF derived zygotes were randomly allocated (3 × 3 factorial design) into WOWs with different combinations of diameter (300, 400 and 500 μm) and depth (300, 500 and 700 μm). While embryos cultured plain in group of 16 served as controls, subsequent developmental competence of embryos was assessed.
Experiment 2: Effect of the microenvironmental embryo density on in vitro development of single bovine zygotes
IVF derived zygotes were randomly allocated into WOWs with different diameters (500, 700 and 900 μm) and fixed depth (700 μm) resulting in different WOW volumes. While embryos cultured plain in group of 16 served as controls, subsequent developmental competence of embryos was assessed in relation to WOW volume (0.137, 0.269 and 0.445 μl) as a function of diameter and depth and thus in relation to microenvironmental embryo density. Blastocysts derived from different microenvironmental densities were analysed for total cell number, number of trophectoderm cells (TE) and inner cell mass (ICM) cell number.
Experiment 3: Effect of macroenvironmental embryo density on in vitro development of single bovine zygotes
IVF derived zygotes were randomly allocated into WOWs within different culture dish volumes (160, 320 and 480 μl) resulting in macroenvironmental embryo per culture dish volumes (macroenvironment) of 1 : 10 μl, 1 : 20 μl and 1 : 30 μl while keeping the WOW volume (microenvironmental density) fixed. Subsequently, the developmental competence of the bovine embryos was assessed.
Statistical analysis
The data for this study were analysed using the Statistical Analysis System (sas) version 8.0 (SAS Institute Inc., NC, USA) software package. Developmental rates of in vitro produced embryos were analysed using Two Way anova (Tuckey Test) and χ2-test. Regression analysis between WOW volume and embryo development was performed by Pearson Correlation. Differences of p < 0.05 were considered significant.
Results
Experiment 1: Effect of WOW diameter and depth on in vitro development of bovine zygotes
When bovine zygotes were cultured in WOWs with different diameters and depths (3 × 3 design), a significant effect of WOW diameter on cleavage rate was observed (p < 0.05). Embryos cultured in WOWs of 500 and 400 μm diameter cleaved more effectively compared with embryos cultured in WOWs of 300 μm diameter and controls (82.9% vs 85.7% vs 72.0% vs 73.4%) respectively. Moreover, embryos cultured in WOWs with a diameter of 500 μm reached a significantly higher blastocyst rate compared with embryos cultured in WOWs of 300 μm or plain (32.5 vs 21.1% vs 20.3%) as shown in Table 1. WOW depth also had significant effects on developmental rates to the blastocyst stage: Embryos cultured in WOWs with 700 μm depth reached significantly higher developmental rates to the blastocyst stage compared with embryos cultured in WOWs of 300 μm depth or control embryos cultured plain (30.6 vs 22.6% vs 20.3%), as shown in Table 2. When embryo development was correlated to WOW volume (function of diameter and depth), a strong positive correlation (r2 = 0.92, p < 0.01) was observed between WOW volume and subsequent developmental rates of single placed embryos as shown in Fig. 2. Correlation of developmental rates to WOW volume was stronger than correlation to diameter (r2 = 0.76, p < 0.05) and the lowest for correlation with depth (r2 = 0.54, without significance) of Wells.
| WOW microenvironment | Replicates | n | Cleaved (%) | Blastocyst (%) | |
|---|---|---|---|---|---|
| Diameter × depth (μm) | Volume (μl) | ||||
| 300 × 300 | 0.021 | 5 | 79 | 55 (69.6) | 15 (19.0) |
| 300 × 500 | 0.035 | 5 | 78 | 57 (73.1) | 17 (21.8) |
| 300 × 700 | 0.049 | 5 | 80 | 60 (75.0) | 20 (25.0) |
| 15 | 237 | 172 (72.6)a | 52 (21.9)a | ||
| 400 × 300 | 0.037 | 5 | 79 | 65 (82.3) | 15 (19.0) |
| 400 × 500 | 0.062 | 5 | 76 | 68 (89.5) | 22 (28.9) |
| 400 × 700 | 0.087 | 5 | 75 | 64 (85.3) | 25 (33.3) |
| 15 | 230 | 197 (85.7)b | 62 (27.0)ab | ||
| 500 × 300 | 0.058 | 5 | 77 | 66 (85.7) | 23 (29.9) |
| 500 × 500 | 0.098 | 5 | 80 | 65 (81.3) | 25 (31.3) |
| 500 × 700 | 0.137 | 5 | 77 | 63 (81.8) | 28 (36.4) |
| 15 | 234 | 194 (82.9)b | 76 (32.5)b | ||
| Control (plain culture) | 237 | 174 (73.4)a | 48 (20.3)a | ||
- Values with different superscripts within columns differ significantly (anova, Tukey test, p < 0.5).
| WOW microenvironment | Replicates | n | Cleaved (%) | Blastocysts (%) | |
|---|---|---|---|---|---|
| Depth × diameter (μm) | Volume (μl) | ||||
| 300 × 300–500 | 0.021–0.035 | 15 | 235 | 186 (79.1) | 53 (22.6)a |
| 500 × 300–500 | 0.035–0.098 | 15 | 234 | 190 (81.2) | 64 (27.4)ab |
| 700 × 300–500 | 0.049–0.137 | 15 | 232 | 187 (80.6) | 72 (31.0)b |
| Control (plain culture) | 15 | 237 | 174 (73.4) | 48 (20.3)a | |
- Values with different superscripts within columns differ significantly (anova, Tucky test, p < 0.5).
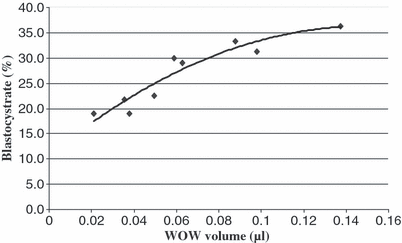
Relationship between WOW volume and developmental rate to the blastocyst stage of single bovine embryos. Check for Pearson correlation confirmed a strong relationship (r2 = 0.92, p < 0.05)
Experiment 2: Effect of microenvironmental embryo density on in vitro development of single bovine zygotes
When zygotes (n = 176) were placed single in different microenvironmental embryo densities [embryo per WOW volume (1 : μl)], embryos placed in microenvironmental embryo densities of 1 : 0.445 μl did not differ compared with embryos cultured in densities of 1 : 0.269 μl and 1 : 0.137 μl. In contrast to this, all embryos cultured in microenvironments reached a significantly higher blastocyst rate than zygotes cultured plain in group of 16 (31.3% vs 29.0% vs 27.3% vs 22.2%) (Table 3). No effects on cleavage rate, total cell count, TE cell count or ICM cell count were observed between embryos cultured in microenvironments and embryos cultured plain (control) as shown in Table 3.
| WOW Microenvironment | Replicates | n | Cleavage (%) | Blastocysts (%) | Cell counts | ||
|---|---|---|---|---|---|---|---|
| Volume (μl) | Diameter × depth (μm) | Total | ICM | ||||
| 0.137 | 500 × 700 | 11 | 176 | 142 (80.7) | 48 (27.3)a | 96.6 | 35.0 |
| 0.269 | 700 × 700 | 11 | 176 | 135 (76.7) | 55 (31.3)a | 99.6 | 31.0 |
| 0.445 | 900 × 700 | 11 | 176 | 138 (78.4) | 51 (29.0)a | 105.9 | 29.2 |
| Control group (plain culture) | 11 | 176 | 139 (79.0) | 39 (22.2)b | 99.3 | 31.8 | |
- ICM, inner cell mass.
- Values with different superscripts within columns differ significantly (p < 0.05).
Experiment 3: Effect of macroenvironmental embryo density on in vitro development of single bovine zygotes
Embryos cultured in fixed embryo per WOW volume ratios (1 : 0.269 μl) reached the blastocyst stage the more effective the lower the macroenvironmental embryo density [embryo per culture dish volume (n : μl)] was (Table 4). Macroenvironmental embryo densities of 1 : 30 μl reached significantly higher developmental rates to the blastocyst stage compared with embryos cultured in a density of 1 : 20 μl and development was the lowest at 1 : 10 μl (33.1% vs 25.0% vs 13.1%).
| WOW macroenvironment | Replicates | n | Cleavage (%) | Blastocysts (%) | |
|---|---|---|---|---|---|
| Dish volume (μl) | Embryo density (μl) | ||||
| 160 | 1 : 10 | 10 | 160 | 128 (80.0) | 21 (13.1)a |
| 320 | 1 : 20 | 10 | 160 | 134 (83.8) | 40 (25.0)b |
| 480 | 1 : 30 | 10 | 160 | 136 (85.0) | 53 (33.1)c |
- Values with different superscripts within columns differ significantly (p < 0.05).
Discussion
In the present study, different WOW properties and embryo densities (micro- and macroenvironment) were tested for their ability to support development of bovine embryos following in vitro fertilization.
Development of embryos using the WOW system was much higher compared with plain culture in group of 16 embryos. This result is in accordance with previous studies reporting that the rate of blastocysts was improved by the WOW system (Vajta et al. 2000; Taka et al. 2005). The explanation for that is the effect of the microenvironment. The microenvironment created by the WOW system allows a suitable concentration of embryonic factors surrounding embryos to be maintained, which compensates against lower embryo numbers in the total culture volume (Vajta et al. 2000).
When bovine zygotes were cultured in WOWs with different diameter and depth in a 3 × 3 factorial design in our experiments, WOW diameter significantly affected cleavage and blastocyst rate with larger diameter (500 μm) leading to higher developmental rates than lower ones (300 μm). One explanation for this result could be that smaller diameter acts like a bottleneck leading to a reduced diffusion of toxic metabolites out of the microenvironment or a decreased influx of nutrients into the WOW. Our findings are in agreement with a recent publication (Taka et al. 2005), which showed that WOW diameter of 1000 μm resulted in higher developmental rates to the blastocyst stage than diameters of 500 μm in the porcine. However, in that study, 4–5 embryos were placed in each WOW, whereas in our experiment, one single embryo was cultured in each WOW. Moreover, it is difficult to convert results from the porcine to the bovine. Similarly, WOW depth significantly affected developmental characteristics with a depth of 700 μm being most effective. The greater depth leads to a larger distance between embryo and microenvironmental opening which may result in a reduced diffusion of auto- and paracrine embryotrophic factors out of the WOW and thereby maintain a high concentration of positive factors surrounding the embryo. Candidates for these factors are IGF-I, IGF-II, platelet activating factor (PAF) and platelet-derived growth factor (PDGF) (for review: O’Neill 2008). On the other hand, greater depth also reduces diffusion of toxic metabolites such as ammonia and oxygen-derived free radicals out of the well. However, the positive effect seems to be more weighty than a hypothetic detrimental effect of accumulated toxic substances. Interestingly, the few publications which have dealt with WOW culture before, have prepared WOWs with a relatively low depth of 250–300 μm (Vajta et al. 2000; Taka et al. 2005; Kamiya et al. 2006; ), which is much lower compared with the best conditions obtained in our study. When combining diameter and depth to the factor WOW volume (pi × (diameter/2)2 × depth), regression analysis confirmed a strong relationship between WOW volume and blastocyst rate. Larger WOW volumes gave a better development compared with smaller ones. Thus, microenvironmental embryo density as defined by embryo number per WOW volume [n/μl] is correlated to embryo development with microenvironmental embryo densities of 1 : 0.137 μl giving much better development than 1 : 0.021 μl. Correlation of developmental rates with microenvironmental embryo density was greater than correlation of blastocyst rate/diameter and was the lowest for blastocyst rate/depth. This can be explained by the fact that diameter and depth are factors of the volume, but the diameter has a higher impact for calculating the volume, as it is a square factor. For reasons of this finding, we aimed to test whether further increase of the WOW volume results in more enhanced development to the blastocyst stage. Therefore, we tested in a second set of experiments whether further decreased microenvironmental embryo densities (1 : 0.445 μl and 1 : 0.269 μl) are more beneficial compared with one of 1 : 0.137 μl. Lower microenvironmental embryo densities were prepared by increasing the WOW volume (increase of the diameter up to 900 μm while keeping depth and embryo number per WOW constant). However, further increase in WOW volume (decrease in microenvironmental embryo density) did not result in higher blastocyst rates and developmental rates reached a plateau when the volume exceeded 0.137 μl. We speculate that a decrease in developmental rates would take place after exceeding a certain WOW volume because lowering the microenvironmental embryo density counteracts the accumulation of parakrine and autokrine factors. However, we did not explore the upper limit for optimal WOW volume. Best developmental rates to the blastocyst stage (31.3%) was observed in our experiments when an embryo per WOW volume of 1 : 0.269 μl was used. This developmental rate is comparable with developmental rates usually obtained in mass embryo production systems using plain culture (for review: Lonergan 2007), but seems to be lower compared with those reported by Vajta et al. 2000;. However, in that work, the authors calculated the blastocyst rate on the basis of putative zygotes, whereas blastocyst rate was calculated on the basis of oocytes in our study. Moreover, results within different publications are difficult to compare when different culture media (CR1aa vs SOFaa) and different oxygen tensions (20% vs 5%) are used (for review: Merton et al. 2003). Furthermore, embryo quality in terms of different cell counts did not differ within embryos derived from microenvironmental embryo densities of 1 : 0.137 μl, 1 : 0.269 μl and 1 : 0.445 μl. This is in accordance with previous publications, which also found no effect of the WOW volume on differential cell counts in the bovine (Vajta et al. 2000) and porcine (Taka et al. 2005; Kamiya et al. 2006). However, that observation is in contrast to experiments performed in plain culture where embryo density was found to affect differential cell count in murine embryos (Stoddart et al. 1996).
The use of WOWs with diameters larger than 700 μm was found to be impractical because embryos easily swapped out of these WOWs and best development to the blastocyst stage was observed when single bovine embryos were cultured in WOWs with a volume of 0.269 μl (700 × 700 μm). It has to be considered that the WOWs used in our study are of cylindrical shape with a subconcave bottom, whereas WOWs used in other studies have a more or less conical shape. As a consequence, the opening of our WOWs is relatively small in relation to the volume of the WOW. As the opening diameter directly affects the arrest of beneficial auto- and paracrine factors within the WOW, the amount of diffusion of toxic substances out of the WOW as well as the influx of nutrients into the WOW, we believe that the best volume for cylindrical WOWs has to be larger than for conical WOWs. The most suitable embryo per WOW volume was 1 : 0.269 μl in our experiments, whereas the best embryo to culture volume ratio has been reported to be 1 : 0.125 μl, termed the ‘effective volume’ (Wheeler et al. 2007). The volume of our WOWs is also larger compared with those used in a recent work (Taka et al. 2005). Moreover, 12–15 Embryos were placed within one Well in that work. In that study, microenvironmental embryo density was 25–30 times higher compared with that in our study. Microenvironmental porcine embryo densities of 1 : 0.015–1 : 0.012 μl resulted in much lower blastocyst rates compared with microenvironmental embryo densities of 1 : 0.060 – 1 : 0.048 μl WOW (Taka et al. 2005). The authors suggested that the embryo density of the first mentioned group was inadequately high and diffusion of toxic substances in the culture medium was probably limited. They concluded that the most important condition for the porcine IVP system is to create a microenvironment that maximizes and maintains embryonic factors and diffusion of toxic substances. Taking into account also culture of bovine embryos in microfluidic platforms, the best embryo to culture volume ratio has been reported to be 1 : 0.125 μl, termed the ‘effective volume’ (Wheeler et al. 2007), which is very comparable with our most successful microenvironmental embryo density (1 : 0.269 μl). However, the comparison between static and fluidic culture systems should be done carefully. Considering also the culture of zona free cloned embryos, it was reported that higher microenvironmental embryo densities supported higher developmental rates compared with lower microenvironemtal embryo densities (Feltrin et al. 2006). Furthermore, the microenvironmental embryo density of the most successful group of that study was much higher compared with the best of our study. However, these embryos did not have a zona pellucida and therefore we suggest that a smaller WOW has to mimic the function of the zona pellucida to enhance the concentration of paracrine substances surrounding the embryo under these conditions. It is obvious that zona free embryos cannot be compared directly with zona enclosed embryos.
In a previous study, best development of bovine embryos was observed when 5 embryos were placed in one WOW of 0.15 μl volume (microenvironmental embryo density of 1 : 0.03 μl) (Vajta et al. 2000). High developmental rates for plain embryo culture were reported at an bovine embryo density of 1 : 8 μl (Holm et al. 1999), whereas others reported an embryo density between 1 : 5 μl (Fujita et al. 2006) and 1 : 1 μl (Ferry et al. 1994) being best for embryonic development of bovine embryos. However, different studies are difficult to compare because of different time-points for developmental check, culture media and oxygen tensions (for review: Lonergan 2007).
Furthermore, we successfully explored if the culture dish volume (macroenvironmental embryo density) is also an important factor for the success of the WOW culture system. Large volumes (lower macroenvironmental embryo densities) allowed a significantly higher development to the blastocyst stage compared with smaller volumes in our study. The best development was observed at a macroenvironmental embryo density of 1 : 30 μl with development being lower in case of higher densities because of smaller total volumes of culture medium. On the contrary, studies in which embryos were cultured plain reported the best development at higher embryo densities (Ferry et al. 1994). The authors of that study reached a blastocyst rate of 18% at a density of 1 : 1 μl (embryo per culture dish volume), whereas the same authors reached a blastocyst rate of 2.5% when culturing embryos in a density of 1 : 10 μl. Thus, the WOW system allows to use low macroenvironmental embryo densities (1 : 30 μl in our case) because of the metabolite accumulating effect of the WOW, which results in high microenvironmental concentrations within the WOWs. Actually, we suggest that culture systems using larger volumes and thereby lower macroenvironmental embryo densities have a greater capacity to remove toxic substances out of the WOW and to provide the embryos with nutrients according to their diffusion capacity, more stable culture conditions and a larger reservoir for nutrients. We believe that the in vitro embryo culture environment has to accomplish conflictive needs: Low embryo densities to enhance accumulation of parakrine substances and a low embryo density for dilution of toxic metabolites while maintaining stable culture conditions and energy reservoirs. Thus, only the WOW system can provide a solution for both needs. In our experiments, we found the best microenvironmental embryo density (1 : 0.269 μl) to be much higher than reported by others. On the other hand, macroenvironmental embryo densities gave the best development when being much lower than reported by others (Ferry et al. 1994; Fujita et al. 2006). Interestingly, embryos cultured in WOWs of different volumes and thereby different microenvironmental embryo densities did not differ in terms of differential cell counts compared with controls. This is in accordance with previous publications, which also found no difference in differential cell counts both for bovine (Vajta et al. 2000) and porcine embryos (Taka et al. 2005; Kamiya et al. 2006) cultured in the WOW system compared with embryos cultured in groups as usual. Although it has been shown recently that culture of bovine embryos in WOWs affect the global gene expression profile (Hoelker et al. 2009), it seems that mitotic dynamics of embryos cultures in WOWs are not different from embryos cultured plain.
In conclusion, we clearly demonstrated that WOW properties affect development of bovine embryos. Micro- and macroenvironmental embryo densities have superior impact on developmental rates of bovine in vitro cultured embryos using the WOW system. A microenvironmental embryo density of 1 : 0.269 μl (WOWs being 700 × 700 μm) in combination with a macroenvironmental embryo density of 1 : 30 μl (16 embryos within 480 μl total culture medium) was most successful to culture bovine embryos in WOWs. Our optimized WOW culture system increased developmental rates of single bovine embryos to the blastocyst stage up to the level of usual mass embryo production (data not presented). While this gives the opportunity to track each embryo over the complete culture period in combination with high developmental rates, no differences in mitotic dynamic was observed. The results of this work will provide benefit for single embryo culture allowing the culture of single IVP embryos of high medical and/or economic interest at high success rates, which could have enormous implications for human-assisted reproduction. However, further experiments should be performed to check whether WOW properties affect the viability of WOW derived embryos after transfer to recipients.
Acknowledgements
We thank Mr. Stefan Knauf, University of Bonn, Germany for sedulous and exact preparation of WOWs of different calibres.
Author contributions
Hoelker, Rings, Lund and Phatsara performed experiments; Hoelker, Tesfaye wrote the paper; Schellander performed supervision and correction.




