Ovarian Responses, Hormonal Profiles and Embryo Yields in Anoestrous Ewes Superovulated with Folltropin®-V after Pretreatment with Medroxyprogesterone Acetate-releasing Vaginal Sponges and a Single Dose of Oestradiol-17β
Contents
In ruminants, superovulatory treatments started at the time of follicular wave emergence result in greater and less variable ovulatory responses and embryo yields compared with the treatments begun in the presence of a large growing antral follicle(s) from the previous waves. The progesterone–oestradiol treatment is routinely used for follicular wave synchronization in cattle. The main objective of this study was to characterize the ovarian responses, hormonal profiles and in vivo embryo production in anoestrous Rideau Arcott ewes (May-June), which were superovulated after pretreatment with medroxyprogesterone acetate (MAP)-releasing intravaginal sponges and a single dose of oestradiol-17β (E2-17β). Six days after insertion of MAP sponges, eight ewes were given an i.m. injection of 350 μg of E2-17β (E2-17β-treated ewes); 10 ewes were given an i.m. injection of vehicle (control ewes). Multiple-dose Folltropin®-V treatment, followed by the bolus injection of GnRH (50 μg i.m.), began 6 days after E2-17β/vehicle injection. Transrectal ovarian ultrasonography revealed that: (i) the interval between E2-17β/vehicle injection and regression of all follicles ≥5 to 3 mm in diameter was shorter (p < 0.01; 2.6 ± 0.4 vs 4.8 ± 0.6 days respectively); and (ii) the interval between injection and emergence of the next follicular wave was longer (p < 0.05; 5.4 ± 0.3 vs 1.2 ± 0.4 days, respectively) in E2-17β-treated than in control ewes. During the 6 days after injection, the mean FSH peak concentration and basal FSH concentration were lower (p < 0.01) in E2-17β-treated ewes. The mean ovulation rate and the number of recovered embryos did not differ (p > 0.05) between the two groups of ewes. However, the number of luteinized unovulated follicles per ewe, and the variability in the number of luteal structures and overall embryo yield were less (p < 0.05) in E2-17β-treated compared with control ewes. In conclusion, the MAP–E2-17β pretreatment significantly reduced the variability in ovarian responses and embryo yields, without affecting the embryo production in superovulated anoestrous ewes.
Introduction
The ovarian response and embryo yield after superovulation in sheep are highly variable (Gonzales-Bulnes et al. 2004), particularly during seasonal anoestrus (Rubianes et al. 1995), which limits the application of superovulation in the embryo transfer programmes and experimental work. Differences in follicular wave status at the outset of superovulatory treatments appear to be the major source of variability in the ovarian response in domestic ruminants (Adams 1994; Mapletoft et al. 2002). In the cow, a follicular wave refers to the simultaneous emergence of a cohort of follicles, followed by selection of an antral follicle that continues to grow to an ovulatory diameter before regression or ovulation (dominant follicle) and suppresses the growth of the remaining (subordinate) follicles (Adams 1999). In the ewe, each follicular wave consists of one to four ovulatory-sized follicles (Adams 1999). In both species, incessant follicular waves can be observed to emerge during the luteal and follicular phases of the oestrous cycle (Adams 1999), and the emergence of waves continues throughout seasonal anoestrus in ewes (Bartlewski et al. 1998). Several reports in cattle and sheep have shown a decrease in the number of ovulations when superovulation was initiated in the presence of a large antral follicle(s) (Guibault et al. 1991; Huhtinen et al. 1992; Rubianes et al. 1995, 1997; Mapletoft et al. 2002; Gonzales-Bulnes et al. 2004). Consistently higher ovulatory responses were elicited when the treatment began on the day of (Nasser et al. 1993; Adams 1994; Rubianes et al. 1995; Mapletoft et al. 2002) or one day after (Rubianes et al. 1997) wave emergence (i.e., early in the growth phase of a follicular wave).
Follicular wave synchronization has become customary in cattle undergoing superovulation (Adams 1994; Bo et al. 1995; Bergfelt et al. 1997; Baracaldo et al. 2000; Mapletoft et al. 2002). Incorporation of the techniques designed to control follicular wave emergence (i.e., ultrasound-guided follicular aspiration or treatment with exogenous progesterone–oestradiol) significantly reduced the variability associated with superovulatory treatments started at random stages of follicular wave development in cows (Mapletoft et al. 2002). In sheep, there have been no earlier attempts to ablate large antral follicles with the progestogen– oestradiol treatment prior to superovulation.
In a recent study in anoestrous ewes, a single injection of oestradiol-17β (E2-17β; 350 μg/ewe) mid-way through a 12-day treatment with MAP-releasing intravaginal sponges enhanced the regression of large antral follicles and caused synchronous wave re-emergence approximately 6 days after injection (Barrett et al. 2002). The aim of the present study was to describe the ovarian responses, endocrine profiles and in vivo embryo production in anoestrous ewes superovulated after pretreatment with MAP-releasing intravaginal sponges and a single dose of 350 μg of E2-17β given 6 days before the beginning of a superovulatory protocol. We hypothesized that the MAP–E2-17β treatment of anoestrous ewes, combined with the superovulatory treatment started in the absence of large ovarian follicles, at or around the expected time of follicular wave re-emergence, would result in augmented and less variable ovulatory responses and embryo yields. In addition, we assessed the effects of the superovulatory treatments and surgical embryo recovery in anoestrous ewes on their lambing rate, lamb birth weight and sex ratios during the ensuing breeding season.
Material and Methods
Animals and treatments
This experiment was performed on 18 clinically healthy sexually mature (aged between 3 and 6 years; mean body weight: 76 ± 2 kg) Rideau Arcott ewes during mid-anoestrus (May-June). Animals used in this study had lambed one to five times (range two to five and one to three for the treated and control ewes respectively), with the average number of lambs born per ewe being 2.6 ± 0.1. Animals were kept outdoors (with an access to indoor facilities) at the sheep research station in Ponsonby near Guelph, ON, Canada (latitude: 43°33′N). They were fed daily maintenance rations of alfalfa pellets with hay, water and cobalt iodized salt licks available ad libitum. All ewes received intravaginal sponges containing medroxyprogesterone acetate (MAP; 60 mg; Veramix®, Pharmacia and Upjohn Animal Health, Orangeville, ON, Canada; day 0) for 14 days (Shipley et al. 2007). Six days after the insertion of MAP sponges, eight ewes were given a single i.m. injection of oestradiol-17β (E2-17β: Longwing International, Oakville, ON, Canada) in 1 ml of sesame oil (350 μg/ewe; E2-17β-treated ewes) and 10 ewes were given an i.m. injection of sesame oil (vehicle; control ewes). The superovulatory treatment in this study consisted of six i.m. injections of porcine FSH (pFSH: Folltropin®-V, Bioniche Animal Health Canada Inc., Belleville, ON, Canada; 2.5 ml × 1 and 1.25 ml × 5), given at approximately 08:00 and 16:00 h, from the afternoon of day 12 until the morning of day 15. Concurrently with the first pFSH injection, all animals received an i.m. injection of 500 IU of eCG (Folligon®, Intervet Canada Ltd., Whitby, ON, Canada) (Barrett et al. 2004; Shipley et al. 2007). Sponges were removed at the time of the fifth pFSH injection. On the afternoon of day 15, all animals received a single injection of 50 μg of GnRH i.m. (Cystorelin®, Merial Canada Inc., Baie d'Urfe, PQ, Canada) and were placed in a fenced paddock with four adult Rideau Arcott rams for 36 h.
In the breeding season (November-December) following the superovulatory treatment, oestrus was synchronized in all ewes by a 12-day treatment with MAP-releasing sponges. The ewes were bred by two Rideau Arcott rams introduced 48 h after sponge withdrawal. The number, sex and birth weight of lambs were recorded at lambing, for both groups of ewes.
Transrectal ovarian ultrasonography
Daily transrectal ultrasonography of ovaries (conducted in all animals from the day of E2-17β/vehicle injections until 1 day after GnRH injection) was performed using a stiffened, 7.5-MHz linear-array transducer connected to a portable B-mode ultrasonic scanner (HS-2000, Honda Electronics Ltd, Toyohashi, Japan). This technique has been previously validated for monitoring ovarian antral follicle dynamics in superovulated ewes (Lopez-Alonso et al. 2005). One experienced operator conducted all examinations. All ovarian images were displayed on the viewing screen of the scanner at 2× magnification. Follicles were measured to the nearest 1 mm using internal electronic calipers and the number and diameter of all antral follicles ≥3 mm were sketched on ovarian charts.
Assessment of ovulation and embryo collection
The number of all luteal structures, including corpora lutea (CL) and luteinized unovulated follicles, was noted at laparotomy 6 days after GnRH treatment. All animals were deprived of food and water from 24 h before surgery. Surgical procedures were performed under general anaesthesia induced with xylazine (Rompun®, Bayer Animal Health, Ethobicoke, ON, Canada; 6 mg i.m.) and ketamine (Bioniche, Bellville, ON, Canada; 10 mg/kg i.v.). The reproductive tract was exposed and CL were identified on the basis of projection from the surface of the ovary and/or presence of the ovulatory stigma (Ireland et al. 1980); all remaining luteal structures were classified as luteinized unovulated follicles (Cognie et al. 2003; Liu et al. 2006). Thereafter, embryos were recovered by flushing both oviducts and uterine horns (flushing medium: PBS + 1% bovine serum albumin + penicillin and streptomycin; approximately 70 ml per ewe). The flushing was performed with a three 1/5 French Tomcat catheter (14 cm in length) inserted into the oviduct approximately 2 cm from the utero-tubal junction, and a paediatric French catheter (silicone elastomer coated, size 10) inserted into the uterine horn at the bifurcation of the uterus and kept in place by an inflatable balloon (max. diameter: 35 mm). Embryos were evaluated morphologically, using a stereomicroscope, at a magnification of 40× and 80×. Embryos that developed to the morula or blastocyst stage at the time of collection were graded from one to four, with one being excellent, two-good/fair, three-poor and four-degenerated (Lindner and Wright 1993; Rubianes et al. 1995); embryo classes one to three were collectively regarded as transferable quality embryos. Following surgery, all ewes were given an i.m. injection of prostaglandin F2α (PgF2α; Lutalyse®, Upjohn, Orangeville, ON, Canada; 15 mg).
Hormone assays
Blood samples (10 ml) were collected by jugular venipuncture into vacutainers (Becton Dickinson, Rutherford, NJ, USA). Samples were collected at 24-h intervals (at approximately 12:00 h), from the day of E2-17β/vehicle injection until 7 days after GnRH injection. The blood sample was allowed to coagulate for 18–24 h at room temperature. After removal of blood clots and centrifugation at 1500 × g for 10 min, serum was harvested and stored at −20°C until assayed.
Daily serum samples taken before and after the superovulatory treatment were analysed by validated radioimmunoassays (RIAs) for concentrations of ovine FSH (oFSH; Currie and Rawlings 1989). The concentrations of the hormone are expressed in terms of an oFSH-SIAFP-RP-2 standard. The ranges of the standards were 0.12–16.0 ng/ml and the sensitivity of the assay (defined as the lowest concentration of hormone capable of significantly displacing labelled hormone from the antibody) was 0.10 ng/ml. The intra- and interassay coefficients of variation (CVs) for the oFSH assay, for reference sera with a mean concentration of 1.53 or 5.10 ng/ml, were 4.8 or 6.0% and 6.4 or 8.6% respectively. Oestradiol-17β concentrations were determined by a validated RIA (Joseph et al. 1992) in serum samples obtained every 24 h for the duration of the study. The range of standards was from 1.0 to 50 pg/ml and the sensitivity of the assay was 1.0 pg/ml. For reference sera with mean E2-17β concentrations of 8.2 or 22.1 pg/ml, the intra- and interassay CVs were 6.5 or 6.5% and 8.4 or 8.4% respectively. Blood samples collected between 1 and 7 days after GnRH injection were analysed for progesterone concentrations (Rawlings et al. 1984). The sensitivity of the progesterone RIA was 0.03 ng/ml and the range of standards was from 10.0 pg/ml to 10.0 ng/ml. For reference sera with a mean progesterone concentration of 0.24 or 1.20 ng/ml, the intra- and interassay CVs were 6.2 or 7.8% and 8.3 or 13.3% respectively.
Data analysis and statistical comparisons
The ovarian data were analysed on a per ewe basis after combining data for both ovaries of each ewe. The following time intervals were noted for each ewe: (i) between E2-17β/vehicle injection and regression of all follicles ≥5 mm in diameter present on the day of injection to 3 mm and (ii) between E2-17β/vehicle injection and the emergence of the next follicular wave. In the present study, a follicular wave (including the first wave emerging after the beginning of the superovulatory treatment) was regarded as a follicle or group of follicles that grew from 3 to ≥5 mm in diameter, with emergence restricted to a 24-h period. The following mean values were also determined for each animal: (i) daily numbers of follicles in three different size classes (3, 4 and ≥5 mm in diameter; small, medium and large follicles, respectively) and (ii) the diameter of the largest follicle (maximum follicle diameter). Peaks in FSH concentrations in individual ewes were identified with the cycle-detection computer program (Clifton and Steiner 1983; Bartlewski et al. 2006). The peak amplitude was defined as the difference between the peak concentration and the nadir preceding the peak concentration. Basal FSH concentrations were calculated by averaging the lowest points (nadirs) between peaks. The mean duration of complete fluctuations (nadir-to-peak-to-nadir) was also determined for each animal. Ovarian follicular data and hormone concentrations were analysed by two-way repeated measures analysis of variance (RM-anova; simgastat®3.0 for windows®, 2003, systat Software, Inc., Richmond, CA, USA) with Fisher's protected least significant difference as a post-anova test for comparisons of individual means. All serial data were assessed for extreme values using the Dixon's Q-test (outlier test). When the normality or equal variance tests were significant, the data were transformed by natural logarithm or square root prior to further analysis. Single time-point observations were compared between the treatment and control groups by Student t-test. Lastly, the variability in ovarian responses and embryo yields was compared between the two groups of ewes using the standard deviation criteria, with a smaller standard deviation indicating lower variability; the comparison of standard deviations was made using F (variance ratio) tests. Differences were considered statistically significant at p < 0.05.
Results
Antral follicular development
The time taken by individual follicles ≥5 mm in diameter detected on the day of E2-17β/vehicle injection to regress to 3 mm was shorter in E2-17β-treated than in control ewes (2.6 ± 0.4 vs 4.8 ± 0.6 days respectively; p < 0.01). The interval between injection and the emergence of the next follicular wave was longer in E2-17β-treated compared with that in control ewes (5.4 ± 0.3 vs 1.2 ± 0.4 days respectively; p < 0.05). When follicular data were analysed for the period from the day of E2-17β/vehicle injection until 5 days after injection, the number of large follicles ≥5 mm in size was greater (p < 0.05) from 1 to 4 days after injection in control ewes (Fig. 1c) and the maximum follicular diameter was greater (p < 0.05) from 3 to 5 days after injection in the control group (Fig. 1d). There were no differences (p > 0.05) between the two groups of ewes in mean daily numbers of small (3 mm; Fig. 1a) and medium-sized (4 mm; Fig. 1b) follicles.
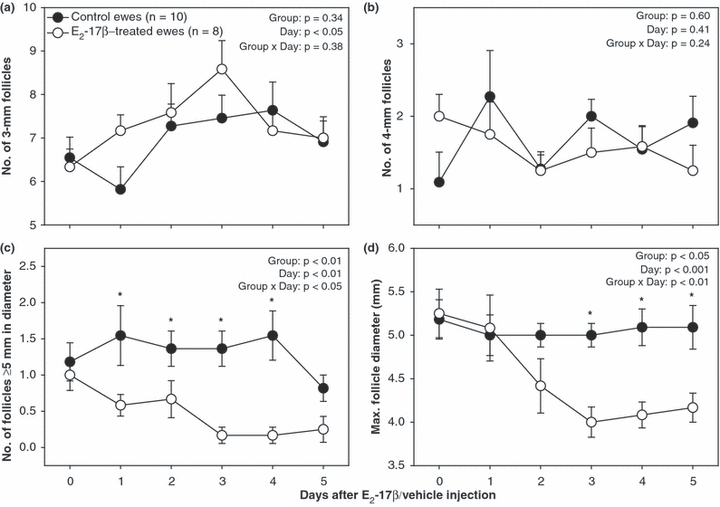
Mean (±SEM) daily numbers of small (a; 3 mm), medium (b; 4 mm) and large (c; ≥5 mm) antral follicles, and maximum follicular diameter (d) determined by transrectal ovarian ultrasonography in anoestrous Rideau Arcott ewes, treated for 14 days with medroxyprogesterone acetate-releasing intravaginal sponges with or without a single i.m. injection of oestradiol-17β (E2-17β) given 6 days after sponge insertion (day 0; E2-17β-treated vs control ewes respectively). Follicular data were aligned to the day of injection and analysed for the period from days 0 to 5. Asterisks indicate significant differences between groups (p < 0.05)
When follicular data were analysed for the period from the day of the first Folltropin®-V injection until 5 days after injection, the number of follicles ≥5 mm in diameter was greater (p < 0.05) in control compared with that in E2-17β-treated ewes 1 day after the beginning of the superovulatory treatment, but it was greater (p < 0.05) in the treated ewes 3 days after the first Folltropin®-V injection. The maximum follicle diameter was greater (p < 0.05) on the day of and 1 day after the first Folltropin®-V injection in control ewes. The difference in mean daily numbers of 3-mm follicles between E2-17β-treated and control ewes approached significance (p < 0.06; Fig. 2a); the treated ewes appeared to have more small antral follicles 1 day and 5 days after the beginning of the superovulatory treatment and there was an increase in the number of small follicles on each of the 2 days after GnRH injection in E2-17β-treated ewes.
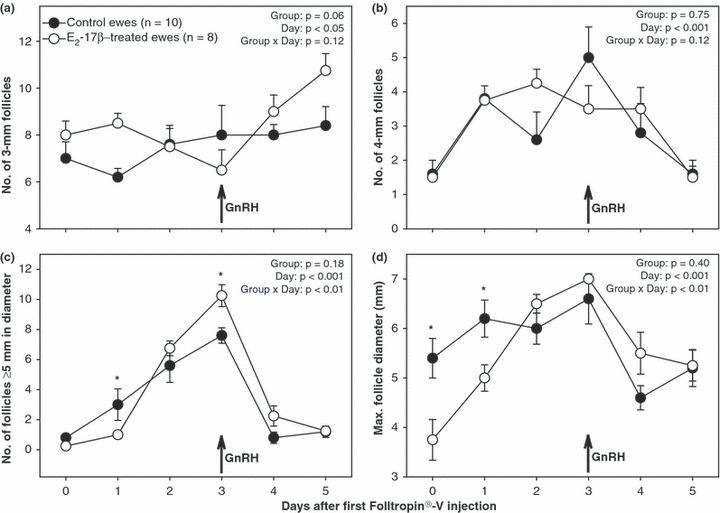
Mean (±SEM) daily numbers of small (a; 3 mm), medium (b; 4 mm) and large (c; ≥5 mm) antral follicles, and maximum follicular diameter (D) determined by transrectal ovarian ultrasonography in anoestrous Rideau Arcott ewes, superovulated with a multiple-dose pFSH treatment (Folltropin®-V) started 6 days after an oestradiol-17β (E2-17β)/sesame oil injection (E2-17β-treated/control ewes, respectively). Data were normalized to the day of first injection of Folltropin®-V (day 0) and analysed for the period from days 0 to 5. Asterisks indicate significant differences between groups (p < 0.05)
Systemic concentrations of FSH, E2-17β and progesterone
During the 6-day period after E2-17β/vehicle injection (day 0), the number of peaks, mean peak amplitude and duration of fluctuations for FSH concentrations, as determined by the cycle-detection computer program, did not differ (p > 0.05) between E2-17β-treated and control ewes (Table 1). Mean FSH peak concentration and basal FSH concentration were lower (p < 0.01) in the ewes pretreated with E2-17β. A 24% and 30% reduction occurred in the mean FSH peak concentration and basal FSH concentration, respectively, in E2-17β-treated compared with control ewes. Mean daily concentrations of FSH were lower (p < 0.05) in E2-17β-treated compared with that in control ewes 1 day after E2-17β injection (data not shown).
| Variable/Group | E2-17β-treated ewes (n = 8) | Control ewes (n = 10) |
|---|---|---|
| Number of FSH peaks/6 days | 1.5 ± 0.2 | 1.8 ± 0.2 |
| FSH peak concentration (ng/ml) | 2.27 ± 0.14a | 3.07 ± 0.23a |
| FSH peak amplitude (ng/ml) | 0.73 ± 0.11 | 0.81 ± 0.16 |
| Basal FSH concentration (ng/ml) | 1.54 ± 0.16a | 2.20 ± 0.21a |
| Duration of FSH fluctuations (days) | 3.0 ± 0.3 | 3.1 ± 0.2 |
- Within rows, means denoted by the same letters are different (p < 0.05).
- E2-17β, oestradiol-17β.
There were no differences (p > 0.05) between E2-17β-treated and control ewes in mean daily concentrations of E2-17β, for the duration of the present study (Fig. 3a), and no differences (p > 0.05) in serum levels of progesterone, from the day of GnRH injection until 7 days after injection (Fig. 3b). In both groups of ewes, serum concentrations of E2-17β reached a maximum value on the day of GnRH injection and progesterone concentrations continued to rise over the 7 days after GnRH injection.
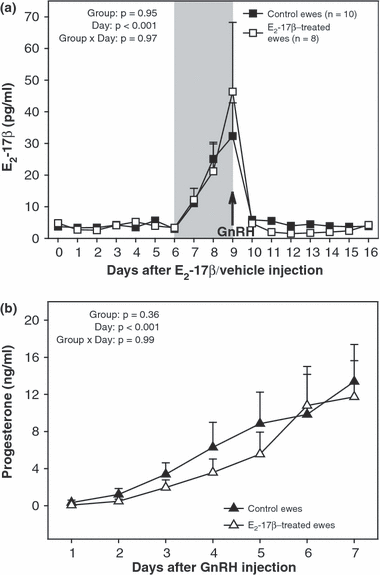
(a) Mean (±SEM) daily serum concentrations of oestradiol-17β (E2-17β), from 7 days before to 10 days after the beginning of the superovulatory treatment (outlined with shading) of anoestrous Rideau Arcott ewes that were pretreated with a single i.m. dose of E2-17β (E2-17β-treated) or sesame oil or (control ewes). (b) Mean (±SEM) daily serum concentrations of progesterone in blood samples collected from 1 to 7 days after GnRH injection
Ovarian responses and embryo yields
The ovarian responses and embryo yields in the ewes of the present study are summarized in Table 2. The number of luteinized unovulated follicles per ewe was greater (p < 0.05) in control than in E2-17β-treated ewes post-treatment. The variability in the mean number of luteal structures, CL and luteinized unovulated follicles, and in overall embryo yields was less (p < 0.05) in the treatment compared with that in control group, as determined using F (variance ratio) test.
| Group/Variable SD | No. of luteal structures | No. of CL | No. of luteinized unovulated follicles | No. of embryos recovered | No. of embryos – grades 1–3 |
|---|---|---|---|---|---|
| E2-17β-treated ewes (n = 8) | 14.0 ± 0.8 | 10.0 ± 0.8 | 4.0 ± 1.3a | 8.2 ± 1.4 | 5.7 ± 1.9 (62%)* |
| 2.3b | 2.3b | 3.7b | 4.0b | 5.4 (40) | |
| Control ewes (n = 10) | 21.3 ± 3.9 | 13.2 ± 2.8 | 13.7 ± 3.7 a | 6.9 ± 2.5 | 3.3 ± 2.0 (54%) |
| 12.3b | 8.8b | 11.7b | 7.9b | 6.3 (42) |
- SD, standard deviation; MAP, medroxyprogesterone acetate; E2-17β, oestradiol-17β.
- Within columns, means or SD values denoted by the same letters are different (p < 0.05).
- *The proportion of transferable quality embryos (grades 1 to 3) relative to the total number of recovered embryos per ewe.
Breeding season lambing rates, lamb birth weights and sex ratios
There were no differences (p > 0.05) in the percentage of ewes that lambed (χ2-test; 100 and 90% respectively) and mean numbers of lambs per ewe (live births: 2.9 ± 0.3 and 3.0 ± 0.3, respectively) between the E2-17β-treated and control ewes, and no differences (p > 0.05) between the groups for lamb birth weights (3.4 ± 0.2 and 3.5 ± 0.1 Kg, respectively) or the percentage of male lambs born per ewe (47 and 70% respectively) during the breeding season after the superovulatory treatment. Within each group, the lambing rates did not vary (p > 0.05) from the pretreatment lambing rates (E2-17β-treated ewes: 2.9 ± 0.3 vs 2.5 ± 0.2; control ewes: 3.0 ± 0.3 vs 2.6 ± 0.2, respectively).
Discussion
In the present study in anoestrous Rideau Arcott ewes, a single injection of 350 μg of E2-17β given during the treatment with intravaginal MAP-releasing sponges enhanced the regression of large (≥5 mm in diameter) antral follicles and suppressed the emergence of follicular waves for 4–6 days after injection. These effects of exogenous E2-17β appeared to be due mainly to reduction in basal FSH levels and peak concentrations of successive increases in FSH secretion (Table 1). Because the endogenous FSH peaks were truncated, the small gonadotropin-responsive follicles might not receive adequate FSH signal to stimulate their entry into a follicular wave. These results are in complete agreement with previous studies on FSH-dependency of follicular wave kinetics in ewes (Duggavathi et al. 2004, 2005; Barrett et al. 2006) and indicate that there exists a threshold FSH concentration that must be reached to induce the emergence of a follicular wave. In most of the treated ewes in this study (five of eight animals), a new follicular wave emerged only after the administration of pFSH had created a sufficient FSH stimulus.
The first injection of pFSH was followed by the emergence of a new follicular wave in all animals in the treatment and control groups, as evidenced by a rise in the number of follicles ≥5 mm in size (Fig. 2c), regardless of the presence or absence of large follicles from the previous waves. Prior to the superovulatory treatment, the pools of small (3 mm) and medium (4 mm) follicles were not affected by the treatment with E2-17β (Figs 1a, b). These observations bring into question the existence of follicular dominance in MAP-treated anoestrous ewes, as seen in cattle (Adams 1999). In cattle, both physiological and supraphysiological concentrations of FSH administered in the presence of a large growing follicle (dominant follicle) fail to trigger the emergence of a new follicular wave and dominant follicles suppress the growth of co-existing follicles (Guibault et al. 1991; Adams et al. 1993).
Overall, a single injection of E2-17β during the treatment with MAP-releasing sponges in anoestrous ewes, suppressed basal oFSH secretion and truncated oFSH peaks to such an extent that the development of follicular waves was blocked for 4–6 days, without affecting the number of small and medium-sized follicles. It would appear that the periodic peaks in endogenous FSH are not essential for maintaining the pool of small antral follicles and their initial growth to 4 mm in diameter, in agreement with recent observations in ewes (Barrett et al. 2006), and even reduced basal serum concentrations of FSH are sufficient to maintain this development of ovarian follicles in anoestrous ewes.
In a study by Barrett et al. (2006), the cyclic ewes treated with subcutaneous implants containing E2-17β between days 4 and 14 after ovulation had no emerging follicular waves during 8 days after implant insertion. During the entire period when E2-17β implants were in place, mean oestradiol concentrations in the treated ewes were approximately 2.7-fold higher than that in the control ewes. A significant reduction was found in the mean FSH peak concentration and FSH peak amplitude in the treated animals, but mean and basal FSH concentrations and pulsatile LH secretion remained unaffected. Injection of physiological doses of oFSH during the treatment with E2-17β implants reinitiated the emergence of follicular waves. It was concluded that the treatment of cyclic ewes with E2-17β implants provided a useful experimental model whereby the characteristics of FSH peaks required to induce follicle waves in the ewe can be studied. Our present treatment of anoestrous ewes, with a single dose of E2-17β and MAP-soaked intravaginal sponges, may provide an experimental model that would allow the examination of the ovarian effects of FSH in the absence of elevated systemic levels of E2-17β. In anoestrous Western White Face ewes that had received 350 μg of E2-17β in 1 ml of sesame oil (i.m.) during the treatment with MAP-releasing sponges, E2-17β reached a maximum concentration in peripheral circulation 6 h after injection and declined to the initial levels 18 h after injection (Barrett et al. 2002).
The mechanisms underlying a reduction in endogenous FSH release after pretreatment with MAP and a single dose of E2-17β remain to be elucidated. One possible explanation is that partial suppression of FSH secretion was due to the presence of biologically active oestradiol metabolites in the peripheral circulation and/or central nervous system (Hochberg 1998). Oestrogen-responsive tissues can esterify oestrogens and certain oestradiol metabolites (i.e., lipoidal derivatives of oestradiol) are found at elevated concentrations only within the tissues. Alternatively, the treatment with E2-17β could have brought about profound molecular and histomorphological changes (i.e., gene expression patterns and neuronal architecture) in various regions of the brain, resulting in diminished FSH synthesis for several days post-treatment (Parducz et al. 2006). More research is needed in this area.
Two days after the superovulatory treatment had been initiated, mean daily numbers of follicles ≥5 mm in diameter did not vary between E2-17β-treated and control ewes (Fig. 2c). Resumption of follicular growth to ovulatory diameters in the treated ewes coincided with a transient increase in the numbers of small (3 mm) follicles (Fig. 2a), confirming that the recruitment of antral follicles into follicular waves resumed primarily after the administration of pFSH. At the time of GnRH bolus injection, the mean number of large antral follicles was greater in E2-17β-treated ewes (Fig. 2c). In spite of the difference in large follicle numbers around the time of ovulation induction with GnRH, there were no differences in the ovulation rate between the two groups of ewes studied (Table 2). It is, therefore, feasible that some of the follicles ≥5 mm in diameter in E2-17β-treated ewes had diminished ability to ovulate in response to GnRH. This diminished ovulatory ability may be due to alterations in the GnRH-induced LH surge; we did not measure LH concentrations in this study. A delayed (Lopez-Alonso et al. 2005) or truncated (Bartlewski et al. 2004) preovulatory LH discharge may result in diminished ovulatory responses in ewes.
Variations in the preovulatory LH surge (Veiga-Lopez et al. 2006) and/or follicular responsiveness to gonadotropic hormones (Bartlewski et al. 2001) could both contribute to the failure of ovulation of some large antral follicles as well as reduction in the number of luteinized unovulated follicles in E2-17β-treated ewes. Luteinizing hormone plays a key role in the control of ovulation and luteogenesis (Baird 1992) and FSH and oestradiol stimulate the synthesis of LH receptors in preovulatory follicles (England et al. 1981). Although E2-17β-treated ewes had more large antral follicles than control ewes around the time of GnRH injection, mean oestradiol concentrations did not differ between the two groups and appeared to be more variable (SEM) in the treatment group (Fig. 3a). Because circulating oestradiol is a reliable marker of follicular secretory activity and health, it is conceivable that some of the ovulatory-sized follicles in the E2-17β pretreated ewes were not fully functional or mature; this might be due to diminished follicular sensitivity to gonadotropins (i.e., FSH/LH receptor populations). Nevertheless, our results do not support the hypothesis that, in anoestrous ewes pretreated with MAP and E2-17β, the superovulatory treatment started around the expected time of follicular wave emergence would result in greater ovulatory responses.
Superovulatory output in the ewes of the present study, in terms of the number of recovered embryos, was not affected by the treatment with E2-17β (Table 2). However, statistical analyses revealed that the variability in ovarian responses (i.e., mean ovulation rate and numbers of luteal structures) and in overall embryo yields, were significantly less in E2-17β-treated compared with control ewes. Therefore, the present MAP–E2-17β treatment resulted in fewer luteinizing unovulated follicles and reduced the variability in ovarian responses and embryo yields, without compromising net embryo production in superovulated ewes in anoestrus. Lastly, the superovulatory treatments and surgical embryo recovery in the ewes of our present study did not appear to affect lamb productivity during the ensuing breeding season.
In conclusion, the administration of a single dose of E2-17β to MAP-treated anoestrous ewes suppressed follicular wave emergence for 4–6 days. These effects of MAP–E2-17β treatment appeared to be due mainly to the partial suppression of FSH secretion. The superovulatory treatment started 6 days after E2-17β injection was associated with a reduced variability in ovarian responses and embryo yields. In addition, there was a significant reduction in the number of luteinized unovulated follicles in E2-17β-treated ewes following superovulation. The present treatment of anoestrous ewes with MAP and E2-17β increased the number of ovulatory-sized antral follicles growing in response to Folltropin®-V. However, a possible prolonged suppression of LH release and/or follicular responsiveness to gonadotropins may have resulted in diminished ovulatory ability of some large follicles in E2-17β-treated ewes. Further studies of the terminal follicular maturation and ovulating capacity appear to be necessary to refine superovulatory treatments in ewes. Finally, we described an experimental model in which the basal FSH levels and peak concentrations of the periodic increases in serum FSH concentrations were altered by the MAP–E2-17β treatment in seasonally anoestrous ewes. This model may be useful for studying the neuroendocrine control of FSH secretion and FSH-dependency of antral follicular wave development in the ewe and other mammalian species.
Acknowledgements
We would like to thank Ms Liz St John and Dr Gianfranco Coppola for help with embryo collection and evaluation; project and summer students (Ms Rachel Caven, Mr Wilson Chan, Ms Qiao Dai, Ms Jennifer Guertin, Mr Ezra Hart and Mr Ivan Stevic) for technical assistance throughout the experiment; Ms Susan Cook (Prairie Diagnostic Services, University of Saskatchewan) for RIAs of FSH and steroid hormones; and staff at the Ponsonby sheep research station (Ms Pam Hasson and Ms Natalie Carter) for care and management of experimental animals and help with surgical procedures. This study was funded by the Canada Research Chair grant (Animal Biotechnology; WAK), the Ontario Ministry of Food And Rural Affairs (OMAFRA) grant (PMB), and by the Department of Biomedical Sciences, Ontario Veterinary College, University of Guelph (PMB).




