Population genetic structure of the endangered limpet Cymbula nigra in a temperate Northern hemisphere region: influence of palaeoclimatic events?
Abstract
Cymbula nigra (da Costa L, 1771) is the largest limpet in Europe and it is at present considered an endangered species. Nevertheless, its biology is poorly understood and there is a lack of knowledge about population genetic structure. A total of 61 C. nigra specimens were sampled and studied for a fragment of the mitochondrial gene cytochrome c oxidase subunit I. The low genetic diversity, the absence of geographic structure, the significant values reported by measures to test for a recent population expansion and a star-shaped phylogeny pattern are here interpreted as forming a hypothesis concerning the influence of palaeoclimatic events in the genetic pool of limpets, considering also the information available on different limpet species.
Introduction
The Family Patellidae comprises four extant genera –Patella, Cymbula, Helcion and Scutellastra. Most species of Patellidae are found in the Eastern Atlantic, but Scutellastra has a wide distribution ranging from Southern Africa to the Indo-Pacific. In the Mediterranean Sea, only two genera, Cymbula and Patella, are recognized. Cymbula nigra (da Costa, 1771) (see Barea et al. 2008) is the only Cymbula species present in the Mediterranean, although it has a Western African origin (Christiaens 1973). Commonly known as ‘black limpet’, C. nigra is mainly distributed in the southernmost coasts of the Iberian Peninsula and North Africa to Angola (Gofas et al. 1988). It was catalogued as an ‘endangered and threatened species’ (Annex II) by the Barcelona Convention (1993), as ‘strictly protected’ by the Berna Convention (1995) and as ‘vulnerable’ by the Andalusian Red List of Threatened Invertebrate Species (Moreno & Arroyo 2008). Little is known about the biology of this species but it seems to be relatively abundant in artificial breakwaters from the Strait of Gibraltar, showing a preference for smooth rocky surfaces (Rivera-Ingraham et al. 2010). Its larval stage and dispersal ability have not been studied in detail. However, Renault & Moueza (1971) reported a reproductive strategy similar to that of Patella caerulea and Patella vulgata (protandrous hermaphroditism), and the duration of the larval period could therefore be similar to these species, at around 10 days (see Dodd 1957). The genetic structure of C. nigra has only been considered in broader studies dealing with limpet phylogenies (see Nakano & Ozawa 2004, 2007; Sá-Pinto et al. 2005, 2008), and little is known about the genetic structure of its populations. The aim of the present study was to explore the genetic relationships of populations of C. nigra in a temperate area of the Northern hemisphere.
Material and Methods
Sampling sites, number of individuals and codes are shown in Table 1 and Fig. 1. We determined the nucleotide sequence of a 658-bp fragment of the mitochondrial gene cytochrome c oxidase subunit I (hereafter COI) using the primer pair described by Folmer et al. (1994). For details on the experimental protocol see Nakano & Espinosa (2010).
| Locality | Code/Station number | No. of individuals | No. of haplotypes |
|---|---|---|---|
| Benzu (Spain) | BE/1 | 5 | 2 |
| Chorrillo (Spain) | CH/2 | 5 | 2 |
| Cabo Negro (Morocco) | CN/3 | 5 | 2 |
| Cap Malabata (Morocco) | CM/5 | 5 | 3 |
| Asilah (Morocco) | A/7 | 5 | 1 |
| Larache (Morocco) | L/8 | 5 | 2 |
| Rabat (Morocco) | R/9 | 5 | 4 |
| Fatna (Morocco) | F/10 | 7 | 2 |
| Alcazarquivir (Morocco) | AL/4 | 6 | 1 |
| Cap Spartel (Morocco) | CS/6 | 3 | 2 |
| Puerto Saladillo (Spain) | PS/11 | 5 | 2 |
| Estepona (Spain) | E/12 | 5 | 1 |
| TOTAL | 61 | 8 |
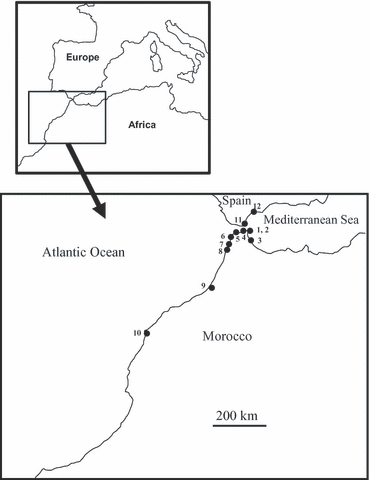
Map of the sampling localities for Cymbula nigra. Numbers correspond to ‘station number’ in Table 1.
DNA sequences were aligned using MACCLADE 4.03 (Maddison & Maddison 2002) referring to translated amino acid sequences. Third-codon positions were retained. All haplotypes of 61 individuals were defined using the ‘Redundant Taxa’ option in MACCLADE 4.03 (Maddison & Maddison 2002), and a minimum spanning tree (MST) (Kruskal 1956; Prim 1957) was constructed using ARLEQUIN version 2.0 (Schneider et al. 2000). The MST is computed from the matrix of pairwise distances calculated between all pairs of haplotypes using a modification of the algorithm described in Rohlf (1973). The MST is convertible to a minimum spanning network (MSN). Several population parameters were also calculated in ARLEQUIN version 2.0. Haplotype (h) and nucleotide (π) diversity values were estimated according to Nei & Tajima (1981) and Nei (1987). Tajima’s D (Tajima 1989) and Fu’s Fs (Fu 1996) measures were estimated to test for evidence of population expansion and Fst fixation index to test for geographical structure. All sequences of Cymbula nigra determined in this study have been deposited in DDBJ and GenBank under accession numbers AB445038 to AB445098.
Results and Discussion
The MSN obtained for the C. nigra samples shows a star-shaped phylogeny with eight different haplotypes (Table 1, Fig. 2). Note that 46 of the 61 individuals analyzed (c. 75% of individuals) had the same haplotype. Values of genetic diversity are low (h = 0.415; π = 0.00077), whereas the analyses used to test for evidence of recent population expansion are significantly negative (Tajima's D: -1.84; Fu's Fs: -5.82). The fixation index is also very low (Fst = 0.00384), indicating the absence of geographic structure. [Correction added after online publication 6th January 2011 - ‘Values of...’ to ‘...geographic structure’ inserted].
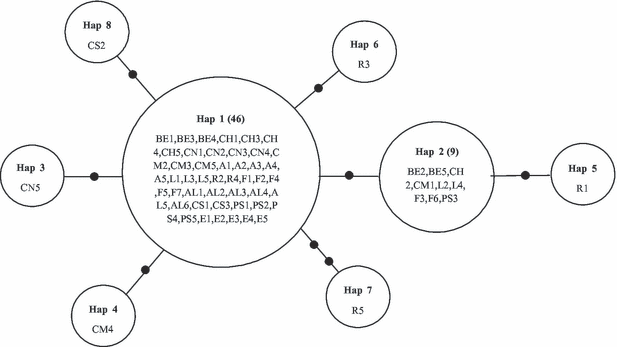
Minimum spanning network of the haplotypes found for Cymbula nigra. For designation of individuals see Table 1. Black spots indicate mutations. Numbers in brackets indicate the total number of individuals with the same haplotype.
The minimum spanning network showed a star-shaped phylogeny. According to Rogers (1995) and Avise (2000) this is a signature of species that have recently expanded in size from small or modest numbers of founders, or have experienced recent colonization events (bottleneck and founder effects). Compared with data on other limpet genetic structure (Espinosa & Ozawa 2006 in Patella ferruginea and Patella caerulea; data from Sá-Pinto et al. 2005 and Mauro et al. 2003 reported in GenBank either in P. ferruginea and P. caerulea or in P. rustica and P. ulyssiponensis), all patellid species from the temperate Northern Hemisphere show a similar pattern of a star-shaped phylogeny. Furthermore, measures of genetic variability may increase in a spatially structured population (Amos & Harwood 1998). On the other hand, the measures may show quite a uniform structure if the population has expanded rapidly in size following a bottleneck, even if there is some spatial structuring of the population (Walker et al. 1998). The postulated expansion was further supported by the significant negative Tajima’s D and Fu’s Fs values, as well as the absence of geographic structure among populations (Fst < 0.05), taking into account that Fst values of 0–0.05 may indicate little genetic differentiation (Hartl & Clark 1989). These results and the presence of shared haplotypes in almost all the sampled populations support the notion of a population expansion of the species related to the colonization of the present distribution area.
Several environmental fluctuations could be responsible for the genetic pattern found. On the one hand, during the Messinian salinity crisis (5.2–6.7 Ma) the Mediterranean Sea nearly dried up (Bover & Alcover 2000; Bianchi & Morri 2000; Taviani 2003) and many species recolonized the Mediterranean basin through the Gibraltar Strait when the sea level rose again, probably causing the founder effects. On the other hand, fluctuations produced by Quaternary glacial–interglacial periods in Europe (see Zazo et al. 2003) may have caused important decreases in population size of patellids and generated bottleneck events. The sea surface temperature (SST) in the Mediterranean Sea ranged from 2.7 to 6.5 °C (winter–summer) to 18–24.4 °C during the Quaternary (González-Donoso et al. 2000). For instance, during the last glacial maximum (28–15 kya) the SST was 8–9 °C mean of the whole year, around 8 °C colder than present values (Melki et al. 2009). These events produced an abrupt modification of the environmental characteristics over relatively short periods and initiated great marine regressions and weak transgressions, changing the shoreline, water/air temperatures and habitats, and affecting the settled species. The low levels of genetic variation are more common among intertidal species that live relatively high on the shore, where exposure times to cold stress in air are longer than for species that live at greater depths (Marko 2004). This is the case for the different limpet species in Europe and nearby North African coasts. Furthermore, several papers have reported the relationship between palaeo-environmental changes and genetic patterns in marine species. For instance, the Chinese shrimp Feneropenaeus chinensis may have undergone a genetic bottleneck during the late Pleistocene global glacial period (Li et al. 2009), whereas Earl et al. (2010) have suggested that Pleistocene/Holocene marine transgressions produced a star-shaped phylogeny in the tidewater goby Eucyclogobius newberry. As Hewitt (1996) pointed out, several species must have gone through a series of bottlenecks and expansions in population size and range depending on climatic conditions. Lately, several papers have documented star phylogenies related to post-glacial colonization events from southern glacial refugia (see Marko et al. 2010 and references herein) or have suggested that a recent population expansion has taken place in Atlantic and Mediterranean basins, most probably following the Messinian salinity crisis in the sea urchin Paracentrotus lividus (Calderón et al. 2008). Therefore, the present genetic pattern in Cymbula nigra could have originated from founder events from tropical areas of West Africa, instead of being influenced by glacial periods in Europe.
From a conservation perspective, the low number of haplotypes in the populations analyzed could represent an additional threat to this endangered species. It would be desirable to promote interbreeding among specimens from different populations to increase genetic variability, as this may enhance the resilience of the species against environmental changes. Further studies on an extensive number of samples from tropical West Africa would be required to determine the genetic structure of the species in areas not influenced by glacial cycles and to test the hypothesis of a greater genetic diversity in these zones.
Acknowledgements
We express our thanks to Dr Emilio Rolán and Dr José Templado (Museo Nacional de Ciencias Naturales, Spain) for contributing specimens studied in this work. Our gratitude to Autoridad Portuaria de Ceuta and Grant-in-Aid for JSPS Fellows no. 207024 to T. Nakano from the Japan Society for Promotion of Science for Financial Support. All material has been collected under appropriate collection protocols and approved ethics guidelines. Thanks to Consejeria de Medio Ambiente-Junta de Andalucía for providing facilities to obtain samples from Alboran Island as well as to Obimasa-Consejeria de Medio Ambiente-Ciudad Autónoma de Ceuta. We also are grateful to Dr Gonzalo Giribet and two anonymous referees who have greatly improved the original version of the manuscript.




