Massive icebergs, alteration in primary food resources and change in benthic communities at Cape Evans, Antarctica
Abstract
The presence of massive icebergs in the Southwestern Ross Sea (Antarctica) in the early-to-mid-2000s changed the regional sea ice regime and phytoplankton productivity. We exploit data on benthic macro- and megafaunal communities collected on six occasions between 2001 and 2009 in the shallow waters adjacent to Cape Evans to link these oceanographic shifts to changes in benthic ecology. Changes in the abundance of individual species and community composition were generally strongest, and mostly negative, in 2002 and 2003, which exhibited the largest decrease in water column productivity and thick and persistent sea ice. This pattern of decreasing numbers from the start of sampling in 2001 to the lowest values in 2003 is consistent with the impact of the icebergs and a lagged response on the part of the benthic populations in response to food shortage. The patterns of stronger effects on macrofauna than on megafauna, and on abundance rather than species richness, are consistent with a change in food supply and/or recruitment for short-lived species, rather than a physical disturbance effect. Nevertheless, recovery patterns are likely to reflect changes in benthic communities associated with larval supply and changes in top-down control after the years of predicted lowest food supply. There are many potential manifestations of climate change in Antarctica, and many of the ecological responses to environmental change are likely to be mediated through the foodweb. Long-term studies in different locations are essential if we are to understand and forecast changes in sea-floor ecosystems.
Introduction
High latitude Antarctic marine environments are generally considered to be stable, due to the very cold water temperatures, ‘protection’ by a covering of fast ice during most of the year, and the general lack of many environmental perturbations associated with humans. Primary production is highly seasonal and coincides with the development of polynyas, the lengthening of daylight hours and the breakout of fast ice during the summer. At other times of the year, coastal production is limited to in situ primary production associated with sea ice algae, microphytobenthos and macroalgae, all of which are influenced by how much sunlight can penetrate the sea ice and the history of fast ice break-out (Dayton et al. 1986). The effects of these limitations on primary food resources are ameliorated by the extreme cold because basal metabolic costs for benthic invertebrates are low (Clarke 1993). Nevertheless, these seasonal events profoundly influence community dynamics, and any perturbations of this will likely influence benthic community structure and function.
One of the many potential manifestations of climate change is an increased rate of calving of large tabular icebergs from ice shelves. For example, the recent collapse of ice shelves on the Antarctic Peninsula has been attributed to warmer sea and air temperatures (Clarke et al. 2007; Raes et al. 2010). Although similar warming has not been observed in the Ross Sea region of Antarctica, calving events from the Ross Ice Shelf (RIS) have occurred in recent decades (Arrigo & van Dijken 2003; Dinniman et al. 2007; Martin et al. 2007). These massive tabular icebergs can be hundreds of kilometres in length and can extend 300–500 m below the water line, scouring the sea floor as they drift north. Importantly, such large icebergs can also change oceanographic conditions, both locally and over hundreds of kilometres (e.g.Massom 2003). Between 2001 and 2006 in the Ross Sea, the presence of three massive icebergs (C-19, B-15A and C-16) caused considerable variability in ice conditions in McMurdo Sound. Acting as physical barriers that prevented sea ice production and dispersal, these icebergs affected the timing and extent of polynya development, surface circulation and deep water formation processes (Arrigo & van Dijken 2003; Dinniman et al. 2007; Martin et al. 2007; N. Robinson & M. Williams, NIWA, personal communication). The following sequence of events influencing McMurdo Sound has been reported (see Fig. 1): C-16 calved off the RIS and became stuck on the eastern edge of Ross Island in November 2000. The massive B-15A (160 × 40 km) collided with C-16 and grounded in January 2001 (Dinniman et al. 2007). B-15A remained here until October 2003, when its northernmost two-thirds broke off and began to drift northwest along the Victoria Land coast (Barber-Meyer et al. 2008) before eventually exiting the Ross Sea in October 2005. The remaining grounded portion of the iceberg (now called B15-J) stuck fast until 2006 (Kooyman et al. 2007). Another massive iceberg C-19 (200 × 32 km) calved off the RIS in early May 2002 and moved directly into the widest part of the Ross Sea polynya (Martin et al. 2007). C-19 drifted north, finally exiting the Ross Sea in June 2003 (Martin et al. 2010). The net results of the presence of these icebergs were apparent in changes in the extent of the Ross Sea polynya and distribution of fast ice over this time (e.g. see Tamura et al. 2008). The polynya decreased in size by about one-third from 1999 to 2003, then slowly increased, reaching pre-1999 levels again in 2006 (Tamura et al. 2008; T. Tamura unpublished data/personal communication).
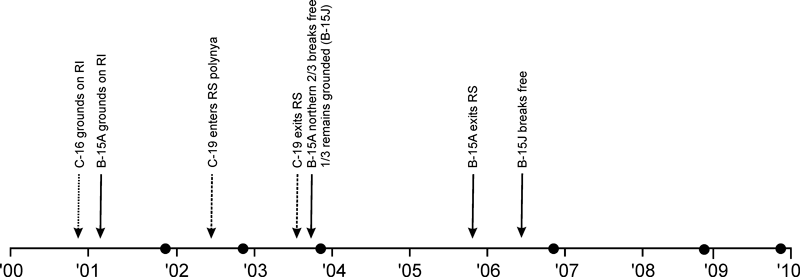
The timing of the major iceberg events in the Southern Ross Sea (RS). The black dots indicate the timing of our benthic sampling at Cape Evans, Western Ross Island (RI). The combined presence of the icebergs had the strongest effect on sea ice conditions from mid-2002 to late 2003.
The presence of these massive icebergs had a major influence on physical and biological water column processes. By physically preventing sea ice from drifting out of the region in spring and summer, B-15A reduced primary production in the Southwestern Ross Sea by 40–95% relative to previous years (Arrigo et al. 2002). The presence of C-19 from mid-2002 to mid-2003 delayed the opening of McMurdo Sound and reduced the size of the Ross Sea polynya; open water was around 25% of its usual levels and rates of primary production were reduced by up to 90% (Arrigo & van Dijken 2003). These changes influenced both food availability and access to feeding and/or breeding areas, and clearly affected the distribution and abundance of a variety of fauna. Adelie penguins altered their migration routes (Arrigo et al. 2002; Shepherd et al. 2005), increased movement rates (Dugger et al. 2010), and exhibited lower breeding success (Lescroël et al. 2009). Numbers of breeding Emperor penguins declined and large mortalities of chicks were observed (Kooyman et al. 2007). Fewer adult Weddell seals returned to their McMurdo breeding areas and, consequently, pup numbers in the area were lower (Siniff et al. 2008). The abundance of the pteropods Limacina and Clione, important consumers in the pelagic foodweb, were negatively affected by the reduced phytoplankton biomass (Siebel & Dierssen 2003). The increased thickness of sea ice in Southern McMurdo Sound (caused by the icebergs preventing sea ice breakout) altered the composition of sea ice microbial communities (Remy et al. 2008). Major changes were also observed in the benthic invertebrate community at Cinder Cones, in Southern McMurdo Sound, which were attributed to changes in the sea ice regime and phytoplankton productivity (Conlan et al. 2010).
In this paper, we exploit data on macro- and mega-faunal communities collected on six occasions between 2001 and 2009 in the shallow waters adjacent to Cape Evans, to extend the work reported by Conlan et al. (2010) both spatially and temporally. We look for changes in macrofaunal and megafaunal community composition and consider the natural history of these species in terms of their sensitivity to loss of phytoplankton resources. The presence of these massive icebergs has a number of potential effects on the Cape Evans benthos, operating on different time scales. In their analysis of mean open water area and annual primary production in the Southwestern Ross Sea from 1997 to 2003, Arrigo & van Dijken (2003) consider 2000/2001 and 2002/2003 to be ‘abnormal’ years (less open water and lower annual production) due to the presence of B-15 and C-19, respectively. More recent work shows the Ross Sea polynya was smaller in size from 2000 to 2005 (T. Tamura, personal communication). Not only was primary productivity changed in the polynya, but shifts in coastal ocean currents due to the icebergs may also have changed the amount of primary production advected past Cape Evans. There was also more pack ice present in Eastern McMurdo Sound in the summers of 2001/2002 and 2002/2003, the latter due to C19 restricting advection of sea ice away from the RIS (Dinniman et al. 2007). Inhibiting the breakout of coastal fast ice from Cape Evans is likely to restrict in situ production. For example, growth of the resident macroalgae Phyllophora only occurs during the short summer period of ice breakout at Cape Evans (Schwarz et al. 2003). Although there is no simple discrete event to which we might expect the benthic community to respond, based on the information presented above, we consider the years 2000 through 2003 to be a time of falling water column productivity, followed by a slow recovery to ‘normal’ productivity levels by 2006. We predict stronger effects on the abundance of macrofauna than on species richness, and stronger effects on these smaller, short-lived species than on megafauna. We also predict more immediate effects on species utilising phytoplankton derived from the polynya (e.g. filter feeders, grazers), with lagged or dampened effects on species at higher trophic levels, or those able to switch to other food resources.
Study site
Our study site was located in front of Scott’s Discovery hut at Cape Evans. The site (77°38.095′ S, 166°24.843′ E) is in about 19 m of water and has a substrate composed of volcanic sandy sediments, rocks and boulders with attached and drift red seaweed Phyllophora antarctica. The site was sampled on six occasions between 2001 and 2009 in the late spring (31 October to mid-November). Figure 1 shows the timing of our sampling relative to movement of the massive icebergs.
Methods
On each occasion the abundance of large megafauna, habitat features, sediment characteristics and macrofauna was described using a combination of video and core sampling along a 20-m transect line, collected using SCUBA. At five random positions along each transect we obtained core samples of macrofauna (one core, 70 mm diam., 100 mm deep) and sediments (one core, 26 mm diam., 50 mm deep) to quantify macrobenthic assemblage composition and sediment characteristics (grain size, organic content, microphytobenthos), respectively. To describe the abundance of large megafauna and the habitat type around each core location, the transect was videoed using a diver-held digital video camera, with the camera lens perpendicular to the sea floor at a fixed height of 40 cm above the bottom (Cummings et al. 2006). Megafaunal abundance and habitat features were quantified in three 50-cm-long quadrats centred on the core location, and scaled to numbers or percent cover m−2 (see Cummings et al. 2006 for more detail). Macrofauna core samples were sieved through a 500-μm mesh, preserved in 70% isopropyl alcohol, sorted and identified to the lowest taxonomic level possible. Chlorophyll a (Chl a) was extracted from freeze-dried sediments by boiling in 90% ethanol. The extract was measured spectrophotometrically, and an acidification step was included to separate degradation products (phaeophytin) from Chl a (Sartory 1982). Sediments for particle size analysis were digested in 6% hydrogen peroxide for 48 h to remove organic matter, and dispersed using Calgon. A Galai particle analyser (Galai Cis – 100; Galai Productions Ltd., Midgal Haemek, Israel) was then used to calculate % volumes for the coarse, medium and fine sand, silt and clay fractions. The organic matter content of the sediment was measured as loss on ignition (LOI) by drying the sediment at 60 °C for 48 h, followed by combustion at 400 °C for 5.5 h.
Differences between years in numbers of megafaunal and macrofaunal species, individuals, and common taxa, and variables indicative of sediment food content (i.e. sediment Chl a, phaeophytin and organic content) were determined using ANOVA, with Duncan’s multiple comparison tests used to identify any statistically significant differences. Data were assessed for normality and homogeneity of variance, and, if these assumptions were not met, the analyses were conducted on transformed data using the appropriate error structures. Linear regressions were used to evaluate the relationship between megafauna or macrofauna and the sediment food content variables.
To investigate trends in the composition of both megafaunal and macrofaunal communities over time we used non-metric multidimensional scaling (MDS) based on Bray–Curtis similarities, performed using PRIMER (Clarke & Gorley 2006). We initially ran the analysis on both untransformed and square root-transformed data. Although both showed the same general pattern, analyses conducted on raw data were clearer, indicating that shifts in species abundance are important components of the temporal response. Consequently, only ordinations based on raw data are presented here. One-way randomisation/permutation tests (ANOSIM, Clarke & Green 1988) were used to test for differences in assemblage structure over time.
Results
The sea urchin Sterechinus neumayeri, the seastars Odontaster validus and Diplasterias brucei, nemerteans and, occasionally, sponges and the soft coral Alcyonium, dominated the megafaunal community. The number of megafaunal species found at this site was low (<3 m−2 on average), and did not vary significantly over time (Fig. 2A; P < 0.6021 rank-transformed data). Megafaunal abundance was 13 and 56 individuals m−2 (in 2003 and 2009, respectively), and varied significantly between years (Fig. 2B): numbers were significantly lower in 2003 than in 2001, 2008 and 2009. Odontaster validus and S. neumayeri were the two most abundant megafaunal species. Odontaster showed stronger temporal variation (Fig. 3A) with the lowest densities in 2003 and 2006 and the highest in 2008. In contrast, Sterechinus densities were lowest in 2002 and highest in 2009; however, the abundance of this urchin exhibited only a marginally significant change over time (Fig. 3B).
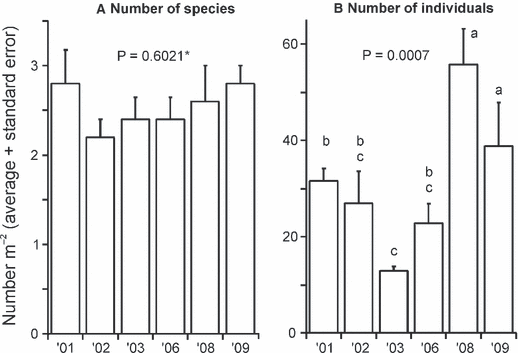
Mean (+ SE) for megafauna species richness (A) and number of individuals (B). Letters indicate statistically significant differences between years (Duncan’s multiple comparison tests); years with the same letters are not significantly different from each other. *The analysis was conducted on rank-transformed data.
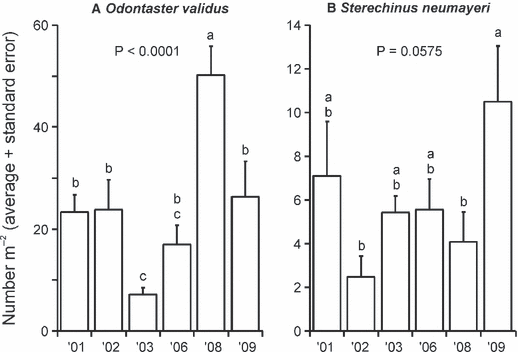
Mean (+ SE) for the two most common megafaunal species at Cape Evans. Letters indicate statistically significant differences between years (Duncan’s multiple comparison tests); years with the same letters are not significantly different from each other.
The number of macrofaunal species found ranged from 8 (in 2003) to 17 (in 2001 and 2009; Fig. 4A) per core. There was a significant change over time, with fewer species found in 2003 than in 2001, 2008 and 2009. Total macrofaunal abundance also changed over time, although these differences are only marginally significant (Fig. 4B). Nevertheless, there is a clear trend of a strong decline in abundance from 2001 to 2003, with low values maintained from 2006 to 2009. Following this decrease in mean abundance is a clear decrease in sample variability (Fig. 4B). The common macrofaunal taxa tended to show one of two temporal patterns: either the lowest or the highest abundance in 2003 or 2006 (Fig. 5). The most abundant macrofaunal species was the archiannelid polychaete Polygordius antarcticus, which was significantly less abundant in 2003 and 2006 than in 2001 and 2002 (Fig. 5A). The tanaid Nototanais sp. (Fig. 5B) and the isopod Austrosignum glaciale (Fig. 5C) were both significantly more abundant in 2002 than in the later sampling years (2006–2009). The abundance of oligochaetes was not significantly different over time (Fig. 5D). The spionid polychaete Spiophanes tcherniai increased in density four-fold in 2006 (Fig. 5E). The syllid polychaete Syllidia inermis show the complementary pattern, with lowest densities in 2003 and 2006 and highest densities in 2001 and 2008 (Fig. 5F). The two most common families of amphipods, phoxocephalids and lysianassids, also show indications of complementarity. The phoxocephalids exhibited lower densities in 2002–2003 than in all other years (Fig. 5G), whereas lysianassids had significantly higher numbers in 2003 than in all other years except 2002 (Fig. 5H). Finally, the common gastropod Onoba gelida did not reveal a significant temporal pattern, probably due to its high spatial variability (Fig. 5I).
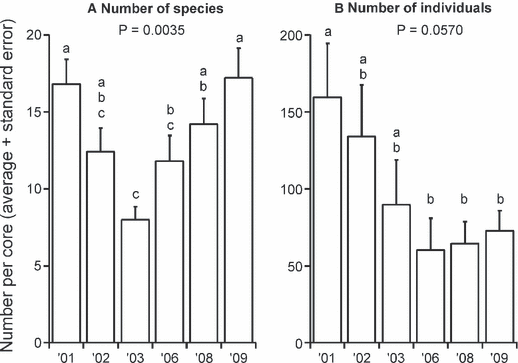
Mean (+ SE) number of macrofaunal species (A) and individuals (B). Letters indicate statistically significant differences between years (Duncan’s multiple comparison tests); years with the same letters are not significantly different from each other.
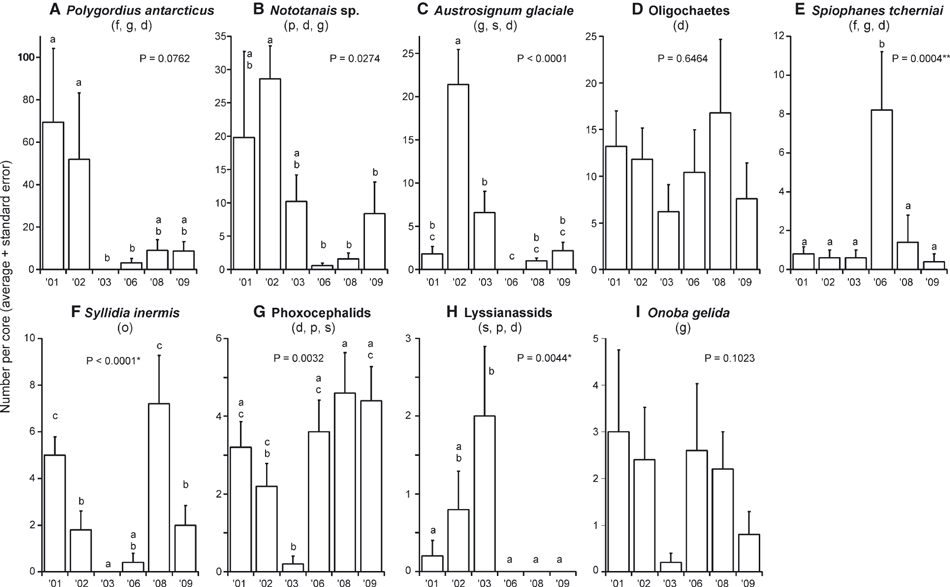
Mean (+ SE) abundance of common macrofauna (per 7 × 10 cm deep core). The feeding mode of each taxon: f, filter feeder; g, grazer; d, deposit feeder; p, predator; s, scavenger; o, omnivore. Letters indicate statistically significant differences between years (Duncan’s multiple comparison tests); years with the same letters are not significantly different from each other. *The analysis was conducted on rank-transformed data. **The analysis used a quasi-likelihood response distribution with a Poisson error structure.
Megafaunal community composition in 2003 was separated from the remaining years in ordination space, as illustrated by their situation on the left of the diagram (Fig. 6A). Although there was considerable overlap between the other years, there is some evidence of a temporal transition: communities in 2001 and 2002 overlapped prior to the shift noted above in 2003. A move back towards a similar community composition to that observed in 2001 occurred from 2006 to 2009. ANOSIM revealed a significant difference in community composition over time (Global R = 0.418; P = 0.001). Inspection of the pairwise comparisons showed that the 2003 megafaunal assemblage was significantly different from that in all other years sampled and, although some recovery was apparent by 2006 (cf. 2001), longer term trends still occurred, matching our interpretation of the ordination (cf. 2001 versus 2008 and 2001 versus 2009, Table 1A). The MDS of the macrofaunal community shows more distinct clustering of years than observed for megafauna, with overlap between assemblages in 2001 and 2002, and in 2008 and 2009. As noted for the megafauna, a temporal trajectory is evident, with assemblage groups moving in a clockwise direction from 2001 (Fig. 6B). ANOSIM revealed a significant difference in macrofaunal community composition over time (Global R = 0.532; P = 0.001). Inspection of the pairwise comparisons showed stronger differences than were apparent for megafauna, with significant differences between all years except 2001 versus 2002, 2008 and 2009, and 2008 versus 2009 (Table 1B).
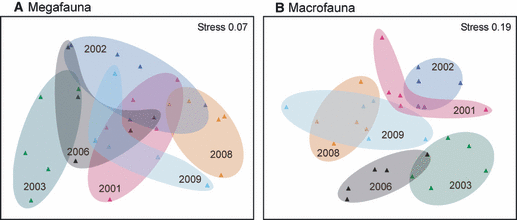
MDS plot of temporal variation in megafauna (A) and macrofaunal (B) community composition. Data from individual samples are shown ( ) for each year with shaded ellipses for ease of interpretation.
) for each year with shaded ellipses for ease of interpretation.
| year | 2001 | 2002 | 2003 | 2006 | 2008 |
|---|---|---|---|---|---|
| (A) | |||||
| 2002 | −0.012 (0.468) | ||||
| 2003 | 0.800 (0.008) | 0.700 (0.008) | |||
| 2006 | 0.008 (0.365) | 0.006 (0.397) | 0.400 (0.016) | ||
| 2008 | 0.520 (0.016) | 0.468 (0.032) | 1.000 (0.008) | 0.720 (0.008) | |
| 2009 | −0.040 (0.556) | 0.120 (0.175) | 0.672 (0.008) | 0.052 (0.270) | 0.516 (0.024) |
| (B) | |||||
| 2002 | 0.128 (0.151) | ||||
| 2003 | 0.580 (0.016) | 0.664 (0.008) | |||
| 2006 | 0.448 (0.008) | 0.844 (0.008) | 0.592 (0.024) | ||
| 2008 | 0.368 (0.160) | 0.900 (0.008) | 0.920 (0.008) | 0.500 (0.008) | |
| 2009 | 0.304 (0.320) | 0.562 (0.008) | 0.632 (0.008) | 0.572 (0.016) | 0.066 (0.262) |
- Data shown are R-statistics, with significance levels in parentheses (P <0.05 bold text).
Sediment food content as indicated by Chl a and phaeophytin concentrations show an interesting lagged temporal pattern, with lowest average values of both pigments in 2003 and 2006, and considerable variation evident between years (Fig. 7A,B). Sediment Chl a content was significantly higher in 2002, 2008 and 2009 than in 2006 and 2003 (Fig. 7A). There were no statistically significant differences in phaeophytin content between years, most likely due to the high between-sample variability in 2001 and 2002 (Fig. 7B). Sediment organic matter content remained low over the sampling period, with significant differences detected only between the years 2006 and 2009 (Fig. 7C). Unfortunately, sediment organic content samples were lost in 2003 due to accidental thawing.
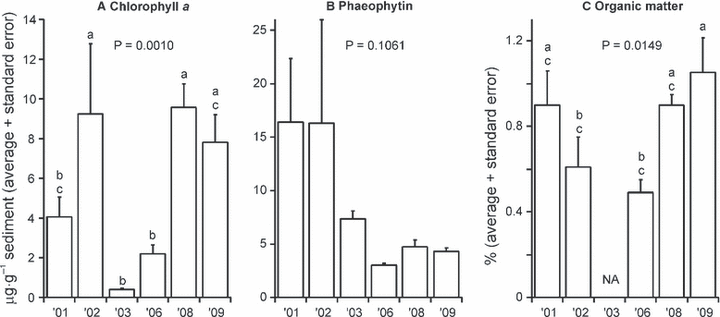
Mean (+ SE) for sediment Chl a (A), phaeophytin (B) and organic matter content (C). Letters indicate statistically significant differences between years (Duncan’s multiple comparison tests); years with the same letters are not significantly different from each other. NA, not available.
We found some interesting relationships between macrofauna and megafauna abundance and species diversity, and these sediment food parameters. For macrofauna, there were very strong correlations between number of individuals and phaeophytin (r = 0.9774), and between number of species and organic matter content (r = 0.9237). The number of megafauna species was also highly correlated with organic content (r = 0.8553), and the number of individuals was well correlated with both sediment Chl a (r = 0.7800) and organic matter content (r = 0.6662).
Discussion
We expected the effects of the massive icebergs near Ross Island to impact on the benthic community at Cape Evans, primarily through decreased water column productivity and thick and persistent sea ice, with these factors showing the largest changes in 2002–2003. Although our results do show different temporal patterns for different community and population response variables, overall these changes in measures of species richness and abundance culminated in distinctly different mega-and macrofaunal community compositions in 2003 (Fig. 6). There was a decline in overall abundance of mega- and macrofauna, and in number of macrofaunal species, in 2002–2003, and a similar pattern in sediment Chl a and organic matter content. This pattern of decreasing numbers from the start of sampling in 2001 to the lowest values in 2003 is consistent with the impact of the icebergs and a lagged response on the part of the benthic populations to food shortage (e.g.Fig. 5: Onoba, Phoxocephalids, Oligochaetes, Syllidia, and Polygordius; although note that not all of these trends were significantly different due to high spatial variation along sampling transects). 2003 was also the most ‘different’ year in both megafaunal and macrofaunal community structure as revealed by the multivariate analysis (Fig. 6). Major changes in productivity in 2003 are further supported by changes in benthic Chl a and organic matter concentrations in the sediments, and the strong correlations between these variables and the abundance of mega- and macrofauna at Cape Evans. The temporal patterns after 2003 show a range of recovery responses, with the densities of some fauna remaining low (e.g. overall macrofaunal abundance, Nototanais sp.) and others bouncing back often to achieve their highest recorded abundance (e.g. macrofaunal species richness, Sterechinus, Odontaster). The overall pattern of stronger effects on macrofauna than megafauna was consistent with our predictions. In addition, the stronger effects on abundance than on species richness are consistent with a change in food supply, rather than a physical disturbance effect. Nevertheless, recovery patterns are likely to reflect changes in benthic communities associated with larval supply and changes in top-down control after the years of predicted lowest food supply.
Natural history information helps to inform our understanding of the variability in response of individual species and provides other lines of evidence that help us relate the effects of the massive icebergs to the benthic community. Many Antarctic benthic species have flexible omnivorous feeding strategies: for example, the urchin Sterechinus and the seastar Odontaster can feed at multiple trophic levels, although both species revealed temporal patterns consistent with the iceberg effects. The variability in the δN15 signature of Sterechinus from Cape Evans is much higher than in locations on the western side of McMurdo Sound and along the Victoria Land Coast (Norkko et al. 2007). Spatial comparisons of trophic relationships between Victoria Land Coast locations with high or low productivity demonstrate that Sterechinus switches to a more detritus-based diet in the absence of primary producers (Norkko et al. 2007). This may dampen the immediate effects of transitory food shortages. In 2 years with contrasting sea ice conditions (and thus food supply) in Terra Nova Bay, Chiantore et al. (2002) showed that the reproductive outputs of Sterechinus and Odontaster were affected, although these effects were mitigated by the ability of these species to adjust their feeding strategies. These results highlight the importance of understanding both environmental change and the potential responses of individual species to changing conditions. Sterechinus and Odontaster are both mobile, which may also have influenced the responses apparent at our monitoring site. Odontaster, a generalist feeding on sponges, starfish, bivalves and detritus (Dayton et al. 1974), is known to respond rapidly to faecal material that accumulates under holes in the sea ice utilised by Weddell seals (Kim et al. 2007). The loss of primary food resources and the thickening of the sea ice restricting access for air-breathing vertebrate predators (Arrigo et al. 2002) may have contributed to the decline of Odontaster in the shallow waters at Cape Evans. Perhaps some individuals were able to find a refuge in deeper water, where they play important roles as starfish predators (Dayton et al. 1974), facilitating population recovery in 2008.
For the macrofauna, we also saw responses consistent with the loss of food resources, but these patterns were not stronger for species with feeding habits more directly affected by changes in productivity (e.g. grazers, filter feeders). It is important to note that as well as responding to bottom-up forcing, top-down (predatory) control of these macrofaunal communities can occur (Oliver & Slattery 1985). Nototanais sp. is a recognised predator of small macrofauna, particularly polychaetes (Oliver & Slattery 1985), and it may have influenced the decline in numbers of Polygordius antarcticus, the most common macrofaunal taxa at this site. Polygordius is a small interstitial polychaete that inhabits a range of sediment types, although high densities appear to be associated with abundant organic debris (Rota & Carchini 1999), emphasising bottom-up influences. Phoxocephalid amphipods are also important in top-down control via their predation on larvae and juvenile macrofauna (Oliver & Slattery 1985). Both the phoxocephalid and lysianassid amphipod families contain species with varied and flexible feeding strategies, but the phoxocephalids tend to be surface deposit feeders and predators (Slattery & Oliver 1986), whereas many lysianassids are scavengers, and it is interesting to consider the complementarity of the temporal patterns in these two families (Fig. 5G,H). Changes in the abundance of Terra Nova Bay macrofauna have also been linked to temporal variation in fast ice, with species responses associated with mobility and reproductive strategies (Gambi et al. 2000).
The spionid polychaete, Spiophanes tcherniai, showed an unusual spike in abundance at Cape Evans in 2006. This is a very different pattern to that apparent further to the south (Conlan et al. 2010). Conlan et al. report on the results of a monitoring programme, initiated in 1988, designed to identify the impacts of McMurdo Station and remedial action taken to reduce anthropogenic impacts. Their ‘upstream’ control site at Cinder Cones (about 13 km north of McMurdo station) does not appear to show any trends associated with anthropogenic pressure and thus provides an important comparison with our sampling site at Cape Evans. Cinder Cones is approximately 20 km to the south of Cape Evans, Conlan et al.’s monitoring site is at about 18 m depth, and activities at the site involved collecting a similar number and size of core samples (see Lenihan et al. 1990) to our monitoring programme. The benthic community at the Cinder Cones site, while generally similar in composition to Cape Evans, is dominated by Spiophanes tcherniai, which is considered a pollution-intolerant species (Conlan et al. 2004). Contrasting data collected at Cinder Cones in 1988–1998 and 2002–2004 revealed about a 50% decline in the abundance of Spiophanes and a three-fold increase in the abundance of the small snail Onoba spp. In contrast, at Cape Evans, densities of Nototanais sp. and Onoba gelida were lower in 2003 (although we did not detect a significant difference in Onoba due to high spatial variance) and the only change in abundance of Spiophanes during our time series was a spike in 2006 (Fig. 5A–C). These differences likely reflect variation in response due to interactions with local habitat features, and in community composition prior to the effects of the massive icebergs on productivity and fast ice persistence becoming manifest. We may also expect earlier and stronger effects at Cinder Cones cf. Cape Evans due to its location further south, where there was a shortened ice-free period during the iceberg years. This is further supported by the fact that Conlan et al. (2010) noted more dramatic effects at a site located even further south than Cinder Cones. Such interactions between local and broad-scale factors driving temporal variability in population dynamics between sites has been revealed by time-series data from temperate coastal ecosystems (Dayton et al. 1999; Hewitt & Thrush 2009). Nevertheless, consistent with the conclusions of Conlan et al. (2010), we consider that the most likely cause of the changes in benthic communities we observed around 2003 was the effect of the massive icebergs on regional primary production and restriction of coastal sea ice breakout.
Defining cause and effect relationships on the basis of ecological monitoring data is not easy and we have tried to use multiple lines of evidence to infer effects. These include available oceanographic data showing effects preceding the major benthic response, some degree of consistency in response at Cape Evans by species with similar biological traits, and the strong correlations between the abundance of both megafaunal and macrofaunal organisms and food resources in the sediments at Cape Evans. Note that the 2002, 2008 and 2009 Chl a concentrations were particularly high for Antarctic coastal sediments compared with those found at other coastal sites in this region (e.g.Cummings et al. 2006; authors’ unpublished data). Our divers noted large quantities of algae floating in the water column and strung off the under-ice surface in 2008 and 2009. Although our time series is short and imperfect, the fact that we see consistent patterns indicates the magnitude of effects to the benthic community due to changes in food resources. Similar to Conlan et al. (2010) and Dayton (1989), we highlight the importance of long-term ecological data both to understand the fundamental community dynamics of these unique benthic communities and to tease apart the effects of natural phenomena and anthropogenic influences. Given the potential for change in this region associated with a variety of anthropogenic impacts, we would encourage the development of an international monitoring programme focused on selected coastal Ross Sea locations to provide some fundamental understanding of how these relatively pristine ecosystems are responding to environmental change.
There are many potential manifestations of climate change in Antarctica (Aronson et al. 2007). Although the Antarctic has been subjected to many environmental changes in the past that have strongly influenced the evolution of its resident biological communities (Clarke & Crame 1992), rapid changes in climate, ocean chemistry and other anthropogenic stressors in the marine environment will profoundly change Antarctica’s ecology. Many of the ecological responses to environmental change are manifested through the foodweb (Dayton 1990). Trophic relationships within coastal benthic communities are complicated, with many species exhibiting flexible and spatially variable feeding strategies and a mix of top-down, bottom-up and slow detrital energy transfer processes (Oliver & Slattery 1985; Dayton et al. 1994; Mincks et al. 2005; Norkko et al. 2007). Such complexity may account for why it has proved difficult to link the flux of organic material to the regulation of benthic communities except in deep water (Barry et al. 2003; Smith et al. 2007). Our results, along with those of Conlan et al. (2010), highlight that changes in shallow coastal benthic communities also occur when stressed by changes in the quantity and quality of primary food resources. Such effects in the context of potential climate change may be particularly important in shallow water because of the availability and importance of sunlight to the sea floor, the potential for under-ice production to fuel benthic systems and the ability of large mobile organisms to move across strong local environmental gradients to exploit resources.
Conclusions
The presence of three massive icebergs in the Southwestern Ross Sea in the early 2000s affected the sea-floor communities by acting as a physical barrier restricting the break out of sea ice, dampening in situ primary productivity, and influencing coastal hydrodynamics. Previous studies have shown clear and immediate direct effects following the presence of these icebergs (e.g. on plankton and access to feeding and breeding sites for penguins and seals). However, effects on the benthos may take longer to manifest and be more complicated due to the interplay of ice cover, polynya size and extent, and modification of oceanic conditions. We have demonstrated changes in benthic mega- and macrofaunal community structure, and sea-floor sediment food content at Cape Evans that were most apparent in spring 2003, almost 3 years after B-15A first grounded off Ross Island and 1.5 years after C-19 first affected the opening of the Ross Sea polynya (Fig. 1). Although our time series is imperfect, the evidence is clear that these icebergs affected benthic community composition, most likely through a combination of effects on availability and supply of food resources and recruitment processes.
Acknowledgements
We thank Paul for his inspiration, for introducing one of us (S.F.T.) to the splendour of Antarctic’s benthic ecosystems; he has consistently sought to develop and encourage international collaboration and we thank him for this. From his PhD research using dog bowls and dynamite on the Washington coast, Paul has been at the forefront of disturbance ecology research in marine systems, and his work is fundamental to our understanding of environmental effects in coastal systems. We would also like to thank our Italian Antarctic colleagues, particularly Riccardo Cattaneo-Vietti and Mariachiara Chiantore, for allowing us the space to begin to understand changes over time in Antarctica. We thank Chazz Marriott and Greig Funnell for video analysis, Ron Ovenden, Sarah Hailes and Scott Edhouse for sediment analyses, and Geoff Read (polychaetes) and Graham Bird (tanaids) for taxonomic assistance. For the diving we thank Greig Funnell, Owen Anderson, Chazz Marriott, Ian Hawes, Anne-Maree Schwarz, Luca Chiaroni, Drew Lohrer Alf Norkko, Neil Andrew and our dive supervisors Steve Mercer, Rod Budd and Jenny Beaumont. Alf Norkko and Neil Andrew are thanked for their valuable input to the early phases of this research, and Takeshi Tamura for unpublished information on changes in the Ross Sea polynya in the years following the icebergs. We are grateful to Antarctica New Zealand for providing excellent logistical support. This work has been funded by the New Zealand Ministry of Fisheries (ZBD2001/02, ZBD2002/01 and ZBD2006/03), NIWA-NSOF and the NZ Foundation for Research Science and Technology International Polar Year project COX01707.




