Spatial patterns of epifaunal communities in San Francisco Bay eelgrass (Zostera marina) beds
Abstract
Epifaunal invertebrate species, such as amphipods and isopods, have been shown to play key but varying roles in the functioning of seagrass habitats. In this study, we characterized patterns in the poorly known epifaunal communities in eelgrass (Zostera marina) beds in San Francisco Bay as a first step in understanding the individual and collective importance of these species, while testing predictions on spatial patterns derived from previous studies in other regions. Surveys conducted at five beds across multiple time periods (April, June, August and October 2007) showed that San Francisco Bay eelgrass beds varied strongly in epifaunal community composition, total, and relative abundance, and that abundance differed markedly among time periods. In contrast to findings by others, morphologically complex flowering shoots frequently harbored greater numbers of epifauna (>2× and up to 10× more individuals) than vegetative shoots, but not different species assemblages. Similar to previous studies, several abiotic factors did not explain patterns in distribution and abundance among beds. The proportion of introduced species was very high (>90% of all individuals), a finding unique among seagrass epifaunal studies to date. Defining numerical patterns in epifaunal communities will inform related efforts to understand effects of epifaunal species and assemblages on eelgrass growth dynamics, seed production, and higher order trophic interactions over space and time.
Introduction
Seagrass beds play a vital role in estuarine systems worldwide by providing substrate, food, refuge from predation, and nursery habitat for a diverse assemblage of associated organisms (Williams & Heck 2001). Mesograzers, mobile epifaunal invertebrates, are important components of seagrass communities, serving as a link in the food chain from primary producers to higher-order consumers (Kikuchi 1974; Edgar & Shaw 1995). Additionally, by grazing epiphytic algae that compete for light, mesograzers can increase seagrass growth by up to 200% (Duffy et al. 2001). Because mesograzers are important in seagrass ecosystem structure and function, community composition and species distributions have been well documented in a wide variety of locations (Orth et al. 1984; Hellwig-Armonies 1988; Gambi et al. 1992; Thom et al. 1995; Bostrom & Bonsdorff 1997; Mattila et al. 1999; Duffy et al. 2001; Arroyo et al. 2006). Epifaunal species play different roles in the structure and functioning of eelgrass systems (Duffy et al. 2001); evaluating patterns in their distribution and abundance aids in the assessment of their functional importance.
Epifaunal species generally show large seasonal fluctuations in community structure and relative abundance (Dugan & Livingston 1982; Edgar 1990; Arroyo et al. 2006; Valentine & Duffy 2006), which has been correlated with seasonal changes in shoot height and density (Arroyo et al. 2006). However, most studies have evaluated only a single site or morphology of seagrass, usually vegetative shoots, over time (but see Nakaoka et al. 2001). Conversely, a few studies have quantified mesograzer community composition and abundance for vegetative versus flowering shoots, but only during a single time period (Hellwig-Armonies 1988; Nakaoka et al. 2008). Vegetative shoots (those that grow clonally through rhizome expansion) have a relatively simple morphology, but flowering shoots exhibit more complex structure as flowers and seeds develop, leading to greater habitat availability for mesograzers (Hellwig-Armonies 1988; Nakaoka et al. 2008). Nakaoka et al. (2008) found a species-specific response to flowering shoots, but no significant difference in abundance or species richness. Phenology and abundance of flowering shoots over time could thus strongly influence mesograzer abundance and community composition, but no studies to date have addressed this idea.
Eelgrass (Zostera marina) is the only seagrass species in San Francisco Bay, with populations ranging as far north as San Pablo Bay (North San Francisco Bay) and south to the San Mateo Bridge (Merkel et al. 2004) (Fig. 1). Most of the beds grow clonally, supplemented by seedling recruitment; however, at least one bed is annual, relying on seeds produced each summer/fall to recolonize in spring. Flowering rates and the timing of flowering and seed development vary markedly among populations (K.E. Boyer unpublished data). Epifaunal species utilizing these eelgrass beds were previously almost unknown (but see Hansen 1998).
The sampled Zostera marina populations in San Francisco Bay. Site abbreviations are as follows: Point San Pablo (PSP), Keller Beach (KB), Richardson Bay (RB), Crown Beach (CB) and Bayfarm Island (BFI).
The objective of this study was to characterize patterns in epifaunal communities within and among eelgrass beds and over time in San Francisco Bay, while testing predictions resulting from research in other seagrass systems. According to Nakaoka et al. (2008) working on Z. marina in Japan, we also hypothesized that epifaunal community composition would differ on flowering versus vegetative shoots. If so, differences in flowering shoot abundances among beds could lead to differences in food choices for higher trophic levels, or perhaps other functional changes. In contrast to the findings of Nakaoka et al. (2008), but consistent with a study by Hellwig-Armonies (1988) on Z. marina in the North Sea, we predicted higher epifaunal abundances on flowering shoots, as preliminary data indicated these offer much greater habitat complexity than vegetative shoots in San Francisco Bay and perhaps more refuge and food availability. Consistent with findings from the Mediterranean Sea by Connolly & Butler (1996, for Cymodeocea nodosa), Arroyo et al. (2006, for Z. marina) and Quiroz-Vazquez et al. (2005, for Z. marina), we further hypothesized that epifaunal species richness and abundance would peak when eelgrass shoots are the largest and when flowering shoots are the most developed; in theory, refuge space and food availability should be maximized during this time period. Thus, based on preliminary data on shoot lengths, flowering frequencies and epiphyte abundance, we predicted this peak to occur in late summer for most beds, consistent with Arroyo et al. (2006), and in contrast to Çinar et al. (1998) (both studies of Z. marina in the Mediterranean Sea). Further, we hypothesized that at the annual bed, epifaunal species richness and abundance would peak in fall since the biomass of vegetative and flowering shoots is greater later in the season due to the bed’s late start from seedlings. Finally, we hypothesized that several important environmental parameters in estuaries such as temperature and salinity would not explain epifaunal distribution patterns, similar to a study by Walsh & Mitchell (1998).
The proportion of introduced epifaunal species has not been described for local eelgrass beds, but is expected to be high due to the heavy invasion of San Francisco Bay overall (Cohen & Carlton 1998). While we can make predictions regarding epifaunal distribution and abundance based on the findings of other studies, the niches and functions of non-native species are difficult to predict in novel environments (Suarez et al. 1999). Elucidating numerical patterns in eelgrass epifaunal species, both native and non-native, presents a first step in evaluating the importance of these species or assemblages to the functioning of San Francisco Bay eelgrass habitats.
Methods
Study sites
Field surveys were conducted in San Francisco Bay in April–October of 2007 at five Zostera marina beds (Fig. 1). Eelgrass beds in San Francisco Bay are sporadically distributed in Southern San Pablo Bay, Central San Francisco Bay and the northern end of South San Francisco Bay in 23 separate locations, ranging in size from 0.1 to 609 hectares, with a total area in the Bay estimated at 1,166 hectares (Merkel et al. 2004). We chose five of the largest beds for our surveys; these sites are also positioned north to south, and along a salinity gradient due to distance from the mouth of the Bay (see Fig. 1 for locations). Point San Pablo (PSP) is the largest bed in the bay, encompassing 609 hectares on a shallow shoal. Crown Beach (CB), an annual bed over most of its distribution, has a total acreage of 270 hectares. Richardson Bay (RB) covers 177 hectares, and Keller Beach (KB) and Bay Farm Island (BFI) cover 43 and 54 hectares, respectively (Merkel et al. 2004). Sampling at all sites and time periods was conducted at a depth between −0.5 and −0.22 MLLW (average of the lower low water height of each tidal day observed, National Tidal Datum Epoch).
Field sampling methods
In April, June, August and October 2007, we measured densities of vegetative and flowering shoots along a 100-m transect, every 5 m, using a 0.25-m2 quadrat. Eelgrass densities were used to calculate areal epifaunal densities. Ten vegetative and 10 flowering shoots were haphazardly collected along or near the transect lines at each time period, except in April, when flowering shoots were very rare. Thus, even if flowering shoots were not present in the sampling quadrats, others nearby were collected for laboratory analyses if available. Each individual shoot was separated from the rhizome at the sediment surface and inserted into a plastic bottle with ambient bay water (Hellwig-Armonies 1988; Bostrom & Bonsdorff 1997; Mattila et al. 1999). Samples were kept cool and immediately transported to the laboratory for further processing. Temperature and salinity of the surface water were recorded at each site and time period using a thermometer and refractometer (Vee Gee A366ATC), respectively. Ten 2-cm sediment cores were inserted randomly along the transect, capped at the top, extracted, placed in a sealed bag and brought back to the laboratory for further analysis.
Laboratory analyses
In the laboratory, each bottle was emptied onto a 500-μm sieve and each shoot was subjected to three 1-min freshwater dips to remove clinging epifauna. This technique was found to remove 92–100% of total epifauna from algae (Holmlund et al. 1990) and 90% from eelgrass (L.A. Carr unpublished data). Sieve contents were preserved in 70% ethanol and identified to the lowest possible taxa, according to Carlton (2007), and enumerated (per shoot). The height of each shoot was also measured and used to determine epifaunal abundances per cm. Epiphyte biomass was quantified using an index, where a subset of the shoots was scraped for epiphytic biomass and the rest were visually assigned a range value. Six vegetative shoots were randomly selected from samples taken at each site in each time period to be completely scraped of epiphytes. Each blade was assigned a value of 0–5 based on visual inspection of epiphyte loads, with a rating of 0 = no visible epiphytes to 5 = spathe completely covered in epiphytes. The rest of the vegetative shoots were assigned values (0–5) but not scraped. Epiphytes were removed from the shoot tissue by scraping with a scalpel in filtered seawater and vacuum-filtered onto Whatman glass fiber filters. Six flowering shoots were randomly selected from each site at each time period when flowering shoots were present for epiphyte scraping. Six spathes from each of the six flowering shoots were chosen for scraping, with selection based on visual determination of epiphyte loads (same as vegetative shoots). Spathes to be scraped were specifically chosen to encompass a range of ratings. After each spathe was scraped, the length and width were measured. Epiphyte biomass was determined using chlorophyll a (chl a) as a proxy, with 90% acetone as the extraction solvent. After extraction, absorbance was read at 630, 647, 664 and 750 nm on a spectrophotometer (Genesys 20; Thermospectronic). Chl a concentration was determined using the formula of Parsons et al. (1984). Concentrations were normalized by dividing the chlorophyll concentration by the area of the blade surface (Duffy et al. 2001).
We determined average chl a for spathes at each index rating (0–5), then extrapolated to the whole shoot (n = 10 for both vegetative and flowering shoots) using the ratings recorded for all spathes or blades on the shoot.
Sediment samples were dried (55 °C), ground and sieved (1-mm sieve). Percent organic matter was determined by combustion at 500 °C for 4 h.
Statistical analysis
Vegetative and flowering eelgrass shoot densities were compared by site using repeated measures ANOVA, with site as a random factor (SPSS v.16). Shoot height, epiphyte biomass, and epifauna per shoot were compared among vegetative and flowering shoots using two-factor repeated measures ANOVA (SPSS v.16), with site and shoot type as factors. Linear regressions were performed on total versus per cm mesograzer abundance for sites with the most abundant mesograzer densities for August and October. Data were tested for homogeneity of variances by Cochran’s test, and log-transformed as necessary.
To examine patterns over time and the effect of site and shoot morphology on the epifaunal community composition, we performed two-way cluster analyses (site, shoot type) using presence/absence data, a Euclidean distance measure and Ward’s method for group linkage, for all taxa on each date (PC ORD v.5).
Non-metric multi-dimensional scaling (nMDS) was employed to graphically depict the distributions of seagrass epifaunal species and their relationships to environmental variables on each date. For the nMDS ordination we used the Bray–Curtis distance measure and followed methods outlined by McCune & Grace (2002). The five sites are displayed as points on the biplots, while the arrows indicate the magnitude of the effect of different environmental variables. Before ordination analysis, species abundance data were square root-transformed to minimize the effect of extremely abundant species. We performed separate nMDS graphs for each season. Correlations between environmental variables and the axes were interpreted according to Pearson and Kendall correlations (r) and the joint bi-plot. The Pearson and Kendall correlation value provides insight into which habitat variables best describe the patterns of epifaunal community composition and abundance.
Results
Flowering and vegetative shoot characteristics
While vegetative shoots were found at all sites at all time periods, flowering shoots were not found in April at any of the sites, and were rare enough in October at two of the sites (RB and BFI) that none were available in the sampling quadrats; hence, there was a significant interaction between time and shoot type (Table 1, Fig. 2A,D). Shoot density varied significantly among sites and time periods; however, there was a significant interaction between site and time (Table 1), as seagrass phenology was different for each site. Some sites peaked in vegetative shoot density in April (KB and BFI), whereas one (CB) peaked in June, another (PSP) in August. RB did not vary in vegetative shoot density across seasons. Flowering shoot density showed similar patterns, with some sites peaking earlier than others (i.e. KB and BFI) (Table 1, Fig. 2A,D). Among sites, vegetative shoot densities were always higher than flowering shoot densities; however, sites differed in the patterns between vegetative and flowering shoot densities, resulting in a significant interaction between site and shoot type (Table 1, Fig. 2A,D). Eelgrass shoot height varied among sites, time and shoot type (Table 1). There was a significant interaction between time period and site, as some sites increased in shoot height over time (KB, PSP), while other sites decreased (BFI) (Fig. 2C,F). Patterns in shoot heights between vegetative and flowering were similar at KB over time. However, CB and PSP both increased in vegetative shoot height over time, but flowering shoot heights peaked in August and decreased in height in October (Fig. 2C,F). Flowering shoots were taller than vegetative shoots at all sites and time periods except at CB, where the flowering shoots did not differ in height from the vegetative shoots (Fig. 2C,F).
| df | F | P | |
|---|---|---|---|
| shoot density | |||
| time | 3 | 11.77 | <0.0001 |
| site | 4 | 21.89 | <0.0001 |
| shoot type | 1 | 11.77 | <0.0001 |
| time × site | 12 | 190.64 | <0.0001 |
| time × shoot type | 3 | 13.39 | <0.0001 |
| site × shoot type | 4 | 16.07 | <0.0001 |
| time × site × shoot Type | 12 | 4.83 | <0.0001 |
| shoot height | |||
| time | 2 | 45.07 | <0.0001 |
| site | 4 | 86.58 | <0.0001 |
| shoot type | 1 | 89.91 | <0.0001 |
| time × site | 8 | 8.94 | <0.0001 |
| time × shoot type | 2 | 2.18 | 0.1160 |
| site × shoot type | 4 | 6.05 | <0.0001 |
| time × site × shoot type | 6 | 0.80 | 0.5720 |
| Chl a | |||
| time | 2 | 3.10 | 0.0490 |
| site | 4 | 9.26 | 0.0001 |
| shoot type | 1 | 121.59 | <0.0001 |
| time × site | 8 | 8.62 | <0.0001 |
| time × shoot type | 2 | 1.81 | 0.1690 |
| site × shoot type | 4 | 6.05 | <0.0001 |
| time × site × shoot type | 8 | 8.61 | <0.0001 |
| log epifaunal abundance | |||
| time | 3 | 160.80 | <0.0001 |
| site | 4 | 25.26 | <0.0001 |
| shoot type | 1 | 4.78 | 0.0320 |
| time × site | 12 | 18.34 | <0.0001 |
| time × shoot type | 3 | 83.59 | <0.0001 |
| site × shoot type | 2 | 6.04 | 0.004 |
| time × site × shoot type | 6 | 2.31 | 0.0350 |
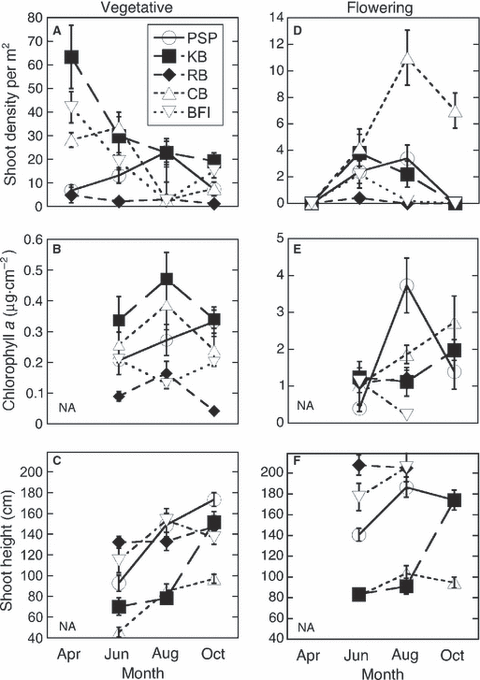
Seasonal (April, June, August and October 2007) and spatial variation among five sites (refer to Fig. 1 for site abbreviations) in A, B, and C: vegetative shoot density, epiphyte biomass (as Chl a) and shoot height, D, E, F: as above but for flowering shoots. April data are not shown as epiphyte biomass and shoot height were not sampled for that time point. Note difference in scale for vegetative and flowering shoots. Error bars are ± 1 SE; n = 20 for shoot density, 10 for epiphyte biomass and shoot height.
Epiphyte biomass
Epiphyte biomass on flowering shoots was, on average, an order of magnitude higher than on vegetative shoots (Fig. 2B,E). Site and time period both influenced the degree to which epiphyte loads differed among shoot types (Table 1, Fig. 2B,E); e.g. PSP had relatively high epiphyte biomass on flowering shoots in August, when BFI and RB had low epiphyte biomass on flowering shoots (Fig. 2E). KB had high vegetative shoot epiphyte biomass at all time periods, although in October, the epiphyte biomass on PSP vegetative shoots was similar to measures at KB (Fig. 2B). Epiphyte biomass on vegetative shoots at PSP increased over time, but on flowering shoots peaked in August and then decreased. At other sites (KB, CB, and RB) epiphyte biomass on vegetative shoots peaked in August and then decreased to October, but on flowering shoots epiphyte loads increased from August to October. Epiphyte biomass on flowering shoots at CB increased steadily from June to October, whereas epiphyte biomass on vegetative shoots at CB peaked sharply in August and then decreased by October (Fig. 2B,E). BFI peaked in epiphyte biomass on flowering shoots in June and then steadily decreased to August, whereas epiphyte biomass on vegetative shoots decreased from June to August and then increased to October (Fig. 2B,E).
Epifaunal community composition and abundance
Sixteen different taxonomic groups of epifauna were identified across all sites and time periods (Appendix Tables 1–2). Juvenile caprellid and gammarids that could not be identified to species were most likely the same as the introduced adult species at each site. Introduced epifauna composed a very large proportion of total numbers at nearly all sites, >90% of all individuals (Fig. 3). The exception was RB, where contributions to abundances were closely balanced between introduced and native species.

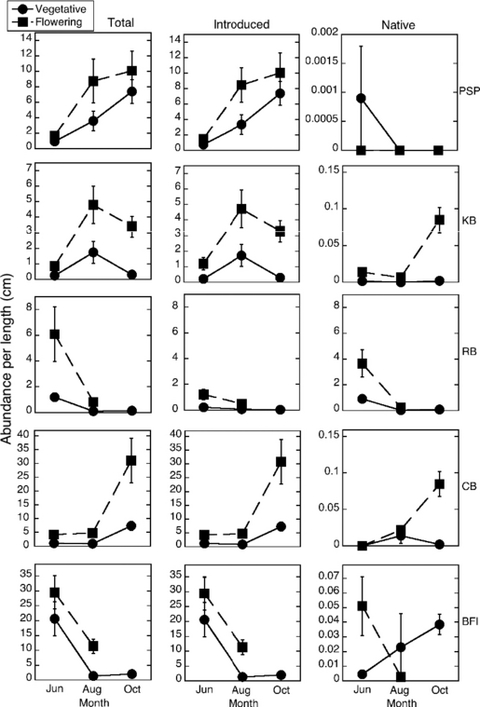
Seasonal and spatial variation in total, introduced and native epifaunal abundance per shoot. Error bars are ± 1 SE; n = 10 samples on each date (April, June, August, October) at each site (see Fig. 1 for locations).
The greatest abundances of epifauna were found on flowering shoots (Table 1, Appendix Tables A1–2, Fig. 3). Although these shoots were significantly taller than vegetative shoots (Table 1, Fig. 2c, f), high epifaunal abundances on flowering shoots were not simply a function of shoot length, as epifauna per unit length was significantly correlated with abundance per shoot at all sites tested (PSP, CB, BFI all R2 ≥ 0.88, Appendix Fig. A1); hence, the tall flowering shoots harbored greater densities of epifauna per unit length than the shorter vegetative shoots (Appendix Fig. A1).
Epifaunal abundances at the five sites varied differentially over time (Table 1, Appendix Tables A1–2, Fig. 3). Although all sites had low abundances of epifauna in April, abundances at BFI and RB peaked in June then fell to very low numbers in October, whereas CB and KB both reached peak abundances in October on vegetative shoots (Table 1, Fig. 3).
Sites varied in their patterns of epifaunal abundance on flowering shoots over time: some sites (KB and CB) increased in epifaunal abundance until October, whereas others (RB and BFI) decreased in epifaunal abundance from June until October (Fig. 3). There was a significant interaction among time, site and eelgrass morphology, with some sites increasing in epifaunal abundance on both flowering and vegetative shoots (CB and PSP), while some (KB) decreased in epifaunal abundance on vegetative shoots from August to October and increased in abundance on flowering shoots from June to October (Table 1, Fig. 3).
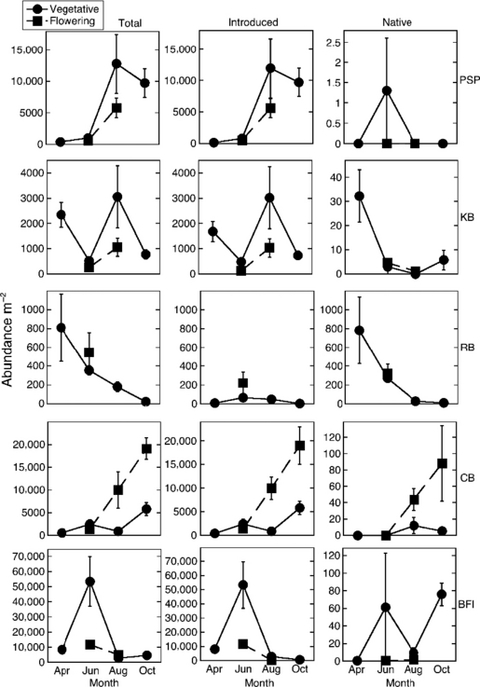
Areal epifaunal abundances varied from 24 to 24.923 individuals per m2. On an areal basis there were greater abundances of epifauna on vegetative shoots, as vegetative shoot densities were higher than flowering shoot densities (Appendix Fig. A2).
Two of the most abundant epifaunal species, the introduced caprellid Caprella cf. drepanochir and gammarid Ampithoe valida, showed markedly different abundance patterns (Fig. 4). Although both reached highest abundances on flowering shoots, C. cf drepanochir was especially abundant at BFI in June, and at PSP and CB in October. Ampithoe valida was abundant at both PSP and CB in August and October, with especially high abundances at PSP in October.
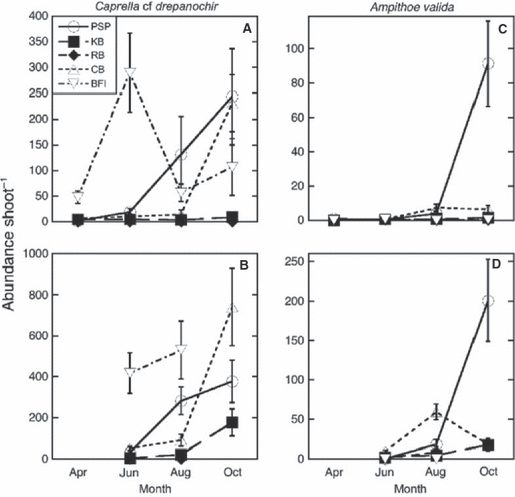
Seasonal and spatial abundance of Caprella cf drepanochir and Ampithoe valida on vegetative (A and C) and flowering shoots (B and D) at five sites (see Fig. 1). Error bars are ± 1 SE; n = 10.
Two-way cluster analyses (Fig. 5) revealed that the epifaunal species groupings were relatively consistent over time. Group A included taxa that were present at nearly all sites: Corophium alienense, juvenile caprellids, C. cf. drepanochir, and A. valida. Group B1 from April to August included taxa that were present at Richardson Bay, including Caprella californica, Jassa marmorata, Paranthura elegans, and Idotea resecata. In October, group B1 consisted of taxa not found at KB. Group B2 from June to October was composed of taxa not found at PSP. In April, group B2 consisted of taxa found at PSP. In June and October, group B2 represented taxa found at KB. In August, taxa not found at KB were represented in group B2.
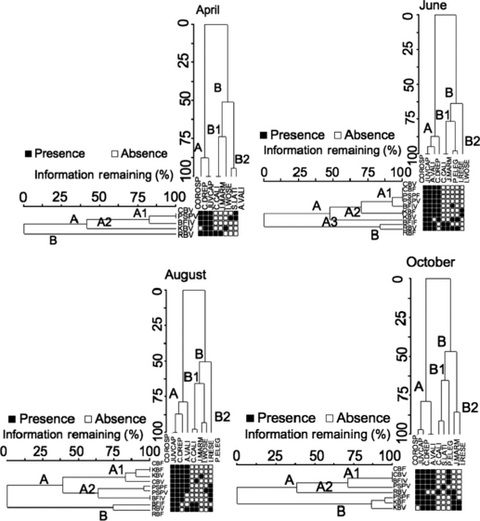
Results of cluster analyses showing similarity among epifauna assemblages by site (see Fig. 1) and eelgrass shoot type (V = vegetative shoots, F = flowering shoots) for each season. Letters are shown to indicate major groupings (A, B, B1, etc.). Squares denote presence (black) or absence (white) of a taxon (refer to Appendix Table A2 for epifaunal species abbreviations). Remaining information remaining ranks the similarity for branches of the groupings.
Generally, the epifaunal community composition grouped more frequently among sites than among similar eelgrass shoot type (vegetative or flowering). However, in June, the BFI vegetative shoot type clustered with group A1, whereas the flowering shoot type clustered with group A2 (Fig. 5). Similarly, in October, the community composition on the PSP vegetative shoot type was more similar to the sites in group A1, whereas the flowering shoot type community was more similar to the sites in group B (Fig. 5). Site group A in April, June and August contained all sites except RB, and in October, included all sites except KB. There was no consistent pattern of similarity over time between sites that are geographically closer to one another (1, 6). In October, sites close to one another grouped together, whereas in June, the northernmost and southernmost sites grouped together (CB and PSP) (Fig. 5).
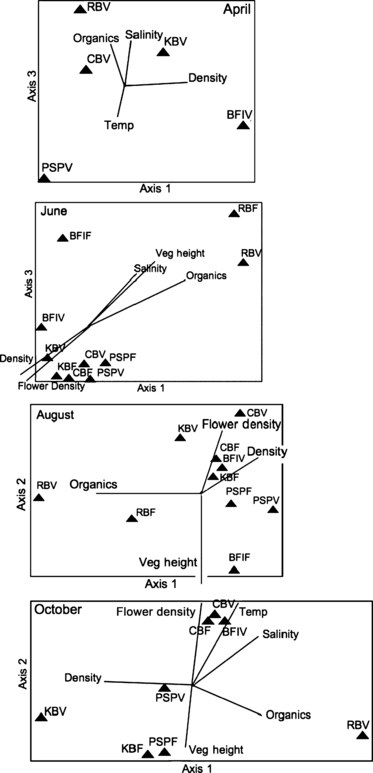
Results of nMDS ordination for epifaunal communities at five sites (triangles) in relation to environmental variables (vectors) for April, June, August, October. Refer to Fig. 1 for site abbreviations. The final ordination possessed no significant axes.
Relationship between epifaunal community composition and environmental variables
A significant nMDS ordination was not detected for any of the time periods. Stress values for the four sampling periods were as follows: 10.2 (April), 19.3 (June), 19.6 (August) and 18.5 (October). However, the plots show some clustering of sites at all time periods. This pattern is driven by soil organic matter (%), the site with the highest organic content (RB) separates out from the other sites (Fig. 6). The environmental variables that best described multivariate patterns of epifaunal community composition and abundance differed seasonally. Total eelgrass shoot density is correlated highly with axis 1 (r = 0.79), while axis 3 represents a salinity gradient (r = 0.71) for the April sampling period. In June, axis 1 is a gradient of soil organic matter (%) (r = 0.98), and vegetative shoot height is correlated highly with axis 3 (r = 0.77). The epifaunal community composition and abundance in August is described by two axes which are negatively associated with environmental variables; soil organic matter (%) is correlated with axis 1 (r = −0.93), whereas vegetative shoot height is correlated with axis 2 (r = −0.92). In October, axis 1 is a salinity gradient (r = 0.73) and axis 2 a gradient of soil organic matter (%) (r = 0.60).
Discussion
Epifaunal communities can play important roles in the functioning of seagrass beds (Kikuchi 1974; Edgar & Shaw 1995; Duffy et al. 2001) but were poorly known previously for San Francisco Bay. The epifaunal community in San Francisco Bay eelgrass beds is characterized by a high abundance of a few dominant species, similar to a study conducted off the coast of Baja California (Quiroz-Vazquez et al. 2005). Additionally, we documented exceptionally high numbers of individuals, and the range of epifauna total abundance across all time periods and sites (24–24,923 individuals per m2) was much wider than expected from previous studies, although referenced studies varied in sampling techniques and time periods. Other studies observed densities ranging from 990 to 1310 (Jacobs 1980) off the European west coast, and in Chesapeake Bay, Orth (1973) reported summer densities of 2350–14,725 individuals m–2. Our high abundances of epifaunal species were largely due to high densities of juvenile caprellids found at all the sites except Richardson Bay. Although extrapolations are subject to errors, our numbers provide the first approximation of epifaunal areal densities in San Francisco Bay. Future studies that examine epifaunal abundances on a per m2 basis while utilizing different sampling techniques, will provide additional insight into the epifauna utilization of eelgrass beds in San Francisco Bay.
Flowering shoots are more structurally complex than vegetative shoots, and in our study, as predicted based on preliminary observations, flowering shoots had higher densities of epifauna than vegetative shoots at all sites and at all sampling periods. This result contrasts with findings of Nakaoka et al. (2008) in eelgrass beds in Japan but is consistent with a study by Hellwig-Armonies (1988), who reported higher abundances of epifauna on the spathes of flowering shoots than on vegetative blades. Counter to our hypothesis, presence/absence data demonstrated that species composition was similar on vegetative versus flowering shoots within sites. In contrast, Nakaoka et al. (2008) found that two of the dominant epifaunal species, a tanaid Zeuxo sp. and a polychaete Platynereis sp., were found on flowering instead of vegetative shoots in their study in Japan. These two species rely heavily on the spathes for feeding and reproduction (Nakaoka 2002). The lack of a strong species-specific response to shoot type in San Francisco Bay eelgrass beds could be due, in part, to a lack of species that use the flowering shoots for specific trophic or other purposes; notably, there were no tanaids and few polychaete worms found. The finding that total abundance of the epifaunal assemblage did not differ significantly between flowering and vegetative shoots in the study in Japan resulted from different species contributing similar total numbers of individuals on the two shoot types (Nakaoka et al. 2008).
Food availability and refuge from predation are two factors that could affect mesograzer abundance on morphologically distinct structures. Flowering shoots had significantly higher epiphytic biomass than vegetative shoots in this study, and epiphytes are known to be an important food source for mesograzers (Valentine & Duffy 2006); furthermore, fluctuations in epifauna abundances have been correlated with patterns of epiphyte biomass (Gambi et al. 1992). Our results suggest that flowering shoots, when abundant, could increase trophic resources for mesograzers; however, estimates of epiphyte biomass even on flowering shoots were much lower than reported in other systems (Borum 1985; Duffy et al. 2001; Hauxwell et al. 2001). Further, morphologically complex structures are generally considered to increase abundance of epifauna by reducing predation (Nelson 1979; Orth et al. 1984). Recent mesocosm experiments simulating San Francisco Bay eelgrass habitats support the value of flowering shoots as refuges for mesograzers when predators are present (Carr 2008, L.A. Carr et al. unpublished data, L.A. Carr & K.E. Boyer in preparation).
Overall, the cluster analyses demonstrated greater similarity of the mesograzer community assemblage among sites than on similar morphological structures (flowering or vegetative shoots) between sites. Sites nearest to each other in proximity exhibited strong similarity in October. At all other sampling periods, the strong dissimilarity of the epifaunal community composition at RB compared to the other sites drove the observed site groupings. This pattern was due to the presence of Caprella californica and the absence of Caprella drepanochir at RB. Seasonal variation among mesograzer community abundances and assemblages is well documented (for reviews see Jernakoff et al. 1996; Valentine & Duffy 2006). Mesograzer abundance in our study fluctuated over time, which may relate to seagrass phenology, as demonstrated by Arroyo et al. (2006) and Connolly & Butler (1996). In contrast to our prediction, epifauna abundance did not peak at the perennial beds during late summer, but did tend to peak after the highest measured flowering shoot density. As we predicted, the epifauna abundance at the annual site (Crown Beach) was highest during the October sampling, perhaps due to the delay of height growth and flowering in plants that began from seedlings in spring. However, in contrast to our hypotheses, epifaunal community composition and species richness were not notably different over time. These results coupled with the cluster analyses suggest that the mesograzer community assemblage is strongly tied to position in the bay and much less so to shoot type. This finding suggests that mesograzer species assemblages differ by position in the bay, and thus their net effects on producer biomass and food web support are also likely to differ among regions of the bay.
Our results clearly show that epifaunal community assemblage is strongly influenced by site and time period. One site parameter, soil organic matter (%), is a strong predictor of epifaunal community patterns. Increased soil organic matter in the soil (%) is known to increase the availability of nutrients to plants, and thus the palatability, which can influence patterns in abundance and distribution of associated organisms (Kelly et al. 1996).
As we hypothesized, measures of physical parameters such as salinity and temperature are poor predictors of community composition. However, temperature was only measured once per site at each sampling period, and we acknowledge that if an average of more detailed temperature measurements had been used for the analyses, a correlation might have been detected. Nevertheless, our results are supported by findings that demonstrated epifaunal larval recruitment and competition were stronger factors influencing variation in caridean shrimp abundance in seagrass beds than were salinity and temperature (Walsh & Mitchell 1998). While the importance in post-larval migration of epifaunal species from seagrass beds has been documented (Walsh & Mitchell 1998), there is also evidence that individuals do not leave a seagrass bed once they have settled (Bell et al. 1988). Our study did not specifically examine settlement and migration, but as the majority of the observed species are live brooders, we speculate that migration would not have a large effect on epifaunal community composition in San Francisco Bay eelgrass beds. Our results suggest that the larval epifauna community is determined by the existing community composition, and by introductions of exotic species.
San Francisco Bay is well known for its high proportion of introduced species (Cohen & Carlton 1998) and we found that eelgrass beds are no exception. Dominant epifaunal species in the studied eelgrass beds were C. cf drepanochir, (an introduced caprellid amphipod from Asia) (C. Brown unpublished data), Corophium alienense (an introduced gammaridean amphipod from Asia) (Ruiz et al. 2000), Ampithoe valida (an introduced gammarid native to the US Atlantic coast) (Cohen & Carlton 1995), and numerous juvenile caprellids and gammarids, probably of the same species. Notably, the introduced caprellid C. cf drepanochir was absent at Richardson Bay, but the native C. californica was relatively abundant there. However, with the exception of Richardson Bay, introduced epifauna were by far more common than native species (93% of individuals), a finding that is unique to seagrass epifaunal studies that we are aware of to date.
Determining identities, abundances and distributions represents a first step toward identifying functionally important mesograzer species and assemblages in San Francisco Bay eelgrass beds. Co-occurring mesograzers in other systems are known to differently influence ecosystem processes, including the production of eelgrass, (Duffy et al. 2001). In Chesapeake Bay, Duffy et al. (2001) found that one isopod species caused an increase in eelgrass biomass accumulation, another caused a decrease, and the third species, a gammaridean amphipod, had no effect. We speculate that there is a similar range of functions among mesograzers in San Francisco Bay eelgrass habitats and thus that the variation in community composition among sites and time periods leads to different net effects. However, we currently understand little of the roles of epifauna in these beds; further, the functions of introduced eelgrass epifauna in their native ranges may be poor predictors for new habitats (Suarez et al. 1999). Preliminary studies suggest damage to eelgrass by one invader (A. valida) may be more common in this system than in the species’ native habitat (Carr 2008, L.A. Carr et al. unpublished data). Such findings have implications for restoration in San Francisco Bay and elsewhere, as the spread of live-brooding invaders might be limited by careful restoration practices.
Declines in fish abundances in the bay and worldwide highlight the need to conserve habitats with high abundances of preferred prey species and refuge potential. But while a conservation focus on areas with high abundances of native species is common, it may be that eelgrass beds with high overall abundances of epifauna, regardless of native versus introduced status, will provide strongest support of higher trophic levels. Experimental evaluation of food web interactions will help to define the functional roles and relative contributions of both native and introduced epifaunal species to higher trophic levels.
Acknowledgements
We thank W. Kimmerer and 2 anonymous referees whose advice greatly improved this manuscript. S. Kiriakopolos and D. Singh provided valuable field and laboratory assistance. Boat access to survey sites was provided through funds from the California Coastal Conservancy and Ocean Protection Council.
Appendix
| Species | PSP | CB | KB | BFI | RB |
|---|---|---|---|---|---|
| April 2007 | |||||
| Juvenile Caprellids (JUVCAP) (c) | 9.7 | 8.3 | 21.3 | 127.6 | 138.7 |
| Caprella cf drepanochir (C.DREP) (c, i) (Mayer, 1890) | 1.3 | 6.5 | 4.5 | 47.8 | 0 |
| Caprella californica (C.CALI) (c, n) (Stimpson, 1857) | 0 | 0 | 0 | 0 | 24.3 |
| Juvenile Gammarids (JUVGAM) (g) | 2.6 | 0.1 | 0.3 | 0.1 | 0 |
| Corophium alienense (COROSP) (g, i) (Chapman, 1988) | 8.3 | 0.4 | 0 | 0.7 | 1.3 |
| Ampithoe valida (A.VALI) (g, i) (Smith, 1873) | 0.7 | 0.4 | 0 | 0 | 0.2 |
| Synidotea lavidorsalis (S.LATI) (i, i). (Benedict, 1857) | 0.2 | 0 | 0 | 0 | 0 |
| Idotea wosnesenskii (I.WOSE) (i, n) (Brandt, 1851) | 0 | 0 | 0.5 | 0 | 0 |
| Nematodes (NEMA) | 0 | 0 | 0 | 0 | 0.1 |
| Cumaceans (CUMACE) | 0 | 0 | 0.1 | 0 | 0.1 |
| Copepods (COPES) | 35.0 | 0 | 9.1 | 4.2 | 0.7 |
| Polychaetes (POLYS) | 0.7 | 0 | 0 | 0 | 3.3 |
| Bivalves (BIVALV) | 1.1 | 4.6 | 0 | 1.1 | 0 |
| Gastropods (GASTRO) | 0.2 | 0 | 0.5 | 0 | 0.1 |
| June 2007 | |||||
| Juvenile Caprellids (JUVCAP) (c) | 9.7 | 56.5 | 9.9 | 2255.3 | 86.3 |
| Caprella cf drepanochir (C.DREP) (c, i) | 18.8 | 10.7 | 4.8 | 289.2 | 0 |
| Caprella californica (C.CALI) (c, n) | 0 | 0 | 0 | 0 | 37.1 |
| Juvenile Gammarids (JUVGAM) (g) | 9.2 | 0.4 | 0 | 19.1 | 9.7 |
| Corophium alienense (COROSP) (g, i) | 23.4 | 3.9 | 1.2 | 215.5 | 12.3 |
| Ampithoe valida (A.VALI) (g, i) | 0.4 | 0.5 | 0.4 | 0 | 0.1 |
| Jassa marmorata (J.MARM) (g, i) (Dana, 1853) | 0 | 0 | 0 | 0 | 8.2 |
| Idotea wosnesenskii (I.WOSE) (i, n) | 0 | 0 | 0.1 | 0 | 0 |
| Paranthura elegans (P.ELEG) (i, n) (Menzies, 1951) | 0 | 0 | 0 | 0.4 | 0 |
| Copepods (COPES) | 13.9 | 0.3 | 0.9 | 0 | 7.2 |
| Polychaetes (POLYS) | 2.5 | 0 | 0 | 0 | 0.7 |
| Bivalves (BIVALV) | 0.1 | 0.8 | 0 | 0 | 0 |
| Gastropods (GASTRO) | 0.1 | 0 | 0.2 | 0.1 | 0 |
| August 2007 | |||||
| Juvenile Caprellids (JUVCAP) (c) | 374.7 | 59.2 | 127.9 | 176.9 | 3.6 |
| Caprella cf drepanochir (C.DREP) (c, i) | 130.9 | 14.1 | 3.9 | 56.4 | 0 |
| Caprella californica (C.CALI) (c, n) | 0 | 0 | 0 | 0 | 2.2 |
| Juvenile Gammarids (JUVGAM) (g) | 3.2 | 0.6 | 0.3 | 0 | 2.6 |
| Corophium alienense (COROSP) (g, i) | 1.9 | 1 | 0.2 | 2.8 | 0.5 |
| Ampithoe valida (A.VALI) (g, i) | 3.8 | 7 | 0.5 | 0 | 0 |
| Jassa marmorata (J.MARM) (g, i) | 0 | 0 | 0 | 0 | 6.1 |
| Idotea wosnesenskii (IWOSE) (i, n) | 0 | 0 | 0.5 | 0 | 0 |
| Paranthura elegans (P.ELEG) (i, n) | 0 | 1 | 0 | 0 | 0 |
| Idotea resecata (I.RESE) (i, n) (Stimpson, 1853) | 0 | 0.1 | 0 | 0 | 0 |
| Nematodes (NEMA) | 0 | 0 | 0 | 0 | 0.2 |
| Cumaceans (CUMA) | 0 | 0.9 | 0 | 0 | 0 |
| Copepods (COPES) | 37.3 | 0 | 1.1 | 0 | 0.6 |
| Polychaetes (POLYS) | 0 | 0 | 0.4 | 0 | 0.6 |
| October 2007 | |||||
| Juvenile Caprellids (JUVCAP) (c) | 969.6 | 527.8 | 27.6 | 201.8 | 4.3 |
| Caprella cf drepanochir (C.DREP) (c, i) | 243.2 | 230.5 | 9 | 106.7 | 0 |
| Caprella californica (C.CALI) (c, n) | 0 | 0 | 0 | 0 | 2.9 |
| Juvenile Gammarids (JUVGAM) (g) | 6.4 | 1 | 0.2 | 0 | 2.6 |
| Corophium alienense (COROSP) (g, i) | 2.4 | 0.2 | 0 | 0.1 | 0.2 |
| Ampithoe valida (A.VALI) (g, i) | 91.2 | 6.2 | 1.3 | 0.2 | 0 |
| Jassa marmorata (J.MARM) (g, i) | 0 | 0 | 0.4 | 0 | 0.9 |
| Paranthura elegans (P.ELEG) (i, n) | 0 | 0.7 | 0 | 0 | 2.1 |
| Idotea resecata (I.RESE) (i, n) | 0 | 0 | 0.3 | 0 | 0 |
| Copepods (COPES) | 0 | 0 | 0 | 0 | 3.6 |
| Polychaetes (POLYS) | 0 | 0 | 0.9 | 0.1 | 1.6 |
| Species | PSP | CB | KB | BFI | RB |
|---|---|---|---|---|---|
| June 2007 | |||||
| Juvenile Caprellids (c) | 32.9 | 251.2 | 25.4 | 4600.7 | 587.1 |
| Caprella cf drepanochir (c, i) | 28.2 | 50.6 | 3.9 | 417.2 | 0 |
| Caprella californica (c,n) | 0 | 0 | 0 | 0 | 221.3 |
| Juvenile Gammarids (g) | 55.3 | 9.9 | 1.8 | 46 | 201.6 |
| Corophium alienense (g, i) | 89.4 | 12.3 | 1.7 | 245.6 | 111.1 |
| Ampithoe valida (g, i) | 0.3 | 9.3 | 1.8 | 0 | 1.4 |
| Jassa marmorata (g, i) | 0 | 0 | 0 | 0 | 233.3 |
| Synidotea lavidorsalis (i, i) | 2.9 | 0 | 0 | 0 | 0 |
| Idotea wosnesenskii (i, n) | 0 | 0 | 1.2 | 0 | 0 |
| Paranthura elegans (i, n) | 0 | 0 | 0 | 3.2 | 1.3 |
| Idotea resecata (i, n) | 0 | 0 | 0 | 0 | 0.9 |
| Cumaceans | 0 | 0 | 0.1 | 0 | 0 |
| Harpacticoid copepods | 31.3 | 0.1 | 31.8 | 0 | 12.3 |
| Polychaetes | 3 | 0 | 0 | 0 | 4 |
| Bivalves | 0.6 | 1.2 | 0 | 0 | 0 |
| Gastropods | 0 | 0 | 0 | 0 | 0 |
| August 2007 | |||||
| Juvenile Caprellids (c) | 1318.9 | 347.1 | 440.4 | 1760.5 | 45.6 |
| Caprella cf drepanochir (c, i) | 281.1 | 89.2 | 20.7 | 530.2 | 0 |
| Caprella californica (c, n) | 0 | 0 | 0 | 0 | 12 |
| Juvenile Gammarids (g) | 11.5 | 2.2 | 1.9 | 3.2 | 20.1 |
| Corophium alienense (g, i) | 17.9 | 0.8 | 0.2 | 2.8 | 0.5 |
| Ampithoe valida (g, i) | 18.1 | 59.1 | 4.3 | 0 | 8.1 |
| Jassa marmorata (g, i) | 3.2 | 0 | 0 | 0 | 74.9 |
| Idotea wosnesenskii (i, n) | 0 | 0 | 0.5 | 0 | 0 |
| Paranthura elegans (i, n) | 0 | 2.2 | 0 | 8 | 1 |
| Cumaceans | 0 | 0 | 0.1 | 0 | 0 |
| Nematode | 0 | 0 | 0 | 0 | 0.5 |
| Harpacticoid copepods | 49.9 | 0 | 13.3 | 0 | 4.9 |
| Polychaetes | 0.3 | 0 | 0.1 | 0 | 4.6 |
| October 2007 | |||||
| Juvenile Caprellids (c) | 904 | 1957.6 | 238.7 | N/A | 0 |
| Caprella cf drepanochir (c, i) | 376.9 | 739.4 | 176.3 | N/A | 0 |
| Caprella californica (c, n) | 0 | 0 | 0 | N/A | 0 |
| Juvenile Gammarids (g) | 40 | 0.8 | 22.1 | N/A | 0 |
| Corophium alienense (g, i) | 32.8 | 0.4 | 92.2 | N/A | 0 |
| Ampithoe valida (g, i) | 200.8 | 17.6 | 17.3 | N/A | 0 |
| Jassa marmorata (g, i) | 0 | 0 | 0.4 | N/A | 0.9 |
| Paranthura elegans (i, n) | 12.8 | 0 | 28.3 | N/A | 0 |
| Idotea resecata (i, n) | 0 | 0 | 14.9 | N/A | 0 |
| Juvenile isopods (i) | 0 | 3.2 | 0.4 | N/A | 0 |
| Harpacticoid copepods | 1.6 | 0 | 2 | N/A | 0 |
| Polychaetes | 0 | 0 | 2.3 | N/A | 0 |