The future of three-dimensional microscopic imaging in marine biology
Abstract
Measuring biodiversity in the oceans and its modifications with time or during climate change requires the accurate description of plankton organisms from viruses to fishes. However, our taxonomic knowledge of this 70% of the Earth is extremely limited. The ultimate way to perform large-scale taxonomical analysis is to achieve full 3D imaging of every specimen collected. Novel 3D imaging techniques are undoubtedly at the forefront of such efforts, and will provide an important tool to identify, classify and generate reference models (or 3D gold standards) for rapid recognition and classification of plankton organisms during community or basin studies. This review gives an overview of recently developed 3D imaging techniques and discusses their limitations and promises.
Introduction
Taxonomy is an important tool in biology, and the capacity to record the true 3D nature of organisms reliably is essential for classifying them. The Convention on Biological Diversity was signed in 1992 at the Rio ‘Earth Summit’. In 1998 the Darwin Declaration further promoted the need to increase taxonomical knowledge of our biotopes; however, those treaties did not take into account the technical requirements for achieving such a complex and challenging task. This is even more important in the context of oceanography, where the knowledge of ocean biodiversity is extremely limited. Plankton is extremely diverse, with many complex structures and semi-transparent organisms. In fact, in the context of marine biology it is extremely difficult to analyze large plankton communities specimen by specimen. This often requires net tows and other collection systems followed by fixation and manipulations prior to analysis. This is labor-intensive and limited, as it is actually impossible to analyze billions of specimens given that only 10% of species have been well documented to date. Most technologies have low throughput and require human intervention. Moreover, to speed up the process, many imaging devices have been created that rely on single two-dimensional (2D) images. Often there is not enough information in such an image to be used by any trained user or machine for classification. Formal identification rules have to be relaxed so that not only non-formal categories of organisms are recorded by crude segmentation and simple geometrical classification but also the introduction of advanced recognition functions such as characteristic extraction (eg counting legs and antennules). The ultimate way to perform such large-scale taxonomical analysis would be to achieve full 3D imaging of every specimen collected. Each ‘morphoscan’ could be matched against a reliable 3D library containing 3D gold standards established from reference specimens, including intra-species variations and key references established by the community. Such a dream will only come true if we can achieve 3D imaging at high-throughput levels. Recently, many 3D microscopy techniques have been developed to assess non-destructively the third-dimension of the biological world. From transmission electron microscopy (TEM) of species in the nanometre size range and viruses, to magnetic resonance imaging (MRI) of entire preserved fishes, there is a range of opportunities for studying marine species in three dimensions. But, as in the case of confocal microscopy, the 3D images are still used with low-throughput approaches, often for single species characterization. Moreover, each technology has its specificity and there is no magical microscope that is able to image centimeter-long transparent objects (salps) or calcium carbonate bases of skeletons of micrometer width (coccolithophores). Not only do we need 3D imaging techniques, but they must be fitted to our needs from underwater 3D imaging of copepods to nanometric precision imaging of picoeukaryote structured walls. Finally, many of such advanced 3D techniques have been developed but are scarcely used in marine biology.
In this review, we will present 3D microscopy techniques that open new possibilities in marine research. We will start with underwater 3D imaging techniques, followed by recent developments in magnetic resonance imaging and X-ray microtomography. Finally, we will introduce two newly developed light microscopy techniques: optical projection tomography and light sheet microscopy.
Underwater 3D imaging
There is a clear need in marine biology for non-invasive and non-destructive methods to measure quantitatively the mass or volume of an organism (fish, sponge, coral or copepod) or its behavior (swimming, feeding, etc.) in its natural habitat. Earlier attempts using sound waves gave limited results in terms of resolution but were used successfully to trace large populations of fishes and submarine organisms. This approach was developed further and gave birth to FishTV, a 3D acoustical imaging system allowing the tracking of hundreds of individual animals at 37 m depth (McGehee & Jaffe 1996). However, sound cannot provide information on color or internal structures (symbionts, chloroplasts, etc.) and has limited applications for taxonomy and identification.
At the mesoscopic level, the 3D reconstruction of the sea floor and its inhabitants can be achieved by combining underwater video and image processing (Cocito et al. 2003), but is limited to well illuminated environments (shallow waters, extra light sources). At the macroscopic and microscopic levels, underwater imaging relies on ordinary floodlights and sometimes laser sources to provide the necessary light. So far, it is nearly impossible to obtain 3D information of a complete animal underwater (i.e. volume) as it moves, and it is usually imaged from only one side (e.g. see video plankton recorder; Benfield et al. 1996). One partial answer to this problem is the use of holography. Invented by Dennis Gabor in 1947 (http://nobelprize.org/nobel_prizes/physics/laureates/1971/gabor-autobio.html) it has received limited attention due to the need for extensive reconstruction to obtain the final image. In fact, holography is a two-step process. First, the hologram is recorded, resulting in a complex interference pattern that does not closely resemble the object. Secondly, light is ‘back-propagated’ through the hologram itself (either physically or computationally) to give rise to a final image. Recently, improvement of the reconstruction algorithms and the development of a submersible digital in-line holographic microscope (DIHM) have shown promising results (Xu et al. 2003).
Even if 3D underwater imaging has been greatly improved over the past few years, the techniques of choice to obtain 3D information of marine species rely on on-land imaging platforms on live or fixed samples to provide reference material.
Magnetic resonance imaging
Microscopic magnetic resonance imaging (micro-MRI, or μMRI; Glover & Mansfield 2002) is one of the best imaging methods available for assessing soft tissue structures and monitoring biological processes as they occur in vivo (Van der Linden et al. 2004). This technology does not rely upon ionizing radiation, yet it offers spatial resolution of tens of micrometers (Glover & Mansfield 2002; Tyszka et al. 2005), and thus has clear advantages over other imaging methodologies such as positron emission tomography (PET). MRI is a non- invasive in vivo (Hart et al. 2003; Pohlmann et al. 2007) and post-mortem imaging technique that allows the study of ‘soft’ tissue (e.g. brain, bone marrow, muscle; Tyszka et al. 2005; Pohlmann et al. 2007). It can be used to map anatomical structure (anatomical MRI, or aMRI; Narasimhan & Jacobs 2002), or as a monitor of organ function [functional MRI (fMRI); Bekinschtein et al. 2009]. Moreover, it is possible with magnetic resonance spectroscopy to track various metabolic processes. The advent of high magnetic field MRI systems for small animals, i.e. with an average magnet bore size that fits an adult rat body, has made this technique accessible for the study of most small animal models (Hart et al. 2003; Tyszka et al. 2005; Null et al. 2008). MRI uses the physical phenomenon of nuclear magnetic resonance (NMR), which exploits the magnetic properties of atomic nuclei (Glover & Mansfield 2002). Certain nuclei, such as hydrogen nuclei, have a weak magnetic moment, or spin. Hydrogen nuclei are naturally abundant in most biological tissues, and thus are used to image live organisms. NMR works by detecting variations in magnetization of atomic nuclei in response to an electromagnetic wave applied at the right frequency, i.e. the resonance frequency. When these nuclei change alignment and return to their original positions, they emit signals (Glover & Mansfield 2002). Technological advances in computing and magnetic fields have taken NMR from condensed matter physics to chemical analysis, and then structural biology, and more recently into medical imaging. MR images can be generated that yield either morphological or functional information. To assess morphology, the signal-to-noise ratio (SNR) and resolution are generally not high enough for microscope samples. If signal differences do not exist between different structures, the image will be flat and featureless. Thus an adequate contrast, or signal-to-background ratio, is required to separate structures of interest. MR techniques are unique in that several contrast mechanisms (e.g. T1, T2, diffusion) may be employed by using different imaging sequences that control the degree of contrast that can be achieved (referred to as the image ‘weighting’). In 1980, Hansen et al. published the first anatomically interpretable MR images of a normal living rat body (Hansen et al. 1980) and thus laid the groundwork for the many MR applications in small animals that quickly followed.
Anatomical MRI
MRI makes it possible to display most body organs visually (Fig. 1D,E,G,I). The resonance of water molecules, under a very high magnetic field, which are naturally abundant in most biological tissues, is used to generate cross-sectional images detailing brain structure (gray matter, white matter; Narasimhan & Jacobs 2002), or body morphology (Kabli et al. 2006; Null et al. 2008; Ziegler et al. 2008). The typical pixel size obtained is between 100 × 100 and 10 × 10 μm2.
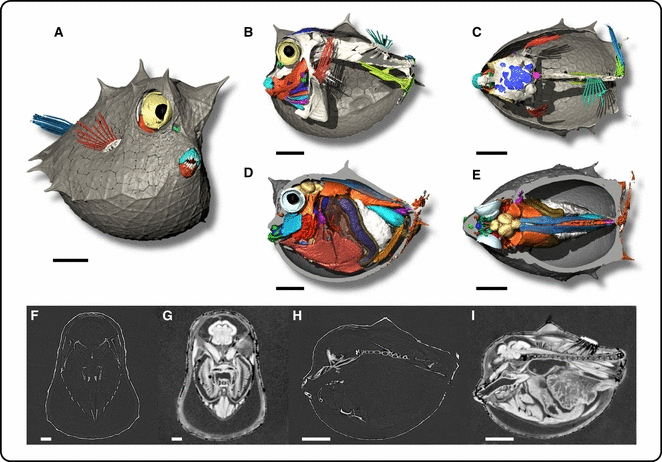
Comparison between synchrotron phase contrast X-ray microtomography and microscopy MRI of scrawled cowfish species, Acanthostracion quadricornis. Volume rendering of scrawled cowfish.: (A) Three-quarter view, (B,D) lateral view, and (C,E) dorsal view show skeleton (in white) with a different labeling color for soft tissues. Virtual and sagittal slices (F,G) show the brain and branchial cavity. (H,I) Parasagittal view. (A,B,C,F,H) X-ray phase contrast synchrotron microtomography obtained at ID19 (ESRF) at 900 mm between sample and camera, at 25 keV, voxel size 7.5 μm (A,B,C,F,H). DEGI MRI experiments were performed using a 9.4-T vertical bore spectrometer (Bruker Biospin, Ettlingen, Germany), voxel size 43 μm. Scale bar: 1 mm.
Functional MRI
The recent acceleration in data acquisition and processing has led to the advent of ‘functional’ MRI (fMRI), which is able to show neural and muscle activity in different regions of the brain or body (Van der Linden et al. 2004; Lowe et al. 2007; Pohlmann et al. 2007). Speaking, reading, moving or thinking activates specific areas in the brain (Boumans et al. 2008). This neural activation triggers a local increase in blood flow in the brain regions concerned. Although it cannot directly detect the local, transient increase in blood flow that neural activity causes, fMRI can estimate the increase of the blood flow by gauging the magnetization of the hemoglobin contained in red blood cells.
Tensor diffusion imaging, or diffusion MRI (DTI, dMRI)
Water diffusion in tissue is an intrinsic physical 3D process that can probe tissue structure. dMRI is a powerful tool providing access to the mean diffusivity, the diffusion anisotropy, and the fiber orientation in tissue (for example, neuronal and muscular tissues). It offers a more direct and non-invasive method of measurement of the local orientations in the tissues compared with other conventionally used 3D imaging techniques (Beaulieu & Allen 1994; De Groof et al. 2006; Aggarwal et al. 2009). The most advanced application of dMRI is fiber-tracking in the central nervous system (Le Bihan, 2003).
Manganese-enhanced magnetic resonance imaging (MEMRI)
Mn2+ has been used in the field of light microscopy in conjunction with fluorescent dyes such as fura-2 to indirectly monitor the influx of Ca2+ ions because Mn2+ is a well known Ca2+ analog. Importantly, Mn2+ is also paramagnetic and causes positive contrast enhancement in T1-weighted MRI images in tissues where it has accumulated. These attributes suggest that a direct assay for neuronal connectivity and function using MRI is possible. Recently, the use of Mn2+ as an in vivo, trans-synaptic, MRI-detectable neuronal tract tracer has been demonstrated. In contrast to previous histological approaches, Mn2+ tracing can be performed repeatedly on the same living animal. MEMRI can be used to enhance the contrast in animal brain cytoarchitecture (Gillet et al. 2010), to probe activity in heart and brain (Yu et al. 2008), and to trace neuronal active connections (Watanabe et al. 2006). Results in the literature indicate that MEMRI provides an efficient and powerful in vivo method for analyzing the physiology and pathology of animal embryos (Silva et al. 2004).
X-ray microtomography techniques
The first practical application of X-rays – more than 100 years ago – was for biomedical imaging purposes. Computed tomography (CT) was developed in the late 1960s and consists of acquiring projections (i.e. radiographs) of an object in different directions and combining them computationally to obtain a 3D reconstruction (using virtual slice stacks) of the object (Cormarck 1963; Hounsfield 1972, 1973). This technique has been used for large-scale biological samples since the 1980s, when it was developed primarily for medical applications with a resolution of about 1 mm (Kalender, 2006). Since the arrival of synchrotron radiation, the resolution of tomography has improved considerably, such that samples can now be imaged at submicrometer resolution (Baruchel et al. 2008), with effective pixel sizes down to about 60–90 nm.
- •
Synchrotrons used for microtomographic applications in biological imaging are distributed worldwide and include the ERSF (France), Diamond (England), Desy (Germany), Elettra (Italy), NSLS (USA), PLS (Korea), SLS (Switzerland) and Spring8 (Japan). Compared to conventional X-ray sources, synchrotrons have the advantages of a wide energy range (4–200 keV), a high photon flux, and a monochromatic (ΔE/E ≈ 10−2–10−4), parallel and partially coherent beam (Betz et al. 2007; Baruchel et al. 2008). The main reasons that synchrotron-generated X-rays are preferred to desktop-generated X-rays are that they offer a significantly higher resolution, a better signal-to-noise ratio, a short acquisition time, the potential for quantitative reconstructions and, last but not least, both phase and absorption contrast imaging (Betz et al. 2007). X-ray imaging techniques are non-destructive and can select a wide range of properties (density, chemical elements, chemical bonds, strains), and 3D morphometric information with up to nanometric resolution. Synchrotron radiation (SR) allows several imaging methods.
- •
Absorption microtomography (using the intrinsic sample contrast or with a contrasting agent).
- •
X-ray phase contrast imaging, which can be classified into (i) free-space propagation methods, (ii) crystal interferometer methods, and (iii) analyzer-based techniques.
- •
X-ray chemical nano-imaging.
Absorption imaging
Natural contrast
X-ray microtomography (Baruchel et al. 2008; Peyrin 2009) is based on absorption, i.e. the attenuation contrast between different tissues. The principle of this microtomography method is similar to that used in medical CT scanners. In conventional X-ray imaging, the observed contrast results from the variation in photon absorption arising from density differences between different tissues, and from the thickness and composition of the specimen. The technique yields excellent results when a highly absorbing structure is embedded in a matrix of relatively weak absorbance (e.g. bone inside a body or shell; Stock et al. 2003; Prymak et al. 2005; Perez-Huerta et al. 2009; Pasco-Viel et al. 2010). However, because higher spatial resolution images require greater X-ray fluxes, there is an inherent trade-off between image quality and tissue damage.
Contrast agents
Several staining methods used in histology for optical and electron microscopy can be used for X-ray microtomography as well (Betz et al. 2007; Metscher 2009). The use of contrast agent will be very efficient: as the X-ray absorption coefficient increases with the electronic density of the material for a given energy, injection of heavy metals in the biological sample will create an artificial contrast, revealing a specific structure. One can then use barium sulfate, gadolinium, nano-gold, iodine, osmium tetroxide (Fig. 2A), silver or uranyl acetate to visualize soft tissues such as nerves (Johnson et al. 2006), muscles (Johnson et al. 2006; Betz et al. 2007) or the vascular system (Plourabouéet al. 2004; Heinzer et al. 2006; Wirkner & Prendini 2007). The K-edge digital subtraction imaging (KES) method can be used. K-edge describes a sudden increase in the attenuation coefficient of photons occurring at photon energy just above the binding energy of the K shell electrons of the atoms interacting with the photons (Adam et al. 2005). The sudden increase in attenuation is due to photoelectric absorption of the photons. To perform K-edge imaging, two images are recorded simultaneously using monochromatic beams with energies that are below and above the K-edge of the atomic element used as contrast agent. The logarithmic subtraction of the image sets produces an image of the stained tissues only (Bayat et al. 2001; Elleaume et al. 2002; Alric et al. 2008; Schultke et al. 2010).
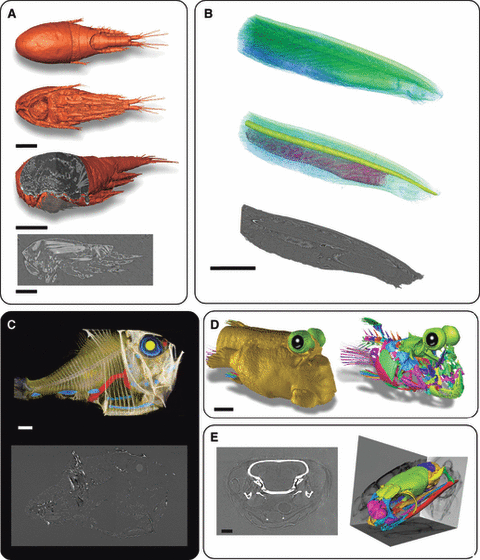
X-ray microtomography analysis of various marine species. (A) Thermocyclops consimilis. Volume rendering of dorsal view (upper), ventral view (middle) and three-quarter view with cross-section in sagittal (lower) and parasagittal plane at level of head and virtual slice in parasagittal plane showing muscle. Scale bar: 100 μm. (B) Branchiostoma platae. Lateral view of anterior part of animal, Upper: volume texturing with green/blue map of general anatomy. Middle: volume rendering with notochord and pharynx with gill slits. Lower: parasagittal virtual slice in phase contrast. Scale bar: 1 mm. (C) Argyropelecus aculeatus. Upper: volume rendering showing lateral view of general anatomy. Lower: parasagittal virtual slice. (D): Periophtalmus barbarus. Three-quarter view showing the head with skin (left) and the labeling of several skull bones (right). Scale bar: 1 mm. (E) Laticauda saintgironsi. Sea snake from New Caledonia (îlot Brosse, November 2003. MNHN 2009.0168 Coll. Ivan Ineich), Sagittal virtual slice of head showing the brain (left) and volume rending in three-quarter view of skull showing labeling of several bones and, in background projection, view of skull. Scale bar: 1 mm. (A) Absorption X-ray synchrotron microtomography experiment obtained at ID19 (ESRF), voxel size 330 nm, using osmium tetroxide to differentially stain tissues. (B–E) X-ray phase contrast synchrotron microtomography obtained at ID19 (ESRF), at 700 and 995 mm between sample and camera, 25 keV. (B,D,E) at ID19 and (C) at BM5 (ESRF).
Phase contrast imaging
Because of the partial coherence of the X-ray beam produced by third-generation synchrotrons, it is possible to observe phase contrast in the resulting image (compare Fig. 1A–C,F, H with anatomical MRI in Fig. 1D,E,G,I and Fig. 2B–E). The arrival of phase-contrast imaging (Snigirev et al. 1995; Cloetens et al. 1996), developed specifically for the imaging of materials with low X-ray attenuation coefficients, such as polymers and many biological materials (Betz et al. 2007; McDonald et al. 2009a,b), opened new perspectives and had resulted in a surge of studies using this approach shortly before the turn of the millennium. Phase contrast imaging, which is hundreds of times more sensitive to contrast than absorption-based microtomography, can reveal dense and soft tissues in a single image. It is therefore of great interest in biological studies as it can show skeletal parts and details of the soft tissues. Synchrotron X-ray phase contrast imaging has the potential to revolutionize the study of internal and external anatomy in small organisms. It has applications in physiology and internal biomechanics as well.
Free-space propagation methods
The principle of X-ray phase-contrast imaging is Fresnel diffraction. Interfaces between materials with different densities (even very small variations) correspond to discontinuities in the refractive index: a monochromatic beam passing through an object constituted of parts with different compositions will produce a diffracted and a non-diffracted component. The interference between these components causes Fresnel diffraction and is manifested by the presence of an intense fringe at the edge of each material (Guigay et al. 2007). This edge-enhancement effect is one of the key features of synchrotron imaging because it improves the sensitivity of structures with only small density differences and absorption contrast (Baruchel et al. 2008; Peyrin 2009). Phase contrast images can be recorded by simply varying the sample-to-detector distance (‘propagation technique’, Snigirev et al. 1995; Wilkins et al. 1996; Cloetens et al. 1999): as the energy of the X-ray beam is high, the angle between the diffracted and the non-diffracted component of the beam is very small. To visualize the interference, it is necessary to let the resulting beam propagate in a free space to increase the distance between the two components in order to visualize edges. The most easily detectable features in phase contrast images are the ‘edges’, corresponding to phase jumps. They provide an important qualitative, or semiquantitative, picture of the sample, but do not allow quantitative extraction of the local phase and limit the spatial resolution through the fringes used to visualize the borders.
Recently, a technique called holotomography (Guigay et al. 2007; Peyrin 2009; Langer et al. 2010) has been developed to quantitatively retrieve the phase from phase contrast images. The technique consists of analyzing the evolution of the fringes while the sample–detector distance is increasing. For this approach, several scans are needed (typically between two and four) at a given energy, from an absorption-dominated scan (sample close to the detector) to a phase-dominated one (the distance needed to realize this kind of scan depends on the energy and the optics used). Based on empirical observations of an object for which the phase is known, the algorithm mixes the different scans to produce a phase-retrieved scan. This method was originally applied in cases where the sample was close to a pure phase object, but nowadays it can also be used for samples with mixed phases (i.e. hard and soft tissues). The refractive index of a material for hard X-rays is directly related to its electron density, and the result is a 3D representation of the electron density that reflects the mass density of the sample (Cloetens et al. 2006).
Analyzer-based imaging
Analyzer-based imaging (ABI) (Bravin et al. 2007; Coan et al. 2008), or DEI microtomography, is used for anatomical studies (Yin et al. 2005; Gao et al. 2006; Kelly et al. 2007; Young et al.2007; Connor et al. 2009; Muehleman et al. 2009). The set-up for ABI consists of a monochromatic beam obtained by means of a silicon crystal system (i.e. a monochromator; Bravin 2003). The monochromatic beam is passed through the sample, and the emerging refracted and scattered X-rays are analyzed by means of another perfect crystal, identical to the monochromator. Only the fraction of the beam satisfying the Bragg law for this analyzer crystal can reach the detector and contribute to the image formation. At the same time, this process suppresses scattered radiation, thereby increasing the signal-to-noise ratio. The angular resolution is provided by the choice of the analyzer crystal and the reflection. The edge enhancement, characteristic of this method, occurs at the interfaces of regions with different refractive indices. The effects of refraction are converted into intensity variations by slightly detuning the analyzer away from the maximum of the reflectivity curve.
A grating interferometer, or Talbot interferometer
Hard X-ray grating interferometry is a relatively new method for wavefront sensing and phase radiography (Pfeiffer et al. 2006, 2008; Weitkamp et al. 2008; McDonald et al. 2009a,b) in the energy range between 8 and 50 keV. The signals from the images are comparable to those obtained from ABI (see above). Different measurement modes such as phase-stepping or Moiré interferometry can be used to obtain quantitative phase maps of X-ray wavefronts and/or objects in the beam. The combination with tomography allows 3D reconstruction of the X-ray refractive index of samples. X-ray grating-based imaging is a method that provides access to multiple contrast modes, including differential phase contrast, X-ray dark-field contrast and absorption contrast.
The set-up for grating-based differential phase contrast (DPC) imaging essentially consists of a phase grating G1 and an analyzer absorption-grating G2. The DPC image formation process is similar to differential interference contrast (DIC) microscopy used with visible light. It essentially relies on the fact that a phase object placed in the X-ray beam path causes slight refraction of the beam transmitted through the object. The fundamental idea of DPC imaging depends on locally detecting the angular deviations. The angle, a, is directly proportional to the local gradient of the phase shift of the object, and can be quantified.
Synchrotron X-ray chemical nano-imaging
X-ray fluorescence CT (XFCT) uses a monochromatic X-ray point beam extracted from SR to scan a sample. XFCT has the key advantage of providing 3D quantitative images of a sample of a few hundreds of micrometers in size in a non-destructive way; none of the other techniques is currently capable of this. The key application of XFCT is chemical imaging of high atomic number (Z) elements inside low Z matrices, i.e. imaging biological objects (Peyrin 2009). The technique is quantitative and also sensitive to chemical effects of the studied elements, such as metal toxicity (Ortega et al. 2007).
A quantitative 3D image (in g·cm−3) of the distribution of each chemical element can be reconstructed by mathematically combining transmission, Compton (http://en.wikipedia.org/wiki/Compton_scattering) and fluorescence tomography. The irradiations of the sample result in the ejection of inner shell electrons, which are replaced by outer shell ones. This phenomenon is accompanied by the emission of X-rays at different energies. Each energy level in the spectrum is characteristic of an element, and the intensity of the emission is related to the concentrations of this element. As the process is repeated at each point of the sample, it provides a mapping of the concentration of the different elements. The incident energy has to be larger than the K-edge of the element of interest. With SR, this technique is sensitive to concentrations on the order of parts-per-million (ppm).
X-ray synchrotron results
In a first step, described methods were tested using a high brilliance X-ray synchrotron beam produced by an undulator source at the European Synchrotron Radiation Facility (ESRF, Grenoble, France). The resulting voxel datasets were visualized with AVIZO 6.3 (SVG, France). To separate and visualize individual anatomical structures, we used the segmentation tools provided by this visualization software. We obtained a complete series of virtual thin sections through the sample which can be used for the inspection of planar sections in the three major sectional planes (i.e. transverse, sagittal and horizontal; Fig. 2A last two images in the column, Fig. 2B last image of the column, Fig. 2C last image of the column, Fig. 2E left image). Monochromatic X-rays of 17.5 keV were used for the experiments (Fig. 2A). Using conventional absorption contrast imaging of an object stained with osmium tetroxide (at 330 nm pixel size), all anatomical details were visible. Although histological details could not be derived from these sections, they were well suited for comparative studies of the general anatomy of complex structures. In such studies, the 3D visualization tools of the relevant software packages make it possible to reconstruct the spatial organization of complex anatomical features (Fig. 2).
Light microscopy 3D imaging techniques
Confocal microscopy
Confocal microscopy is an established technique for 3D imaging of biological objects (Pawley 2006). Its high resolution (typically 0.5–2 μm) makes it well suited for imaging at the sub-cellular or cellular level (Chandler & Volz 2004). This technique is well developed and documented and does not require further introduction here.
Light sheet microscopy
Light sheets have been used in microscopy for more than a century. Heinrich Siedentopf, then a young optical engineer at the Carl Zeiss Company in Jena, and the chemist Richard Zsigmondy designed a very early light sheet-based microscope in 1903 to visualize dispersed nano-sized colloidal particles (Siedentopf & Zsigmondy 1903). The apparatus could visualize particles even when they were considerably smaller than the wavelength of the light used, and was therefore named the ultramicroscope. Recently, the same principle was used for studies of oceanic microbial population distributions (Fuchs 2002). The thin light sheet microscope (TLSM) creates a light sheet several micrometers thick by focusing an Argon laser beam through a cylindrical lens. Microscopic organisms traversing the light sheet were then detected by a wide-field microscope and a charge-coupled device (CCD) camera. As in the ultramicroscope before, the detection of microscopic objects became possible by the high contrast inherent in the orthogonal light sheet illumination.
The fundamental idea behind light sheet microscopy is to achieve optical sectioning with a wide-field detection scheme, by illuminating only a single, thin section of a fluorescent specimen (Fig. 3). The object is thus illuminated from the side, orthogonally to the detection optical axis. The emitted fluorescence light is detected with a standard wide-field fluorescence microscope. In a properly aligned microscope, the illuminating light sheet overlaps with the focal plane of the detection objective lens (Fig. 3E). The fluorescence emission generated in the light sheet will therefore originate from a volume overlapping the focal plane of the detection lens, and will therefore form a well focused, high-contrast image.
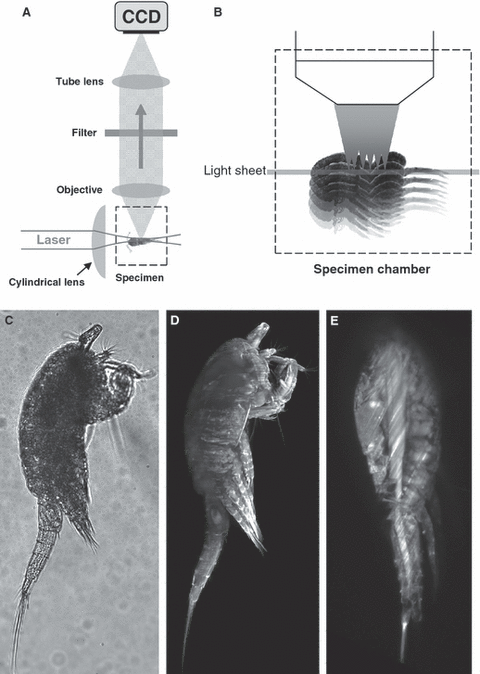
The light sheet microscopy principle. (A) The light sheet is illuminating the sample at an angle of 90° in relation to the detection axis. The sample is maintained in a chamber that is filled with a water-based buffer. (B) The sample is scanned by moving the sample through the light sheet and images are acquired at each single position. (C) Example of a transmission image of Tropocyclops confinis. (D) Projection of a complete stack of a T. confinis in fluorescence mode objective (Zeiss Achroplan 10×, 0.30 W, 70 planes at 1-μm spacing). (E) A single plane extracted from the previous stack.
Such a microscope has a number of advantages over other commonly used optical-sectioning microscopes such as the confocal microscope. As no parts of the specimen outside the light sheet are illuminated, they are not subject to the undesirable effects of high light intensities, i.e. fluorophore photo-bleaching and more general photo-toxic effects. The reduced per-image photo-damage allows one to increase the duration, dynamic range, sampling rate and frame-rate of a recording without increasing the impact on the specimen. Moreover, as a single one- or two-dimensional image (i.e. millions of picture elements in parallel) is acquired at a time, and since a CCD camera with high quantum efficiency is used instead of photomultiplier tubes, light sheet microscopy produces images with a better signal-to-noise ratio than typical laser-scanning confocal microscopes (Fig. 3).
In photography, a method called photomacrography employs a light sheet to image extended objects with effectively infinite depth of field. Light sheet microscopy can be regarded as a combination of 3D light scanning macrophotography with fluorescence microscopy. An early reported use of a light sheet illumination for fluorescence imaging was the orthogonal-plane fluorescence optical sectioning (OPFOS) system that was used to reconstruct a 3D structure of a guinea pig cochlea. More recently, selective plane illumination microscopy (SPIM), objective coupled planar illumination microscopy (OCPIM) (Holekamp 2008) and digital scanning light microscopy (DSLM) have been introduced (Keller et al. 2008).
Currently, these microscopes are mostly used with biological specimens, which usually require water-based media. This is why water-dipping lenses are popular with users of light sheet microscopes. A dipping lens obviates the need for additional air/water/glass interfaces between the objective lens and the object, but requires the use of medium-filled chambers that enclose the objective front lens and the specimen. This is a clear advantage when imaging marine species. Moreover, the water-dipping lenses used have smaller numerical apertures (NAs; up to 1.1) compared with oil immersion lenses (up to 1.45), and also relatively long working distances, which makes specimen handling during imaging considerably easier.
Light sheet microscopy applications in biology are still limited but, compared with confocal microscopy, light sheet microscopy can give more complete mechanical access to the sample and greater possibilities for 3D imaging in water-based media (Fig. 3).
Optical projection tomography (OPT)
OPT is an imaging technique ideally suited to mesoscopic (from a few hundred micrometers to ∼15 mm in size) biological samples. The usefulness of OPT has been demonstrated on both fixed (Sharpe et al. 2002) and live (Boot et al. 2008) biological samples. It is capable of imaging in either transmission (in which the contrast arises from the absorption of light by the sample) or fluorescence (where the signal is due to fluorophores within the sample) modes.
In transmission, OPT is a direct analog of X-ray CT (see section X-ray microtomography techniques above), except that it functions at visible (or near-infrared) wavelengths rather than in the X-ray regime of the electromagnetic spectrum. Contrast can be generated either by the intrinsic absorption of the sample, e.g. through absorption by the exoskeleton of an adult fruit fly, or by the introduction of external absorbing dyes that specifically label features of interest, e.g. by β-galactosidase staining (McGurk et al. 2007). In either case, the sample is illuminated from one side by a visible light source (typically an incandescent lamp or light-emitting diode), and microscope optics on the other side image the sample onto a digital camera. Optics with a large depth-of-field are used, so that the image captured by the camera is effectively a projection through the sample. The sample is mounted on a rotation stage so that these projection images can be captured from many viewing orientations of the sample. Once a dataset has been collected, these projections can be computationally combined by back-projection algorithms similar to those used in X-ray CT. The result is a quantitative 3D voxel dataset that can be analyzed or visualized by a variety of modern image processing software packages.
In addition to the transmission mode, OPT can also be implemented based on the fluorescent properties of the sample. Again, these can be either intrinsic (i.e. autofluorescent properties of the sample (Sharpe et al. 2002) or due to external labeling (e.g. antibody labeling with fluorescent dyes; Sharpe et al. 2002), or through the use of genetically encoded fluorescent proteins (Boot et al. 2008). In any case, the apparatus is the same as that described above for transmission OPT, except that instead of the visible light source, a lamp designed for fluorescence excitation, in combination with the appropriate filters, is used. The relationship between transmission and fluorescence OPT is exactly the same as that between transmission and epifluorescence imaging in classical wide-field microscopy.
Figure 4 shows virtual sections through two stages in zebrafish (Danio rerio) development: a 72-h post-fertilization (hpf) embryo in (A), and a 16-mm long adult fish in (B) (Bryson-Richardson et al. 2007). The contrast arises from the autofluorescence of the sample (excitation around 525 nm, emission detected above 480 nm), which allows details of the anatomy to be visualized. Because OPT is a non-destructive technique there are no artifacts due to the misalignment of physically sectioned slices, and the reconstructed dataset can be virtually sliced in any desired plane. A large initiative called FishNet (http://www.fishnet.org.au/, Bryson-Richardson et al. 2007) is using OPT to realize a complete 3D collection of fishes, and their web-interface (see 5, 6) gives the possibility to access any part of a chosen sample and download the datasets for analysis, comparison or even segmentation. Such an online approach is an example which should be followed to provide full access to reference 3D collections (4-6).
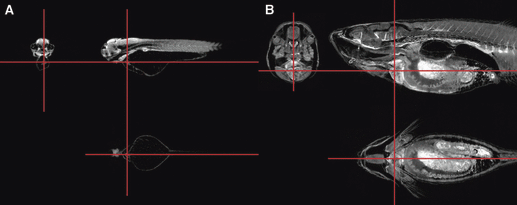
Virtual zebrafish sections by optical projection tomography. Orthogonal virtual sections through the reconstructions of OPT scans of (A) a 72-hpf zebrafish embryo, and (B) a 16-mm fish. Red lines indicate the relative positions of the sections, which are centered on the cardiac ventricle (reproduced from Bryson-Richardson et al. 2007).
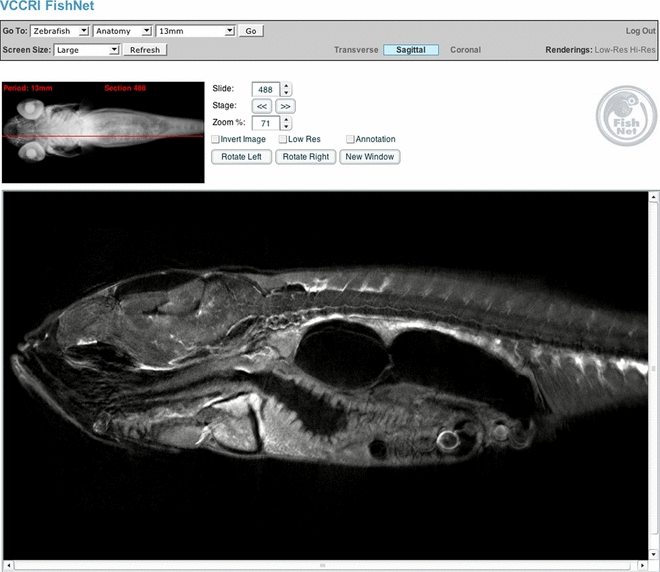
The FishNet interface. Users can determine the plane of section and the position of the section by looking at the volume-rendered image at the top left of the screen. Sections can be selected by dragging the red bar on the volume-rendered image using the cursor keys or by typing in the number of the section to be viewed (reproduced from Bryson-Richardson et al. 2007).
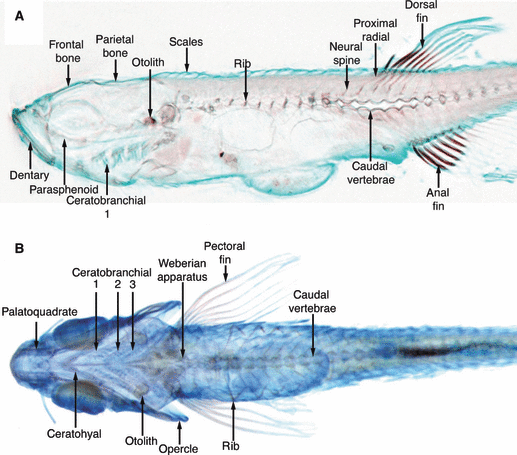
Virtual zebrafish sections by optical projection tomography. The cartilage and bone of a 10-mm fish is shown with Alcian blue and alizarin red staining, respectively. The three separate reconstructions relating to the red, green and blue channels are combined to form a single OPT image allowing a combination of brightfield stains to be used. (A) Sagittal section. (B) Colour volume rendering showing the complete developing skeleton of the fish (reproduced from Bryson-Richardson et al. 2007).
Conclusion and future prospects
Assessing the marine plankton for its contribution to global carbon fluxes is best achieved through bulk analysis methods. Current imaging methods are not effective enough for today’s information consumers. They do not approach the capacity needed to feed large-scale analyses across the range of scales from local basins to oceanic and global models. These are scales that the physical oceanographic community regards as routine [e.g. large-scale online data collection systems such as the Argo buoy network (http://www.argo.net/)]. The same is needed for biological oceanography.
Currently, the only technologies that are potentially able to achieve large-scale analysis of plankton community are metagenomic and cytometry approaches. However, in the former case the low numbers of marine genomes available and the complexity of those biotopes have limited their use to restricted groups (Bacteria and Archea, for example in Craig J Venter Sorcerer Expedition, Rusch et al. 2007). Moreover, DNA is only the blueprint of a specimen and positioning it on the Tree of Life based only on a 16S fragment is not enough, especially when you begin to include previously unknown species. So phenotype must be linked to genotype and imaging is the key technology to do so. The latter technology, cytometry, has also great potential, especially when it can be coupled to imaging. There are portable versions (e.g. Accuri cytometers, FlowCam cytometers) and even an underwater system (Imaging FlowCytobot; Olson & Sosik 2007) and several machines are currently on board research vessels. Newly developed cytometers (Amnis, Alpha Technology) mainly used in medical research are now being used in marine research (https://www.amnis.com/oceanography.html) and their capabilities (speeds of up to 10,000 particles per minute) and fluorescence imaging) represents a promising future. But this will require extensive use by marine scientists and eventually the development of an underwater version, or at least a boat version. Moreover, these new cytometers only provide 2D images, sorting is not fully implemented and specimen recovery to further characterize unknown species will be difficult.
Two-dimensional images are a common standard in marine biology imaging and are often taken in transmission or projection modes. This has several drawbacks. First, 2D images do not encompass the entire taxonomical information of a particular specimen. Often, some features are hidden or the imaging modalities restrict their appearance. For example, semi-transparent organisms generate important lensing effects which distort the resulting image, and many features cannot be seen as they appear blurred. There are solutions: manual handling of the specimen (copepod dissection to unravel specific characteristics (fifth pair of legs), bulk imaging followed by specific segmentation and geometrical classification based on the group physiology, etc. But these are time-consuming or an approximation, and the last has a limited capacity to distinguish small differences within a group. Interestingly, fragmented specimens are generally treated as unclassified and therefore not analyzed, and so biomass calculations are partially inaccurate. It is also important to comment here that fixation procedures and transport can affect shapes and volumes of samples, and may induce biases that are group specific (jelly fish versus decapods) and further affect biomass calculations. However, publications demonstrating the use of automated imaging and plankton identification instruments are now showing some maturity, handling up to 50 functional groups for laboratory-based analyses (e.g. PhytoImage, ZooImage; for review and discussion see Culverhouse et al. 2006). In summary, there is a need for improvement. The use of fluorescence imaging has already been extensively explored in cell biology and medical sciences, and its high resolution using specific dyes in combination with specific technology (confocal, two photon) has driven our knowledge of cellular structures a step closer to nanometric resolution (super resolution microscopy; for review see Huang 2010). Therefore, it will be important to promote the uses of fluorescence imaging in marine biology to enhance the quality of the image obtained, which in turn will greatly improve image processing, segmentation, and feature recognition.
We believe that 3D microscopy can help in this matter. In fact, 3D images give full access to the specimen and can be easily rotated to display features of interest. They contain more information (volumetric measurements in the three dimensions), and can be used to develop further reference training datasets using various imaging angles, texture generation and artificial blurring to quantify automated plankton analyzing systems as well as human experts. However, there are several limitations. First, 3D models are large datasets (up to 2000 images per stack) that require advanced computing systems. These are currently being developed and made available online (e.g. FishNet; (http://www.fishnet.org.au/, Bryson-Richardson et al. 2007). Moreover, the falling price of central processing units (CPUs) and servers will quickly make this limitation less of a constraint. The widespread use of 3D imaging systems and the improvement of the related literacy in the marine biology world will greatly favor the development of specific tools (automated analyzing systems etc.) and, in particular, references. As in classical taxonomy, it is necessary to possess a reference specimen, in this case virtual, that can be used for comparisons. This gold standard is a key factor. There are already around 350–400 specimens available as 3D model images from different image modalities (MRI, X-ray, confocal microscopy, etc.) but they are not stored centrally and often require specific software to be handled, and they are mainly of macroscopic organisms (fishes especially). Therefore, 3D imaging of organisms of specific groups and sub-groups is needed to create a reference library. This innovative approach, currently being tested on some organisms provided by the Tara Oceans expedition (http://oceans.taraexpeditions.org/), can give us access to highly defined ‘morphoscans’ containing all the elements necessary for identification. This gold standard library will allow the creation of training datasets for humans and computers using any output format. Furthermore, these will include unlimited possibilities to modify the information in the image [e.g. noise and blur addition, wrapping, deformation correction (fixation induce shrinkage)] and create model datasets. A community effort to perform 3D imaging of planktonic organisms and provide the final dataset to the central library will definitively open a new era to biological oceanography.
Acknowledgements
The authors would like to thank Ivan Ineich, curator of reptiles, MNHN, Paris for his assistance as well as Dr Phil Culverhouse for his useful comments. We thank Pascal Deynat for kindly loaning material from personal collection and Paul Tafforeau for his help in this study. We are also grateful to the CEMIM team. Supported by a European Synchrotron Radiation Facility Postdoctoral Project through allocation of beam time.




