Effect of Exogenous GnRH at the Time of Artificial Insemination on Reproductive Performance of Awassi Ewes Synchronized with Progestagen–PMSG–PGF2α Combination
Contents
This study was carried out to investigate the efficacy of PGF2α for oestrus synchronization (ES) in Awassi ewes to which were administered the progestagen–PMSG combination, and to evaluate the effect of the exogenous GnRH administration immediately after the artificial insemination (AI) on their pregnancy rate and lambing performance during the breeding season. The ewes (n = 33) were treated with an intravaginal sponge impregnated with 30 mg fluorogestane acetate for 12 days and were injected with 500 IU PMSG at the time of removal of the sponge. The ewes were then divided into three equal groups of 11 ewes each. One millilitre of physiological saline (0.9% NaCl; placebo) was administered to each ewe in Group 1 at the time of second AI. Approximately 4 μg GnRH (busereline) was injected to each ewe in Group 2 immediately after second AI. A total of 150 μg PGF2α (cloprostenole) was injected at the time of sponge removal on day 12 and 4 μg GnRH immediately after the second AI was also treated to each ewe in Group 3. Intracervical AI with diluted fresh semen was performed twice at 12 and 24 h following the onset of oestrus. The injection-oestrus onset and injection-oestrus-end interval in Group 3 was significantly (p < 0.001) shorter than both Groups 1 and 2. Although the pregnancy rates of Groups 2 and 3 (81.8%; 9/11) were numerically higher than of Group 1 (63.6%; 7/11), the difference among the groups was statistically insignificant. The multiple birth rate of Group 3 was found higher than Groups 1 and 2. However, the number of single lambs of Group 1 was also higher than Groups 2 and 3 (p < 0.05). Despite the litter sizes of Groups 2 (1.27; 14/11) and 3 (1.55; 17/11) being numerically higher than Group 1 (0.73; 8/11), the differences among all the groups were statistically insignificant. In conclusion, the administration of PGF2α at the time of removal of the sponge shortens the injection oestrus-onset and oestrus-end interval in Awassi ewes treated with progestagen–PMSG. Additionally, exogenous GnRH treatment immediately after the AI increases the multiple birth rate of Awassi ewes synchronized with progestagen–PMSG–PGF2α combination.
Introduction
The Awassi is the main type of sheep in the Middle East, Asia and Europe, where it is raised for meat, milk and wool. It is known for its hardiness and adaptability to the local environment, and, in the case of the Improved Awassi, also for its high milk production. Yet, the prolificacy of the Awassi is low – approximately 1.2 lambs born per ewe lambing. As lamb production is an important source of income in all flocks, increasing the fecundity of the Awassi has always been an important breeding goal (Gootwine and Goot 1996). To increase the productivity, flock management techniques employ a variety of methods, including oestrus synchronization (ES), artificial insemination (AI) and in vitro fertilization (IVF) in ewes (Wildeus 1999; Abecia et al. 2001; Dixon et al. 2006). For more than half a century, attempts have been made to synchronize the period of sexual receptivity, or oestrus, in farm animals. Oestrus synchronization can save labour, and is a key component in AI programmes (Knights et al. 2006). The technology of AI reduces the incidence of venereal diseases and greatly increases the genetic merit (Foote 1999). Therefore, ES together with AI in ewes is important in the improvement of reproductive efficiencies and management processes (Gordon 1999).
Intravaginal sponges impregnated with progesterone and/or its analogues, or implants, PGF2α alone or combined with gonadotropins are extensively used for the ES methods in sheep during breeding or non-breeding season (Boscos et al. 2002). Different ES methods and afterwards single or double natural mating or AI have been successfully used to improve upon the reproductive management of sheep flocks worldwide (Gordon 1999; Paulenz et al. 2003). Synchronized breeding results in synchronized lambing, thus concentrating and reducing the labour requirements at lambing. Synchronized lambing, in turn, results in a more uniform lamb crop, which facilitates both the management and marketing of lambs (Knights et al. 2006).
Sponges impregnated with progesterone provide ES by extending the luteal phase during the treatment period in ewes (Wildeus 1999; Whitley and Jackson 2004). After the withdrawal of sponges, luteal regression and follicles development occur. PGF2α is the luteolytic factor for sheep, and the use of PGF2α and/or one of its analogues causes faster regression of CL than normal luteolysis in sheep having a functional CL at the time of treatment (Gordon 1999). The use of PGF2α and/or its analogue alone, a double injection system 9 and/or 11 days apart, is the most widely used approach in sheep (Wildeus 1999), progesterone and/or its analogue treatment combined with the injection of PGF2α are also used (Dixon et al. 2006). Beck et al. (1994) and Dixon et al. (2006) have reported that ewes treated with progesterone–PGF2α combination exhibit a greater oestrus response than those of receiving PGF2α alone. Similarly, Dogan and Nur (2006) also documented that the time between the sponge withdrawal and the oestrus onset in Kivircik ewes treated with progesterone–PMSG and/or progesterone–PMSG–PGF2α combinations was more shorter than those of receiving progesterone alone and/or progesterone–PGF2α.
Lower dosages of progestagen have produced persistent ovulatory follicles. The pregnancy rate was inversely correlated with the duration of the growth of the ovulatory follicles or to the dosage of progestagen (Viñoles et al. 2001; Dixon et al. 2006). To stimulate follicle development and number, and afterwards to increase the ovulation rate and litter size, gonadotropins, such as PMSG (Gordon 1999; Dogan and Nur 2006), ECG, FSH (Boscos et al. 2002) and GnRH (Cam and Kuran 2004) are co-treated with intravaginal sponges. It has been reported that the use of the GnRH–PGF2α combination in ES (Ataman and Aköz 2006), and progestagen together with post-mating GnRH administration (Cam and Kuran 2004; Khan et al. 2006) positively effect the fertility parameters of sheep.
The aim of this study was to investigate the efficacy of PGF2α for ES in Awassi ewes, which were administered the progestagen–PMSG combination, and to evaluate the effect of exogenous GnRH administration immediately after the AI on their pregnancy rate and lambing performance during the breeding season.
Material and Methods
Hormonal drugs
Intravaginal sponges impregnated with 30 mg fluorogestane acetate (FGA), a progestagen analogue (Chrono-gest/sponge®; Intervet Drug Industry, Istanbul, Turkey), PMSG (Chrono-gest/PMSG®, 6000 IU/flacon) and GnRH (Receptal 10 ml, busereline acetate 4 μg/ml) were purchased from Intervet Drug Industry (Istanbul, Turkey). PGF2α (Dalmazin 10 ml, cloprostenole 75 μg/ml) was obtained from Vetaş Drug Industry (Istanbul, Turkey).
Animals and location
In the present study, a total of 33 clinically healthy, free of reproductive disorders and once lambed adult Awassi ewes (2–3 years old, weighing 35–45 kg), and six healthy adult Awassi rams (3–4 years old, weighing 55–65 kg) were used. The study was carried out between October 12 and November 11, which is the breeding season for sheep in Elazığ province of Turkey located at latitude of 38°40′N, in the Centre of Education, Research and Application at the Faculty of Veterinary Medicine, Fırat University. During the experimental period, the ewes were kept away from the rams to prevent voluntary mating. The ewes were kept indoors at night, and allowed to graze at pasture on the farm throughout the day. When ewes were kept indoors, they were fed a diet of barley and Lucerne hay, supplemented with grass. Fresh drinking water was provided ad libitum.
Treatment schedule
The treatment schedule is illustrated in Fig. 1. And, 500 IU PMSG was administered to all the ewes in each group following the intravaginal sponges insert for 12 days. Then, the animals were randomly divided into three equal groups of 11 ewes each. One millilitre of physiological saline (0.9% NaCl, placebo) was administered to each ewe in Group 1 at the time of both the sponge removal and second AI. Approximately 4 μg GnRH was injected to each ewe in Group 2 immediately after second AI. A total of 150 μg PGF2α was injected at the time of the sponge removal on day 12, and 4 μg GnRH immediately after the second AI was also treated to each ewe in Group 3. The doses of hormones, used in the study, were selected according to some previous studies (Cam and Kuran 2004; Aköz et al. 2006; Dogan and Nur 2006).
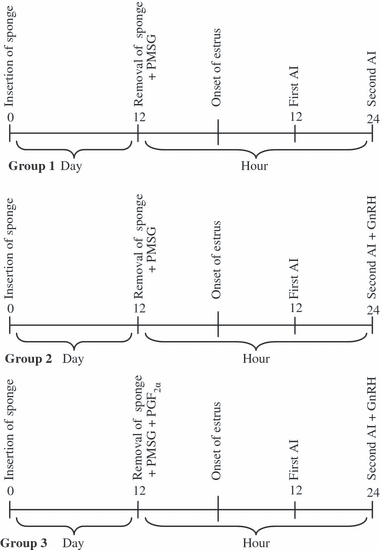
The schematic presentation of administration plan in all group ewes
Oestrus detection
In order to determine the time of the onset, the end and the duration of oestrus, all the ewes were monitored every 6 h from 12 to 96 h following the sponge withdrawal with the aid of the six teaser rams equipped with an apron. The onset of oestrus was confirmed by the passive ability of the ewes to stimulate the interest of the ram, and was usually quantified by measuring his behaviours (the frequency of approach behaviour, smelling or licking of the genitalia, flehmen, vocalization and grinding jaws). The end of oestrus was determined by the refusing of immobility in front of the ram by ewe. The duration of oestrus was evaluated by measuring the movements or postures of ewes (immobility or standing in response to a mounting ram as most obvious sign, lordosis and even leaning or pushing back towards the ram; Ucar et al. 2005).
Semen collection, processing and insemination of the ewes
Semen was collected from all the rams with aid of an electroejaculator twice at 12-h intervals. Each ejaculate was immediately evaluated to determine the motility of the semen. The percentage of sperm motility was evaluated using a light microscope with heated stage (Bearden et al. 2004). For this process, a slide was placed on a light microscope with a heated stage warmed up to 37°C, and then several droplets of this Tris buffer solution were dropped on the slide, and a very small drop of semen was added on the Tris buffer solution, and mixed with a coverslip. The percentage of sperm motility was evaluated by rating the motile spermatozoa which move their own power, to other spermatozoa which have circular, reverse, vibrating and rocking movements. Motility estimates were performed from three different fields in each sample visually at 400× magnification. The mean of the three successive estimates was used as the final motility score. Sperm concentration was determined by the hemocytometric method using the standard haemocytometer (Improved Neubauer, Deep 1/10 mm, Labart, Germany) slide and dilution pipette designed for counting the red blood cells (Bearden et al. 2004; Gür et al. 2005). All the ejaculates having >70% or higher progressive motility, were then pooled and diluted at a 1 : 4 ratio (semen : diluent) at 37°C with the Tris extender containing 3.63 g tris (hydroxymethyl) aminomethane, 0.50 g glucose, 1.99 g citric acid, 15% egg yolk and 100 ml distilled water (Sönmez and Demirci 2004). Then, 1000 IU sodium G penicillin and 1000 μg dihydrostreptomycine sulphate was added to the 1 ml of the diluted semen (Dogan and Nur 2006). The diluted semen was kept at 37°C in a water bath until insemination. Each ewe was intracervically inseminated twice at 12 and 24 h following the onset of oestrus using a specific insemination catheter containing 0.25 ml of diluted semen (approximately 100 × 106 spermatozoa), because it has been recommended that double AI with 12 h apart results in higher fertility and pregnancy rate in synchronized ewes (Paulenz et al. 2003).
Determination of pregnancy and other reproductive parameters
The pregnancies of ewes were determined by transrectal ultrasonography using B-Mod Real-Time ultrasound with a 7.5 MHz rectal probe (Falco Vet, Pie Medical, Maastrciht, the Netherlands) on day 25 after the removal of sponges. Oestrus response (Number of ewes showing oestrus/Total treated ewes in each group × 100), the pregnancy rate (Number of pregnant ewes/Number of inseminated ewes in each group × 100), the lambing rate (Number of lambing ewes/Number of pregnant ewes in each group × 100), multiple birth rates (Number of ewes lambing twin or triplet/Total number of lambing ewes in each group × 100), litter size (Number of total lambs/Number of lambing ewes in each group × 100) and the female or male lamb rate (Number of female or male lamb/Total number of lambs × 100) were recorded (Ataman and Aköz 2006).
Data analysis
The spss/pc program (Version 10.0; SPSS, Chicago, IL, USA) was used for data analysis. Data are presented as mean ± SEM, and a value of p < 0.05 was considered as significant. Values concerning sponge removal-oestrus interval and gestation period were compared by one-way analysis of variance (anova) and post hoc Tukey – HSD test. Chi-squared test was performed to determine the differences among all groups concerning the other reproductive traits measured.
Results
Oestrus response, gestation period and pregnancy rate
Oestrus response rates, gestation periods and pregnancy rates of all the groups are shown in Table 1. The oestrus response rate of each group following the removal of sponges was 100% (11/11). No significant differences were observed among any of the groups in terms of the gestation period. Although the pregnancy rates of Groups 2 and 3 (81.8%, 9/11) were numerically higher than Group 1 (63.6%, 7/11), the difference among the groups was statistically insignificant. The onset, the end and the duration times of oestrus after removal of the sponges are illustrated in Fig. 2. While PGF2α administration at the time of the sponge removal significantly (p < 0.001) shortened the injection-oestrus onset and injection-oestrus-end interval, it had no significant effect on the duration of oestrus.
| Variable | Group | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Number of ewes | 11 | 11 | 11 |
| Oestrus response (%) | 100 (11/11) | 100 (11/11) | 100 (11/11) |
| Pregnancy rate (%) | 63.6 (7/11) | 81.8 (9/11) | 81.8 (9/11) |
| Gestation period (days) | |||
| Single | 150.8 ± 4.5 | 153.6 ± 3.6 | 146.0 ± 6.0 |
| Twin | 151 | 148.7 ± 1.2 | 153.3 ± 4.4 |
| Triplet | 144 | 146.5 ± 0.5 | |
| Lambing rate (%) | 100 (7/7) | 100 (9/9) | 100 (9/9) |
| Multiple birth rates (%) | 14.3% (1/7)a | 44.4% (4/9)ab | 77.8% (7/9)b |
| Number of lambs | |||
| Single | 6 (85.7%)a | 5 (55.5%)ab | 2 (22.2%)b |
| Twin | 1 (14.3%) | 3 (33.3%) | 6 (66.6%) |
| Triplet | 1 (11.1%) | 1 (11.1) | |
| Litter size (%) | 0.73 (8/11) | 1.27 (14/11) | 1.55 (17/11) |
| Female lamb rate (%) | 50% (4/8) | 42.9% (6/14) | 52.9% (9/17) |
| Male lamb rate (%) | 50% (4/8) | 57.1% (8/14) | 47.1% (8/17) |
- Data are expressed as mean ± SEM.
- The difference among the values bearing different superscript (a, b) is statistically significant (p < 0.05).
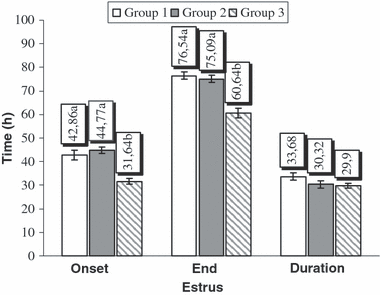
The onset, end and duration times of Oestrus after removal of sponges (Group 1: progestagen + PMSG + post-insemination NaCl; Group 2: progestagen + PMSG + post-insemination GnRH; Group 3: progestagen + PMSG + PGF2α + post-insemination GnRH). The difference among bars bearing values with different lower cases (a, b) is statistically significant (p < 0.001)
Lambing performance
The values concerning reproductive parameters are presented in Table 1. The lambing rates of each group were 100%. The multiple birth rate, the number of twin lambs and the litter size of Group 3 were found higher than Groups 1 and 2. However, the number of single lambs of Group 1 was also higher than Groups 2 and 3. When the multiple birth rates and the number of single lambs were statistically compared an insignificant difference between Groups 1 and 2 as well as between Groups 2 and 3, a significant (p < 0.05) difference between Groups 1 and 3 was found. However, the differences observed among any of the groups in terms of the number of twin lambs, litter sizes and female and male lamb rate were statistically insignificant (p > 0.05).
Discussion
Oestrus synchronization in livestock focuses on the manipulation of either the luteal or the follicular phase of the oestrous cycle. In ewes and does, the opportunity for control is greater during the luteal phase, which is of longer duration and more responsive to manipulation. Strategies can be employed to extend the luteal phase by supplying exogenous progesterone or to shorten this phase by prematurely regressing the existing CLs (Wildeus 1999; Whitley and Jackson 2004). For this purpose, many different protocols were used (Cline et al. 2001; Boscos et al. 2002; Sözbilir et al. 2006). Successful techniques must not only establish tight synchrony, but also provide an acceptable level of fertility upon AI or natural mating (Godfrey et al. 1999; Wildeus 1999; Khan et al. 2006). Therefore, ES constitutes the basis for successful AI and embryo transfer programmes.
In efforts to extend the luteal phase for ES, various forms of progestagens and different administration methods have been used in seasonally polyestric animals (Wildeus 1999). Progestagen administration is common, especially in sheep and does, and has been used with and without accompanying treatments such as prostaglandin analogues or gonadotropins (Whitley and Jackson 2004). The use of gonadotropin is routinely incorporated into intravaginal device synchronization systems used in ewes and does induce ovulation. The most commonly used product is PMSG at the time of sponge withdrawal. One limitation of PMSG is its long-acting biological activity, causing it to continually recruit antral follicles, which results in a large number of unovulated aged follicles (Armstrong et al. 1983; Wildeus 1999). The detrimental effect of ovulation from aged follicles is less clear and may be less critical to fertility in sheep. However, the extent of follicular ageing, and thus the effect on fertility may vary in relation to the dosages of progestagens or the duration of the treatment. Lower dosages of progestagen have produced persistent ovulatory follicles. The pregnancy rate was inversely correlated with the duration of the growth of the ovulatory follicles or to the dosage of progestagen (Viñoles et al. 2001; Dixon et al. 2006).
Dogan and Nur (2006) suggest that sponge withdrawal-oestrus onset interval in Kivircik ewes treated with progesterone–PMSG and/or progesterone–PMSG–PGF2α combinations was more short than those of receiving progesterone alone and/or progesterone–PGF2α in Kivircik ewes during the non-breeding season. Ewes treated with progesterone and PGF2α exhibit a greater oestrus response than those receiving PGF2α alone (Dixon et al. 2006). Husein and Kridli (2003) have alleged that progestogen–GnRH–PGF2α combination is more effective than GnRH–PGF2α in terms of the oestrus response and the pregnancy rate of ewes. Mutiga and Mukasa-Mugerwa (1992) have reported that no difference was observed in cyclic Menze ewes in the oestrus response following the PGF2α injection (2.5 mg, 12 days apart) and sponge (FGA, 40 mg for 12 days) treatment, but PGF2α-treated ewes exhibited oestrus (p < 0.05) earlier (−6 h) than the sponge-treated ewes. Evans et al. (2001) reported that ewes receiving prostaglandin analogue on day 6 of the cycle and the single sponge from day 6 to 20 came into heat (p < 0.05) earlier than those that received multiple sponges. In the present study, the oestrus response rate in ewes with or without PGF2α administration after sponge withdrawal was 100%. While PGF2α administration at the time of the sponge removal significantly (p < 0.001) shortened the injection-oestrus onset and injection-oestrus-end interval, it has no significant effect on the duration of oestrus. This result is compatible with the findings of Mutiga and Mukasa-Mugerwa (1992). Cloprostenole has potency more 200 times than PGF2α (Rubianes et al. 2003), and its induced luteolysis causes a more rapid reduction in progesterone synthesis by luteal cells than does natural luteolysis (Barrett et al. 2002). Furthermore, a sudden decline in progesterone synthesis after the PGF2α treatment results in a faster increase in LH surge when compared with the normal luteolysis (Parfet et al. 1989). Based on these hypotheses, the possible explanation for a shorter injection-oestrus onset interval in Group 3 is that the administration of the exogenous PGF2α analogue at the time of the sponge removal possibly caused the faster regression of the CL than natural luteolysis. Thus, the injection-oestrus onset interval in ewes that received PGF2α following the sponge withdrawal shortened because, the effect of progesterone, which may still be secreted at the time of the sponge removal due to the presence of a still functional CL that regressed at different time in different ewes (Viñoles et al. 2001), was abolished by exogenous PGF2α at an earlier time.
In terms of reproductive performance, the success of AI with fresh and frozen-thawed semen synchronized with different protocols or non-synchronized ewes generally lower than does in cows. It is believed that the detrimental effect of different synchronization protocol on sperm transport in the enital tract of ewes (Dogan and Nur 2006), and particularly embryonic losses (Cam and Kuran 2004) leads to this low reproductive performance. In the present study, intravaginal sponges were inserted into all the ewes in each group for 12 days, and PMSG was injected at the time of the sponge removal. Then, while the ewes in Group 1 received saline alone, GnRH was injected to the Group 2 immediately after second AI; the ewes in Group 3 were also administered post-insemination GnRH along with the PGF2α injection at the time of sponge withdrawal. Indeed, the pregnancy rate, the twinning rate and the litter size of Groups 2 and 3 were numerically (but not statistically) higher than Group 1. Additionally, no statistically significant differences were observed among any of the groups in terms of gestation period, the lambing rate, plus the female and the male lamb rate. However, the multiple birth rate of Group 3 (77.8%; 7/9) was statistically (p < 0.05) higher than both Groups 1 (14.3%; 1/7) and 2 (44.4%; 4/9), while for number of single lamb was opposite (Group 1 higher than Groups 2 and 3, p < 0.05).
Dogan and Nur (2006) reported that the pregnancy rate of ewes, which synchronized with MAp + PMSG + PGF2α and then artificially inseminated, was numerically (but not statistically) lower than those of synchronized with MAP, MAp + PGF2α or MAp + PMSG. Ataman and Aköz (2006) have suggested that no significant difference was found between Akkaraman cross-bred sheep synchronized with GnRH–PGF2α or PGF2α–PGF2α in the pregnancy rate and the litter size in the breeding season. Sönmez and Gür (2004) reported that the improvement in the fertility of heifers given a single injection of gonadorelin (a GnRH analogue) on the day of insemination was probably the result of ovulation and luteinization occurring at the appropriate time relative to insemination. It has been reported that the gonadotropin administration on day 12 post-mating improves the reproductive performance of synchronized (Beck et al. 1994) and non-synchronized (Cam and Kuran 2004) ewes. A study made by Khan et al. (2003) has documented that a single injection of hCG given at the mating time increased ovulation rate, improved the conceptus growth, the implantation, the conception rate and the litter size. The findings of these researchers concerning the conception rate and the litter size are in agreement with our findings obtained from ewes that were administered GnRH immediately after the second AI. There are a number of possible explanations regarding the effect of gonadotropin on embryonic development. It may have done so through a direct effect on the maturing oocyte that is carried over into embryogenesis and implantation or alternatively, on day of insemination hCG or GnRH treatment-stimulated LH surge might have advanced ovulation and the formation of accessory CLs, thereby allowing more time for embryo growth, development and survival (Cam et al. 2002; Khan et al. 2003). In this study, the possible reason for the higher pregnancy rate (but not statistical) observed in Groups 2 and 3 when compared with the Group 1 may depend on the survival of the embryo. However, results from this preliminary study could warrant further investigation.
The low dosage of progestagen administration (the lowest dose of progestagen analogue, 30 mg FGA, was used in the present study) leads to the occurring of persistent follicles in ewes (Viñoles et al. 2001; Dixon et al. 2006). It is also evident from the results that GnRH-treated ewes (Groups 2 and 3) had a higher multiple birth rate (in terms of twin and triplet lambs) than the animals in Group 1 which had not received GnRH. This situation may be explained with the ovulation of progestagen-induced persistent or unovulatory follicles by the injection of GnRH, which causes to increase the secretion of LH at the time of insemination.
In conclusion, the administration of PGF2α at the time of the sponge removal shortens the injection oestrus-onset and injection-oestrus-end interval in Awassi ewes treated with progestagen–PMSG. Additionally, exogenous GnRH treatment immediately after the AI increases the multiple birth rate of Awassi ewes synchronized with progestagen–PMSG–PGF2α combination during the breeding season.




