Evaluation of ‘Section-Ligation-Release (SLR)’ Technique Devised for Castration in the Stallion
Contents
A novel technique [Section-Ligation-Release (SLR)] was evaluated for castration in the horse. Clinical traits, serum testosterone concentrations after challenge with human chorionic gonadotrophin (hCG), and histopathological changes of the testicular tissue were assessed. Five stallions, aged 24–48 months, were castrated using SLR technique under general anaesthesia. Both spermatic cords in each stallion were exposed at the scrotal neck by two 5-cm long incisions, followed by sharp dissection through the parietal vaginal tunic. Both vascular and non-vascular structures in the cords were triple clamped, transected and ligated. Both testes were left in situ. Serum testosterone concentrations were measured pre-operatively and at 2 months after castration following IV administration of 1 × 104 IU of hCG. Both testes from each castrate were collected at 2 months for histopathologic examination. SLR castration was successfully achieved. Moderated scrotal and preputial swelling was the only experienced short-term complication. Serum testosterone concentrations were significantly lower than basal pre-operative levels at 2 months after castration, and did not respond to hCG. On histopathology, hyalinization of the seminiferous tubules and loss of testicular interstitial tissue were indicative of complete avascular necrosis. This novel primary closure castration technique of stallion is a simple practical method, with minimal post-operative complications; and could be safely advocated as an alternative to the traditional castration techniques allowing for second intention healing of scrotal wounds.
Introduction
Castration is one of the most common surgical procedures performed by equine veterinarians. Over hundreds of years castration has been used to control masculine/aggressive behaviour in the male horse. In medieval times, kings rode stallions, and people of lesser stature often were described as riding geldings (Cable 2001). The expression of sexual behaviour by intact male horse in training or competition is undesirable because it can negatively affect their level of performance and may endanger their rider, handler or others. Several techniques have been described for equine castration (Searle et al. 1999). Traditional castration techniques allow for second intention healing of scrotal wounds, which causes potentially serious complications in more than 5% of the treated stallions (Turkstra et al. 2005). Castration complications represent one of the major categories of malpractice claims in equine practice (Thomas et al. 1998). For this reason, some have encouraged primary closure castration techniques. Some owners do not want to care for the incision sites, or wait for the open incision sites to heal. Closing the castration incisions eliminates most of the complications of castration such as infection, maggot infestation, delayed healing, and intestinal prolapse. The procedure is, of course, more expensive than a routine castration; however for some, the saved time and peace of mind are very much worth the extra cost. Until now, several castration techniques have been challenged based on primary intention healing in the male horse (Roberts 1964; Lowe and Dougherty 1972; Cox 1984; Barber 1985; Sedrish and Leonard 2001). In all these methods, regardless of the position of the incision, the testes were separated from their attachments, removed and discarded.
The objective of this study was to describe Section-Ligation-Release (SLR) technique as a novel primary closure method for castration in the horse by rendering the testes non-functional in situ. We hypothesized when testes are left in situ in the scrotum after transection and ligation of the spermatic cord, the testes undergo ischemic necrosis. The surgical removal of the testes by conventional primary closure techniques of equine castration undoubtedly needs a more invasive procedure and accordingly takes more time and renders the gelding more prone to the post-castration complications caused by inevitable excessive surgical trauma. We assumed that the SLR technique would provide a simple, safe, and reliable method with minimal surgical trauma for castration of stallions with a reduction in post-operative complications compared with the standard traditional techniques of surgical castration.
Materials and Methods
Five stallions aged 24–48 months, weighing 450–585 kg, were used in this study. The stallions had a standard pre-operative clinical examination including careful palpation of the external reproductive tract to confirm the presence of two normally descended scrotal testis. All stallions had normal vital signs, and normal hematological profile. All experimental procedures were reviewed and approved by our institutional Animal Care Committee. Food but not water was withheld for 12 h before surgery. Each stallion had received tetanus toxoid (5 × 103 IU/intramuscular). Castration was performed with the stallions anesthetized. Surgical time was measured from the time of making skin incision to placement of the last skin suture.
Castration technique
After pre-medication with xylazine hydrochloride (0.5–0.75 mg/kg IV), general anaesthesia was induced using sodium thiopental (6–8 mg/kg IV). Stallions were maintained at a surgical plane of anaesthesia with halothane in oxygen using a semi-closed circle system. Lactated Ringer’s solution (10–15 ml/kg/h, IV) was administered throughout the procedure. All stallions were administered butorphanol tartrate (0.03 mg/kg, IM or IV) intra-operatively to provide additional analgesia. The stallions were positioned in right lateral recumbency with the upper hind leg tied cranially. The scrotal base was clipped and aseptically prepared for surgery. Two 5-cm parallel incisions were made through the scrotal skin at the scrotal base over the spermatic cords. Each spermatic cord, which was contained within common tunic, was bluntly dissected and freed from the loose interlaced connective tissue of the spermatic fascia and exteriorized using a curved haemostatic forceps. The spermatic fascia plus parietal vaginal tunic were then carefully incised. Ductus deference was identified and then bluntly separated from the vascular portion of the cord (Fig. 1). Subsequently, three Rochester-Carmalt forceps were placed across the former structure (triple-clamping technique) (Fig. 2a) and it was transected between both distal forceps. After that, using no. 2 polyglactin 910, a circumferential ligature was placed at the site of the most proximal forceps. A transfixing ligature was then placed below the first ligature. A second circumferential ligature was placed proximal to the testis, at the site of the most distal forceps. All the three ligatures were placed on the grooves made by the applied forceps. Then, the vascular portion of the cord was triple clamped (Fig. 2b), severed, and ligated in a similar manner. The cord stumps were inspected for haemorrhage and re-ligated if necessary. The two skin portals were closed in two layers (vaginal tunic/spermatic fascia, and skin). Both testes were left in situ (in the scrotum) (Fig. 3). The geldings were recovered in a padded recovery room. Once the geldings were standing and able to walk, they were individually confined to a stall on a deep clean straw bed in conjunction with daily controlled hand walking for 20–30 min. No post-operative medications were administered to the geldings.
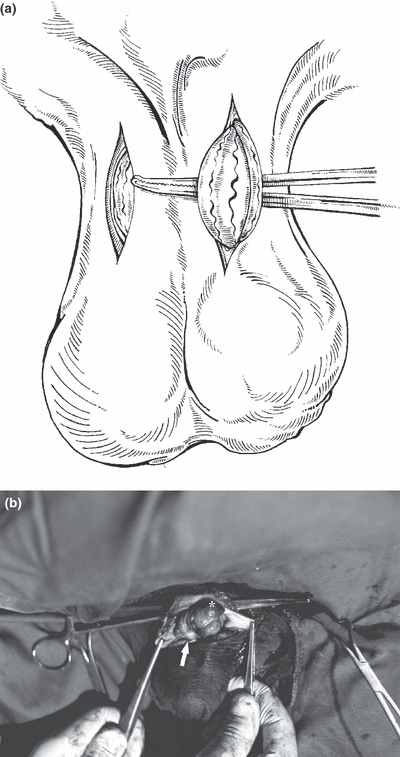
Skin incisions on scrotal neck and exteriorization of the spermatic cord by a haemostatic clamp are shown (a). Vascular portion (asterisk) and ductus deferens (arrow) are visible within the sharply-incised spermatic fascia/parietal tunica vaginalis (b)
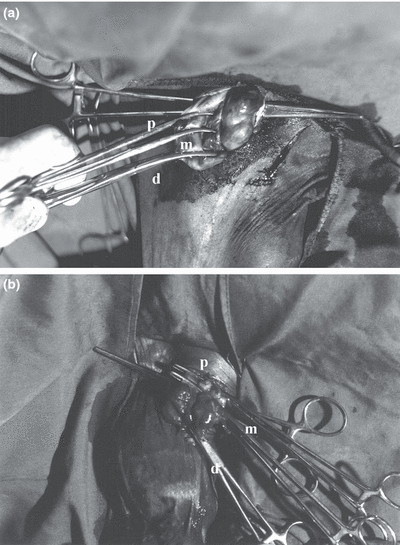
Intra-operative view of triple-clamping of ductus deferens (a) and the vascular portion of the cord (b). Proximal (p), middle (m), and distal (d) clamps are shown
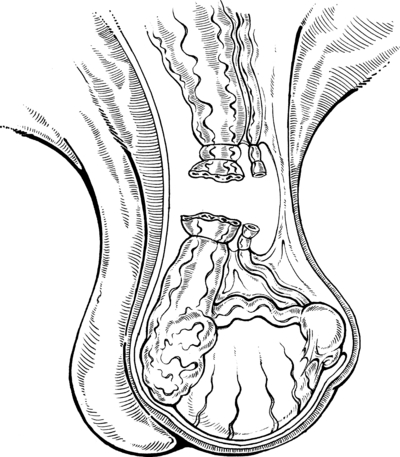
The testis is left in situ (in the scrotum) after transection and ligation of the spermatic cord
Assessments
Scrotal and preputial dimensions and condition
The stallions were evaluated before surgery and several times post-operatively using specific subjective testing criteria to quantify post-castration scrotal and preputial swellings. Dimensions of scrotum (circumference and length) and prepuce (width and length) were measured once daily on the day before castration (pre-castration), and on days 3, 6, 9, 12 and 15 after castration. On pre-castration day, scrotal and preputial lengths were determined by measuring the distance from the base of the scrotum to either the most distal end of the scrotum or the preputial ring, respectively. A location equivalent to 50% on the scrotal and preputial lengths from the scrotal base was permanently marked with a tattoo and was used as an orientation point for measuring scrotal circumference and preputial width. A spring tensioned measuring tape was used to help minimize operator variability between measurements (Fig. 4). Mean scrotal and preputial lengths and girths were determined from three consecutive measurements.
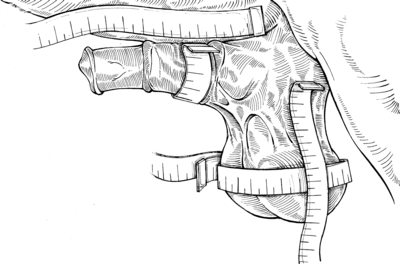
Scrotal and preputial dimensions are taken by a spring tensioned measuring tape
The scrotal and preputial condition (swelling, sign of inflammation, exudation, wound dehiscence and granulation) was noted by visual assessment of the relevant regions.
Testosterone assay
Serum testosterone concentration was measured in each stallion, pre-operatively. To quantify serum testosterone in the castrates, hCG stimulation test was performed two months after castration by taking three jugular venous blood samples, before and 1 and 24 h after intravascular administration of 10 000 IU of human chorionic gonadotrophin (Chorulon®; Intervet, Boxmeer, the Netherlands) (Strong et al. 1997; Searle et al. 1999). The blood samples were centrifuged at 700 g for 7 min within 1 h of blood sampling. The sera were separated and stored at −20°C until testosterone analysis. The testosterone concentrations were determined using radioimmunoassay by a LKB gamma counter. (Wallac OY, Turku, Finland).
Testes histopathology
Two months after castration, both testes of each castrate were removed by scrotal approach under general anaesthesia. Testes were fixed in a 10% neutral buffered formalin solution. The samples were embedded in paraffin wax; tissue sections were stained with haematoxylin and eosin, and examined microscopically for histopathological analysis of the castration success.
Statistical analysis
Data were treated by repeated measures analysis of variance (GLM procedure) followed by pair-wise comparisons among periods using the Bonferroni test. Differences were considered significant when p < 0.05. SPSS Version 9.0 for Windows, SPSS Inc., Chicago, IL, USA was used for analysis.
Results
In the anesthetized stallions, mean procedure duration was 36.5 min (range, 25–45 min). Pulse and respiration rates and body temperatures remained within normal limits during evaluation period. None of the castrates showed any serous, sanguineous or purulent secretions from both the scrotal incisions. Wound healing took place uneventfully with no signs of dehiscence and resultant granulation. During the 1st post-operative week, all castrates developed a mild scrotal and preputial swelling.
Scrotal and preputial dimensions and condition
Section-Ligation-Release technique of castration resulted in moderate scrotal and preputial swelling, with the maximum occurring on day 3 after castration. Mean (±SD) scrotal circumference and preputial width of all stallions before castration were 40 ± 5.34 cm and 20.4 ± 3.43 cm, respectively. On day 3 after castration, they were significantly (p < 0.05) increased to 56.2 ± 7.56 cm and 35 ± 9.67 cm, correspondingly. Scrotum and prepuce showed a significant increase in length on day 3 after castration (p < 0.05) (24.8 ± 3.96 cm vs 15.2 ± 1.64 cm, and 15.7 ± 1.61 cm vs 9 ± 0.89 cm, respectively). Thereafter, the scrotal and preputial size began to decrease and around 15 days after castration, the swelling was no longer obvious (Fig. 5).
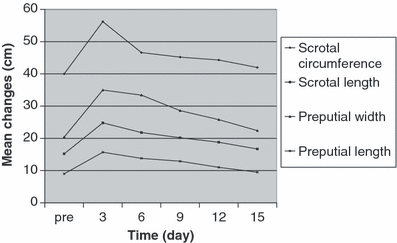
Scrotal and preputial dimensions (mean ± SD; n = 5) before and after castration. Significant increase in dimensions was seen on day 3 after castration (p < 0.05)
Testosterone assay
The testosterone concentrations after hCG challenge are depicted in Fig. 6. The mean pre-operative serum testosterone concentration was 1.35 ng/ml (range, 1.03–1.86 ng/ml). All the castrates showed a significant (p < 0.01) decrease in testosterone level 2 months after castration as compared to the pre-operative value (0.36 ± 0.16 ng/ml vs 1.35 ± 0.34 ng/ml). Following hCG administration, the serum level of testosterone showed a slight, non-significant increase on 1 h post-treatment (0.56 ± 0.18 ng/ml), and reached around the baseline value (0.4 ± 0.13 ng/ml) after 24 h.
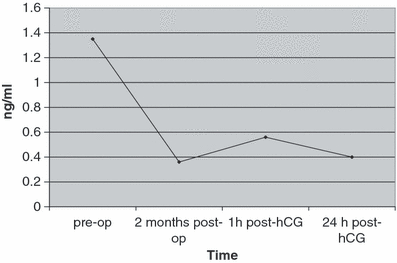
Effect of hCG administration on the levels of testosterone in the castrates serum (mean ± SD; n = 5). The castrates showed a significant decrease in testosterone level 2 months after castration (p < 0.01)
Testes histopathology
The testicular parenchyma was totally affected by coagulative necrosis caused by ischemia. The cells of the seminiferous tubules had lost their nuclei and cellular outline, resulting in complete hyalinization of the tubules. Testicular interstitia were widely necrotized. Diffused oedema was observed in the interstitium and the Leydig cells were totally absent (Fig. 7). In the outer layer of the testes, fibrous tissue was present and visceral and parietal layers of the tunica vaginalis were joined (Fig. 8). No signs of spermatogenesis were evident in the examined testes.
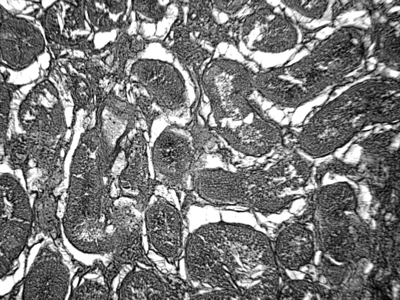
Photomicrograph of a cross-section of the testicular parenchyma. Seminiferous tubules exhibit hyalinization. The interstitium is completely necrotized with diffused oedema. The Leydig cells are totally absent. H&E; X 150
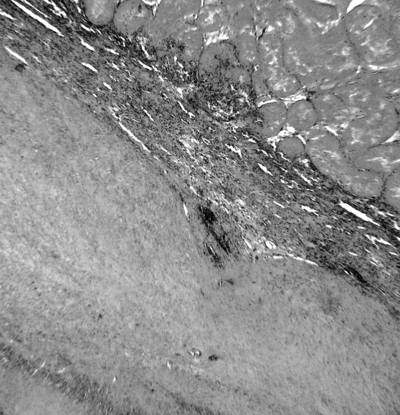
Photomicrograph of testis. Coagulative necrosis of testicular parenchyma surrounded by fibrovascular connective tissue. H&E; X 100
Discussion
Section-Ligation-Release castration technique was readily accomplished in anesthetized recumbent stallions. The spermatic cord was easily recognized, occluded and transected through rather small skin incisions at the scrotal neck without major complications. The present study is the first documented study describing a primary intention healing technique for surgical castration of stallions while leaving the testes in the scrotum.
The surgical removal of the descended testis can be performed using an open or closed technique. There is also a variation on the closed technique referred to as half-closed or semi-closed castration (Searle et al. 1999). The conventional equine castration techniques have been followed by healing by second intention, a process that is necessarily characterized by oedema, soreness, and secondary infection (Cox 1984). Accordingly, some veterinary surgeons have advocated primary closure in equine castration (Roberts 1964; Lowe and Dougherty 1972; Cox 1984; Barber 1985; Sedrish and Leonard 2001). Roberts (1964) reported that when an adequate standard of asepsis was maintained, no untoward sequelae followed partial suturing of the scrotal wounds, which were produced when adult stallions were castrated. In 1972, Lowe and Dougherty reported minimal oedema and rapid convalescence in horses castrated by a closed technique involving suturing of the skin incision. A technique of equine castration with primary closure and scrotal ablation was originally introduced by Barber (1985). Early return to work, minimal post-operative management and a more favourable and early cosmetic outcome with no possibility of eventration are the reported advantages of the latter technique (Barber 1985). Modifications of Barber’s technique have been reported by Sedrish and Leonard (2001), and Osinski (2004). Laparoscopic techniques, w/wo linear stapler, have also been reported for primary intention sterilization of stallions and male donkeys with a reduction in post-operative complications compared with standard techniques of surgical castration (Fischer and Vachon 1998; Wilson 2002; Pepe et al. 2005). Ponvijay (2007) described pinhole castration, a novel minimally invasive technique for in situ castration of bull calves, and proposed the technique be readily adaptable in larger animals with thicker spermatic cords, e.g. horses. Section-Ligation-Release technique, as evaluated in the present study, proved to be a safe and fairly quick method for castrating stallions through scrotal neck incisions followed by primary closure of the skin.
Transection of the spermatic cord induced avascular necrosis of the testicular tissue, without revascularization of the testes as shown by serum testosterone concentrations 2 months after surgery. Testicular coagulative necrosis was confirmed in all testes examined histopathologically at 2 months post-castration. In a study by Smith (1955), it was shown that after 4 h of induced testicular ischemia in dogs, spermatogenesis had ceased and in some testicles the Sertoli and the Leydig cells were also damaged, while 10 h or more of ischemia resulted in elimination of all Leydig cells; and the testicular elements were replaced by connective tissue. In a later study, also in dogs, it was demonstrated that after 12 days of vascular occlusion, seminiferous tubules were replaced by an ‘amorphous mass’ and had no basement membrane. Leydig cells at this time had become atrophied (Dixit 1977). In some studies, marked testicular degeneration was noted after ischemia for 60 min in the rat (Carmignani et al. 1983; Costentino et al. 1986). Local infarction in the testicles and infertility has also been reported in rams after experimental ischemia of the testicular artery (Markey et al. 1994). Our observation of testicular necrosis in the castrates are thus in agreement with the previous observations. In our study, vascular occlusion and transection blocked up the blood flow to the testicles producing testicular necrosis as a result of the hypoxia. An inevitable ischemic necrosis or infarction of the testicles is produced either when occlusion of the arterial supply or when the venous drainage of the tissue occurs (Slauson and Cooper 2002). In the present study, application of the ligatures and transection of the spermatic cord apparently impeded the blood flow to the testes with a subsequent quick hypoxia response of the testicular cells ending in coagulative necrosis.
Endocrine analysis provides a useful non-invasive technique for determining the presence of testicular tissue in suspect cryptorchids and questionable animals (Morrow 1986; St Jean et al. 1992; Cox 1993). The hCG stimulation test is common practice in cases of equine cryptorchidism with the observed increase in testosterone concentration in animals with testicular tissue being thought to result in increased diagnostic accuracy of endocrine testing (Cox 1993). Stimulation of Leydig cells with hCG has been widely used and is often considered the gold standard (McEachern et al. 2004). The test is based on the Leydig cell elaboration of testosterone in increased amounts in response to the injection of hCG (Morrow 1986). hCG acts in a manner similar to LH, stimulating the Leydig cells to produce testicular steroids (Zamaratskaia et al. 2007). The hCG stimulation test has been reported to be 95% accurate in diagnosing equine cryptorchidism (Cox et al. 1986). Arighi and Bosu (1989) reported peaks in testosterone concentration at 60 min and 24 h after intravenous injection of 10 000 IU hCG to intact or cryptorchid stallions with the 24 h concentration representing a 2 to 10 fold increase over the 60 min concentration. These authors postulated that a 24-h interval after hCG for the follow-up sample may be preferable to the 30-min interval recommended by Cox et al. (1986) (Arighi and Bosu 1989). Although a normal stallion should have a greater than three fold increase in serum levels following hCG administration (Milliken et al. 1995), the injection of hCG into a gelding has only very slight effect on plasma testosterone concentration (Roberts 1991). In this study, no significant changes in testosterone concentrations were detected in the castrates, thus the post-hCG stimulation serum testosterone concentrations could be in consistent with successful sterilization.
Antibiotics and anti-inflammatory drugs were not administered in this study in order that the post-operative responses to SLR technique over 2 months could be evaluated entirely.
The most common complications following traditional castration of horses are excessive swelling, haemorrhage, incisional infection, scirrhous cords and eventration (Hunt 1991). The technique reported here, was associated with minimal complications. The SLR technique was associated with moderate scrotal and preputial swelling, reached the highest point on day 3 post-operative. Some preputial and scrotal oedema is normal, usually greatest 3 to 6 days after castration and subsiding by 9 days (Hunt 1991). Excessive swelling is a common complication of equine castration and it typically occurs with inadequate wound drainage, inadequate post-operative exercise, poor lymphatic drainage, excessive surgical trauma or infection (Searle et al. 1999). In a series of 21 primary closure castrations reported by Lowe and Dougherty (1972), only one of 21 animals developed moderate oedema and four of 21 developed fluid distention of the scrotum, which decreased in time with exercise. Haemorrhage may occur during, immediately after or several days after castration (Hunt 1991). Although previous reports have described an incidence of haemorrhage following conventional surgical methods of equine castration (Hunt 1991; Schumacher 2006); as a result of cord ligation and closing the incisions, we did not experience this problem. As a result of closing the incisions and not having a continued source of contamination of the surgery site, we believe that this technique could decrease the occurrence of incisional infections and scirrhous cord formation. Although we did not have any post-operative infections in the cases reported here, the numbers are too low to evaluate. Moreover, eventration of any abdominal viscera did not occur. By far the most serious complication of castration is eventration (Thomas et al. 1998). We believe that in this method, even when the eventration occurs, the skin sutures are able to maintain the viscera in a sterile environment until the horse could be re-operated. As a result of not having the viscera exposed to the external environment, the subsequent peritonitis that usually occurs with eventration was prevented and the horse made a quicker and safer recovery (Sedrish and Leonard 2001).
In the present study, SLR castration was conducted by making incisions at the scrotal neck, over the spermatic cord. We suppose that the position of the incision might prevent contamination from dirt or bedding. This is in agreement with Lowe and Dougherty (1972) reports on primary closure castration in 16 horses and five ponies, using an incision between the scrotum and the inguinal ring. Cox reported the primary closure of 311 scrotal testes removed from horses and donkeys by making scrotal incisions. The results of this technique were good; however, slight swelling or oedema in the surgical area was reported and 12 castrations also resulted in appreciable haemorrhage (Cox 1984).
In SLR technique, the structures in the spermatic cord were crushed and ligated using synthetic absorbable material. During the first quarter of the 20th century, a time-honoured teaching was that on no account should ligatures be used for spermatic cords, because of the persisting infection they provoked. With the adoption of catgut in animal surgery, the ligature method was re-introduced (Wright 1963). Undoubtedly, cord bleeding is prevented and controlled by ligatures and with due attention to surgical asepsis, the presence of small foreign bodies in the form of ligatures, which would be absorbed shortly, would not cause serious complications. Moreover, ligation of the entire spermatic cord proximal to the point of division has been considered important in preventing post-operative swelling when primary closure is employed (Schumacher 2006). In Lowe and Dougherty (1972) report, the spermatic cords were transfixed and transected rather than emasculated. Primary closure castration was reported by Cox in 262 horses and donkeys in which, the structures in the spermatic cord were either ligated or emasculated. Better results with ligation compared with emasculation were concluded (Cox 1984). Barber (1985) and Palmer and Passmore (1987) reported using a median scrotal ablation technique for removal of descended and non-descended testicles. The two techniques differed in that the spermatic cord was transected via emasculation or transected after ligation. Both authors reported good results, but both techniques were time consuming. Osinski (2004) reported on an original method of primary closure castration. In this method, post-operative bleeding was prevented by the controlled degree of spermatic cord squeezing by twisting a loop of cerclage wire around the cord near the external vaginal ring. No complications, except for a mild post-operative wound oedema, were reported.
Synthetic absorbable material used for cord ligation in the present study, is absorbed by a process of hydrolysis, releases antimicrobial substances during absorption and provokes very little tissue reaction and oedema.
The potential benefits of SLR castration in horses include decreased post-operative morbidity, rather smaller incisions, controlled haemorrhage, and decreased post-operative recovery compared with conventional surgical castration. Revascularization of the testicular tissue was not encountered in SLR castration based on the post-hCG stimulation serum testosterone concentrations and the absence of active testicular tissue in histopathology.
It should be noted that this method may have several limitations. Asepsis is essential with this technique, otherwise the introduction of infection into a scrotal cavity would result in severe complications (Kent et al. 1996; Searle et al. 1999). Although in SLR castration technique, the aesthetics of the scrotum will improve, the client should be informed about the post-castration presence of the non-functional testes in the scrotum to avoid potential confusion regarding the status of gelding on the animal’s identification. The SLR technique does not seem to be an apt option for standing castration in horse. Then, disadvantages of recumbency, cost and patient risks of general anaesthesia should be taken into account when the technique is considered.
Despite the fact that geldings in this report had none of the major complications reported with traditional castration methods, a more comprehensive long-term comparative study should be undertaken to understand fully the pro et contra of this technique.
Based on the results of clinical, hormonal and histopathological evaluations of SLR technique presented in this study, the novel technique reported here is proved to be a safe and fairly quick primary closure method for castration in stallions through scrotal base incisions. The technique was associated with minimal complications, and could be brought into consideration as an alternative to the conventional equine castration methods allow for second intention healing of scrotal wounds.
Acknowledgements
The authors gratefully acknowledge the financial support of this study by the Urmia University College of Veterinary Medicine. We are especially thankful to Dr. Mojtaba Hadian for reading the manuscript and for his helpful suggestions.




