Expression of Vascular Endothelial Growth Factor Receptors in Bovine Cystic Follicles
Contents
Cystic follicles have excess fluid derived from blood flow in the theca interna of the follicle; therefore, the vasculature network is related to cystic follicle formation. Vascular endothelial growth factor (VEGF) is a potent stimulator of blood vessel permeability and angiogenesis. The aim of this study was to examine the expression of VEGF receptors proteins and mRNA in cystic follicles to elucidate the VEGF system in cystic follicles. The expression of protein for VEGF receptors; fms-like-tyrosine kinase-1 (Flt-1) and foetal liver kinase-1 (Flk-1) was detected by the immunohistochemical method. The mRNA expression of Flt-1 and Flk-1 in cystic follicles was determined by RT-PCR. Concentration of oestradiol-17β and progesterone in the follicular fluid of cystic follicles was determined using ELISA. Flt-1- and Flk-1 proteins were localized in granulosa and theca interna cells and endothelial cells of theca layers. The intensity of Flt-1 and Flk-1 immunoreaction was similar among cystic follicles with various ratios of oestradiol-17β/progesterone concentrations. The expression of Flt-1 and Flk-1 mRNA was similar, regardless of the ratio of oestradiol-17β to progesterone in follicular fluid. These results demonstrate that cystic follicles have both VEGF receptors in the granulosa and theca interna layers, which may be responsible for the increased permeability of microvessels, causing the accumulation of follicular fluid in cystic follicles.
Introduction
Ovarian cysts (≧25 mm in diameter) are a significant cause of reproductive failure in cattle (Kesler and Garverick 1982). The disease process is a consequence of a mature follicle that fails to ovulate at the appointed time in the oestrous cycle (Peter 2004). Follicular cysts are reportedly caused by an endocrine imbalance in the hypothalamo-hypophyseal-gonadal axis (Lopez-Diaz and Bosu 1992; Hamilton et al. 1995). Inappropriate production of LH, steroid hormone and steroid-synthesizing enzyme has been observed in cows with cystic follicles (Hamilton et al. 1995; Bigelow and Fortune 1998; Ribadu et al. 2000; Calder et al. 2001; Hatler et al. 2003; Isobe et al. 2003a,b; Isobe 2007).
A cystic follicle is characterized by the accumulation of an excess amount of follicular fluid inside the follicle. Recent publications suggested that cystic follicles had a highly developed vasculature network in the theca interna (Isobe et al. 2005a) and that those microvessels were younger and more active than those of healthy follicles based on the distribution of the von Willebrand factor, whose expression in the endothelial cells was significantly less in the immature blood vessels than in the mature ones (Augustin et al. 1995; Isobe et al. 2002). Therefore, the developed microvasculature plays a crucial role in the accumulation of follicular fluid. Serum components in the vasculature permeate through the basement membrane into follicular fluid. Vascular endothelial growth factor (VEGF) is a potent mitogen for endothelial cells (Ferrara and Davis-Smyth 1997) and a stimulator of vascular permeability (Connolly 1991; Senger et al. 1993). It is reported that VEGF mRNA was expressed and that its protein was localized in the granulosa and theca layers of bovine cystic follicles (Isobe et al. 2005a). Taken together, the expression of VEGF as well as developed vasculature are associated with the accumulation of follicular fluid, resulting in the formation of cystic follicles (Isobe et al. 2002, 2005a).
Vascular endothelial growth factor binds to two related receptor tyrosine kinases, VEGF receptor-1 (fms-like-tyrosine kinase-1, Flt-1) and VEGF receptor-2 (foetal liver kinase-1, Flk-1). Both Flt-1 and Flk-1 have seven immunoglobulin-like domains in the extracellular domain, a single transmembrane region and consensus tyrosine kinase sequence that are interrupted by a kinase-insert domain (Shibuya et al. 1990; Terman et al. 1991). The expression of mRNA and protein of Flt-1 and Flk-1 was up-regulated during follicle maturation in the bovine ovary (Berisha et al. 2000). As a cystic follicle express VEGF, VEGF receptors may be expressed as well in the cystic follicle; however, there is no information about VEGF receptor expression in cystic follicles.
Cystic follicles having granulosa cells are characterized by the high concentration of oestradiol-17β and low concentration of progesterone in their follicular fluid as well as in plasma (Yoshioka et al. 1996; Calder et al. 2001; Isobe et al. 2005a); however, the disappearance of granulosa cells reduces oestradiol-17β concentration and increases the progesterone concentration in the follicular fluid (Calder et al. 2001; Isobe et al. 2005a). Even if cystic follicles had granulosa cells, oestradiol-17β concentration in follicular fluid varied (Isobe et al. 2005a). As oestradiol-17β originates from granulosa cells, the reduction of oestradiol-17β concentration may be caused by the functional deficiency and/or disappearance of granulosa cells; therefore, the level of oestradiol-17β concentration is an important indicator to determine the stage of cystic follicles (Isobe et al. 2005a).
The aim of this study was to examine the VEGF system in the cystic follicle to elucidate the mechanisms by which cystic follicles expanded by excess influx of follicular fluid from serum components. The expression of protein and mRNA for the VEGF receptors was investigated using immunohistochemistry and RT-PCR, respectively, in cystic follicles whose steroid hormone profiles were monitored in follicular fluid.
Materials and Methods
Collection and preparation of tissues
Ovaries with cystic follicles (n = 19) were collected from 19 Holstein-Frisian cows at the slaughterhouse. Follicular cysts were diagnosed based on macroscopic characterization without the co-existing corpus luteum in both ovaries (range: 25–40 mm, mean: 29 mm in diameter). Some cows had cystic follicles in the bilateral ovaries. But, only one ovary was used from one of the animals. Ovaries with cystic follicles had various sized Graafian follicles. The cystic follicles with thin and translucent follicular walls were used to exclude cystic follicles containing luteinized cells. To collect the fluid from cystic follicles, a needle attached to a disposable syringe (10 ml) was inserted into a follicle and the fluid inside was aspirated gently. The follicular fluid was centrifuged (1700 × g, 15 min) and stored at −30°C until hormone measurement. For the isolation of RNA from cystic follicles, theca interna and granulosa layers (n = 5) were isolated using forceps (Isobe et al. 2005a) and frozen at −80°C until use. Another piece of follicular walls was dissected from the remaining portion of cystic follicle and was fixed with 10% (v/v) formalin in phosphate-buffered saline (PBS; pH 7.4) and processed in paraffin sections. The sections (4-μm thick) were air-dried on Matsunami Adhesive Slide (MAS)-coated slides (Matsunami Co. Ltd, Osaka, Japan) for immunohistochemistry.
Immunohistochemistry for Flt-1 and Flk-1
After deparaffinization, the sections were boiled in 0.01 m boric acid (pH = 6.0) with an autoclave at 121°C for 1 min to retrieve the antigen. Sections were then blocked with 2.5% casein milk in PBS and incubated with rabbit polyclonal antibody to human Flt-1 (C-17, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) at 2 μg/ml or rabbit polyclonal antibody to mouse Flk-1 (Ab-1; Lab Vision Corporation, Fremont, CA, USA) at 10 μg/ml at room temperature for 3 h. After washing with PBS, the immunoreactions were detected using a HISTOFINE MAX-PO(MULTI) kit (Nichirei Co., Tokyo, Japan) according to the manufacturer's protocol. Briefly, the sections were incubated with peroxidase-conjugated anti-rabbit IgG (100 μg/ml) for 60 min. Immunoreaction products for Flt-1 and Flk-1 were visualized by incubating the sections with a substrate solution consisting of 1 mg 3′,3′-diaminobenzidine and 10 μl 2.5% H2O2 in 5 ml 0.05 m Tris–HCl (pH 7.5) and then counterstained with haematoxylin. Control slides were prepared in an identical manner except that the first antibody was replaced with 1 μg/ml normal rabbit IgG (Vector Laboratories Inc., Burlingame, CA, USA).
Enzyme immunoassay of oestradiol-17β and progesterone
Granulosa cell-containing cystic follicles were selected on the basis of the immunohistochemical results. The follicular fluid of only granulosa cell-containing cystic follicles was measured with steroid hormone. The procedure for enzyme immunoassay of progesterone and oestradiol-17β was performed, described previously (Isobe and Nakao 2003; Isobe et al. 2005a,b). After dilution of follicular fluid with H2O, progesterone and oestradiol-17β were extracted with petroleum ether and diethyl ether respectively. Reconstituted samples were applied to wells of a microtitre plate for the assay. Antibody for progesterone was raised with rabbit in our laboratory (Isobe and Nakao 2003) and that for oestradiol-17β was purchased from COSMO BIO Co. (Tokyo, Japan). Standard for both steroids were purchased from Sigma Chemical Co. (St Louis, MO, USA). Sensitivity, intra-assay and intra-assay CV were 5.5 pg/ml, 8.6–8.7% and 12.2–12.7% for progesterone, and 16 pg/ml, 8.5–9.3% and 9.5–10.5% for oestradiol-17β respectively.
RT-PCR for Flt-1 and Flk-1
Preparation of total RNAs
Total RNAs were extracted from the theca interna of cystic follicles using Sepasol RNA I Super (Nacalai Tesque, Kyoto, Japan) as described previously (Chowdhury et al. 2004). Briefly, tissues were homogenized in 500 μl of Sepasol RNA I Super on ice, 100 μl of chloroform was added and the mixture was centrifuged at 12 000 g for 10 min at 4°C. The colourless upper aqueous portion was transferred to another tube and 250 μl of isopropanol was added to pellet the RNA. The mixture was then centrifuged at 12 000 g for 10 min at 4°C. The RNA pellet was washed with 75% ethanol and again centrifuged at 12 000 g for 5 min at 4°C. The supernatant was removed and the RNA pellet was dried to remove the ethanol. The pellet of RNA was resuspended in TE buffer, the concentration was measured with Gene Quant pro (Amersham Biosciences Corp., Piscatamay, NJ, USA) and stored at −80°C.
RT-PCR
RT-PCR was performed by ReverTra-PlusTM (Toyobo, Osaka, Japan) with minimal modifications (Kitabayashi and Esaka 2003). The RNA preparation was reverse transcribed using ReverTra Ace according to the manufacturer's instructions. Briefly, 10 μl of reaction mixture composed of 2 μl of 5 × RT buffer, 1 μl of dNTP mixture, 0.5 μl of RNase inhibitor, 0.5 μl of Primer hexanucleotides, 0.5 μl of ReverTra Ace, 4.5 μl of RNase free H2O and 250 ng of total RNA sample was placed in a GeneAmp PCR System 9700 (Applied Biosystems, Forest City, CA, USA). RT was carried out with 10 min annealing at 30°C, 60 min extension at 42°C and then 5 min denaturing at 85°C. PCR was performed by using a 1 μl reverse transcription reaction solution as a template in a volume of 50 μl of PCR reaction mixture. The primers used are shown in Table 1. The PCR mixture contained 5 μl of 10 × PCR buffer, 3 μl of 25 mm MgSO4, 1 μl of dNTP mixture, 1 μl of KOD-Plus (Toyobo) and 1.5 μl of each primer. Amplification was performed in a GeneAmp PCR System 9700 (Applied Biosystems) under the following conditions: 94°C for 2 min; then 35 cycles for 10 s denaturing at 98°C, 30 s annealing at 55°C and 15 s extension at 68°C. To exclude any contaminating genomic DNA, all experiments included controls lacking the RT enzyme. As a negative control, water was used instead of RNA for RT-PCR to exclude any contamination from buffers and tubes. PCR products were separated by electrophoresis in a 2% (w/v) agarose gel and 0.4% ethidium bromide was added during gel preparation. Ubiquitin was used as an internal control for reaction efficiency and variations in the concentrations of mRNA in the original reverse transcription reaction.
| Gene | Primer | Sequence | Fragment size (bp) |
|---|---|---|---|
| Flk-1 | Forward | 5′-TGGCCCAACAATCAGAGCAG-3′ | 154 |
| Reverse | 5′-GAACGGAGCCCATGTCAGTG-3′ | ||
| Flt-1 | Forward | 5′-GAAGGACGGGATGAGGATGC-3′ | 186 |
| Reverse | 5′-ATGGCGTTGAGCGGAATGTA-3′ | ||
| Ubiquitin | Forward | 5′-ATG CAG ATC TTT GTG AAG AC-3′ | 189 |
| Reverse | 5′-CTT CTG GAT GTT GTA GTC-3′ |
Results
Oestradiol-17β and progesterone concentrations ratio of fluid of cystic follicles containing granulosa cells are shown in Table 2. Nine of 19 follicles had a low ratio of oestradiol-17β/progesterone (<1.0). In the remaining 10 follicles, the ratios were above 1.0.
| Oestradiol-17β/progesterone | Number (percentage) of follicle |
|---|---|
| <1.0 | 9 (47.4) |
| 1.0–2.0 | 4 (21.1) |
| 2.0–3.0 | 4 (21.1) |
| >3.0 | 2 (10.5) |
| Total | 19 |
The expression of Flt-1 and Flk-1 was examined by RT-PCR using five cystic follicles having various ratios of oestradiol-17β/progesterone. All cystic follicles expressed a similar level of Flt-1 and Flk-1 PCR products regardless of the ratio of oestradiol-17β/progesterone.
In the immunohistochemical study, Flt-1-positive cells were localized in both granulosa and theca interna cells of cystic follicles (Fig. 1a, b) and healthy follicle (Fig. 1c). Granulosa and theca interna cells expressed Flt-1 in both cystic follicles with high and low ratios of oestradiol-17β/progesterone concentrations were detected. Endothelial cells in the theca interna were immunostained positively to Flt-1 antibody in both cystic follicles having low and high ratios of oestradiol-17β/progesterone concentrations (Fig. 1a, b). No staining was observed in the control slide, which was incubated with normal rabbit IgG in place of the first antibody.
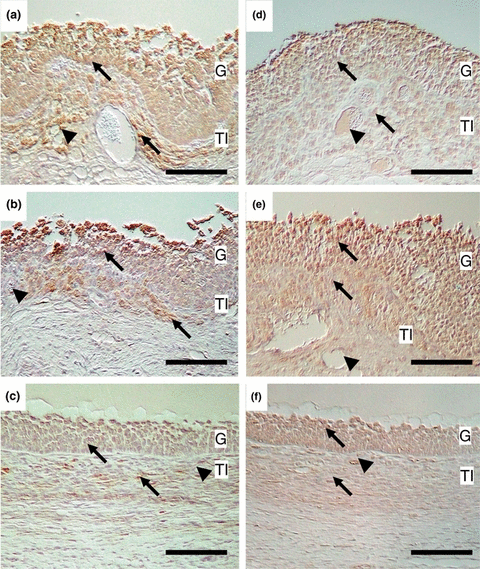
Micrographs of cystic (a, b, d, e) and healthy (c, f) follicles sections immunostained with anti-Flt-1 (a, b, c) and Flk-1 (d, e, f) antibodies. Ratio of oestradiol-17β/progesterone was >1 in the cystic follicles of (a) and (d) and <1 in (b) and (e). Arrows and arrowheads represent immunopositive cells and immunopositive microvessels. G: Granulosa layer; TI: Theca interna; Bars = 50 μm
Similar to Flt-1, both granulosa and theca interna layers contained Flk-1 immunopositive cells in the cystic follicle and healthy follicle and intensity was stronger in the granulosa than theca interna layers (Fig. 1d–f). Theca interna had Flk-1-positive endothelial cells in both cystic follicles having low and high ratios of oestradiol-17β/progesterone concentrations (Fig. 1d, e). No staining was observed in the control slide, which was incubated with normal rabbit IgG in place of the first antibody.
Discussion
Cystic follicles have a highly developed microvasculature and sufficient VEGF, a stimulator of vascular permeability, in the theca interna, which causes the active influx of serum components to inside the follicle (Isobe et al. 2005a). The biological activities of VEGF are mediated by two high affinity receptor tyrosine kinases (Ferrara and Davis-Smyth 1997) and this study was undertaken to identify those receptors.
The present study demonstrated that cystic follicles expressed protein and mRNA for VEGF receptors, Flt-1 and Flk-1. Both receptors were immunodetected in the granulosa and theca layers of cystic follicles like healthy follicles. Taken together with the fact that VEGF is expressed in the granulosa layer and theca interna of cystic follicles (Isobe et al. 2005a), it is suggested that VEGF is produced and reacts in the granulosa and theca interna of cystic follicles in an autocrine or paracrine manner. Moreover, Flt-1- and Flk-1 were detected in the endothelial cells of the theca interna. As VEGF is a stimulator of vascular permeability, VEGF produced in the cystic follicles must have a pivotal role in the influx of serum components into the follicular antrum, leading to the expansion of follicles followed by the formation of cystic follicles.
In bovine maturing follicles, Berisha et al. (2000) reported that VEGF receptors are expressed weakly in the granulosa layer but highly in the theca interna. The target for VEGF produced in the granulosa layer and theca interna could be endothelial cells in theca interna where both VEGF receptors are found (De Vries et al. 1992; Terman et al. 1992). These are supported by the present study showing that endothelial cells in the theca interna are immunostained with Flt-1 and Flk-1 protein. However, in the present study, cells other than endothelial cells in the theca interna and granulosa cells were also positive for Flt-1 and Flk-1. It has been reported that VEGF prevents apoptosis (Yuan et al. 1996; Gerber et al. 1998) by induction of the expression of anti-apoptotic proteins Bcl-2 and A1 in endothelial cells (Gerber et al. 1998). The granulosa and theca interna have only a few apoptotic cells in cystic follicles (Isobe and Yoshimura 2000a); therefore, VEGF may have an important role in apoptosis inhibition in cystic follicles. Suppressed apoptosis of granulosa and theca interna cells may cause the disorder of degenerative changes in the follicle, resulting in cystic follicle formation (Isobe and Yoshimura 2000b).
The localization of Flt-1- and Flk-1-positive cells in the cystic follicles were similar in follicles with different ratio of oestradiol-17β/progesterone. Therefore, the expression of VEGF receptors is constant during the development of cystic follicles. It is possible that the potential sensitivity of VEGF receptors and/or ligand concentration are/is changed during the development of cystic follicles. However, further study is required to investigate this concept.
In conclusion, our results demonstrate that cystic follicles have VEGF receptors in the granulosa and theca interna cells. The binding of VEGF to its receptors is likely to be associated with the upregulation of permeability in endothelial cells and then influx of the serum component into the follicular antrum, resulting in the excess expansion of cystic follicles.
Acknowledgements
This study was supported by a Grant-in-Aid from the Japan Society for the Promotion of Science (JSPS).




