Structure and dynamics of distinct fish assemblages in three reaches (upper, middle and lower) of an open tropical estuary in Brazil
Abstract
We collected fishes and environmental variables in three zones (upper, middle and lower) of a small open tropical estuary during flood tide. The aim was to test for differences in fish assemblages along a gradient from freshwater to marine waters and to detect any seasonal variation in fishes and environmental variables across these zones. A total of 111 species (18 in the upper, 50 in the middle and 66 in the lower estuary) were recorded, forming three distinct fish assemblages, with the family Eleotridae dominating in the upper, Gerreidae in the middle, and Sciaenidae in the lower estuary. Only two species (Geophagus brasiliensis in the upper and the middle zones, and Eucinostomus argenteus in the middle and the lower zones) composed more than 1% of the total number of individuals in more than a single zone. Short-term (tidal) changes in salinity in the middle estuary were associated with different assemblages in the three estuarine zones, even in winter, when the differences in salinity are lowest between the middle and the lower zones. Seasonal variation in salinity was irrelevant, except in a protected sidewater lagoon in the middle estuary. Low salinity seasonal change may be related to the lack of seasonal variation in the structure of fish assemblages in all estuarine zones.
Introduction
Estuaries are transitional systems where seasonally fluctuating freshwater river flows meet the daily fluctuating marine tides to create conditions of highly variable salinity and other environmental factors that influence fish assemblage structure (Haedrich 1983; Whitfield 1999; Blaber 2000). Three distinct estuarine zones have been established for the estuarine systems based on the dynamics of these two flow variables: a riverine zone (upper estuary) in the upper limit of tidal influence, a coastal zone (lower estuary) with the estuarine plume, and an intermediate mixing zone (middle estuary), whose features constantly change due to waters of different characteristics (Kjerfve 1987). Although some species may occupy all three zones at times, many different fish species tend to adapt to a particular estuarine zone, thereby forming or changing assemblage structure throughout the longitudinal estuarine extent according to the environmental conditions in each zone.
Research on fish assemblages in estuaries has shown that salinity plays a major role in shaping assemblage structure (Wagner & Austin 1999; Whitfield 1999; Martino & Able 2003; Barletta et al. 2005; Selleslagh & Amara 2008), although only a few studies encompass the full salinity gradient, i.e. from ocean to tidal freshwater. Estuarine fishes are able to cope with salinity fluctuation but their ability to do so varies from species to species and hence may influence their distribution (Blaber 2000). Besides salinity, other environmental variables such as temperature, depth and turbidity can play important roles in determining fish assemblages (Blaber & Blaber 1980; Blaber 2002). Thiel et al. (1995) reported salinity to be the best predictor of fish richness, and temperature for fish abundance in temperate estuaries. However, temperature is seldom a structuring factor in tropical areas, as it remains relatively stable during the whole year, whereas oxygen may restrict the distribution and movement of fishes (Araújo et al. 1998, 2002). Blaber et al. (1984) suggested that low dissolved oxygen values contributed to the impoverished fish fauna in the Tongati Estuary. Tropical estuaries generally have high turbidity (Blaber 2000), which is also considered an important characteristic of rearing grounds for juvenile fishes (Robertson & Blaber 1992) because visual predators are less effective in the lowered light levels (Blaber & Blaber 1980). Finally, in coastal estuarine areas, assemblage structure depends more on depth (Araújo et al. 2002), but this factor is additionally correlated to substrate type (Horne & Campana 1989). The relationship between environmental variables and the distribution of organisms within estuaries has been studied at length in large estuaries exposed to human pressure (Marshall & Elliott 1998; Whitfield 1999; Akin et al. 2005); however, at present we lack information on small and less impacted estuaries.
Protected areas in the estuarine zone feature particular fish assemblages and serve as rearing grounds for fishes (Beck et al. 2001; Lazzari et al. 2003). Estuaries with habitat heterogeneity usually have higher species richness than homogeneous systems (Whitfield 1983). When occupied by aquatic macrophytes and wood debris, estuarine margins lead to high structural complexity and spatial heterogeneity (Keefer et al. 2008). Mangroves are dominant habitats in tropical estuaries and their structural complexity provides shelter and food as well as decreased predation risk for fish, making them ideal rearing grounds for juvenile fishes (Laedsgaard & Johnson 2001).
The Mambucaba River has a relatively well-preserved estuarine area on the Rio de Janeiro coast, in Southeastern Brazil. This open estuary still shows minor flow alteration or other human interference in river geomorphology. No information is available about fish assemblages in small open estuaries in Southeastern Brazil. This study aims to address this lack of knowledge by describing the structure and dynamics of fish assemblages in three zones of the Mambucaba estuary (upper, middle and lower) and by testing the hypothesis that distinct fish assemblages occur along the estuarine zones as the result of changes in environmental variables throughout the longitudinal gradient. We also examine the variability of the fish community structure in each zone across seasons and in association with environmental variables.
Material and Methods
Study area
The Mambucaba River (23°01′37.30″ S, 44°31′15.22″ W), located on the coast of the state of Rio de Janeiro, Southeastern Brazil, has a small open estuary (Fig. 1). The estuary is 5 km long with a mouth width ranging from 20 m at low tide during the dry season to 40 m at high tide during the wet season, and a maximum width of 10 m on the main channel. The region has semi-diurnal tides, with a mean variation ranging from 0.1 m at neap tides to 1.3 m at the highest tides, and is considered a micro-tidal estuary according to the McLusky & Elliott (2004) classification. Coastal littoral transport accumulates sediment at the estuary entrance and changes the main channel position. The water circulation is mainly dependant on the tides and on a small freshwater input of about 13.8 m3·s−1 in the dry/winter season to 37.9 m3·s−1 in the wet/summer season (Francisco & Carvalho 2004). Averaged accumulated annual rainfall is 1770 mm, ranging from 180 mm in the dry/winter season (June–August) to 750 mm in the wet/summer season (January–March). Temporal changes in rainfall and in river flow result in two seasons of comparatively low (winter) and high (summer) river influence, and two intermediate seasons (spring and autumn).
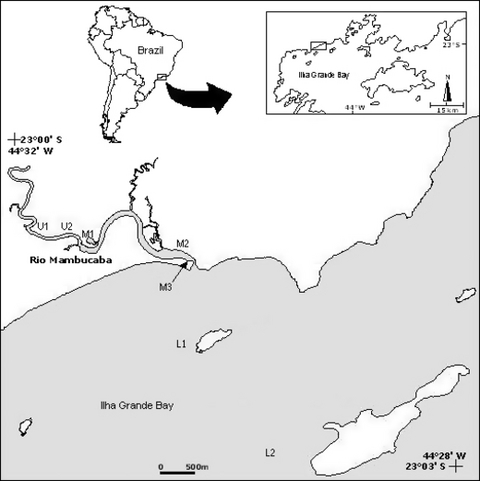
Map of the Mambucaba estuary with indication of the sampling sites: upper estuary (U1 and U2), middle (M1, M2 and M3) and lower (L1 and L2).
The study area covers the whole estuarine gradient (sensuCameron & Pritchard 1963; McHugh 1967), encompassing the transition from a freshwater/oligohaline estuarine system to the adjacent coastal area in three zones (upper, middle and lower) defined according to Kjerfve (1987). These estuarine zones were defined by measuring bottom and surface salinity, which detected both the limit of tide influence in the upper estuary and the estuarine plume in the lower estuary. The upper estuary comprises the fluvial reaches with predominant river characteristics and is approximately 40 m wide and 5 m deep. As this area is the upper limit of tidal influence, salinity is approximately zero. Margin cover is composed of grass and medium-sized trees, with muddy-sand substrate and shelters made up of wood debris, snags and stones. The middle estuary is the most dynamic estuarine reach where both tide and river flows interact more intensively. Sandbanks divide the main water flow during low tide into two channels approximately 3.5 m deep. The middle estuary is approximately 120 m wide. The substrate is predominantly sand with the margins surrounded by sparse mangrove formation at the lower reaches and small villages at the upper reaches. A protected lagoon is connected to the main channel by a narrow channel of approximately 2 m in the upper reaches. The lagoon has a surface of 0.7 ha, and has a muddy substrate with margins comprising mangroves, ripraps and a small sandy beach. The lower estuary has a plume extending approximately 2.3 km from the mouth of the estuary and a maximum depth of 17 m. The substrate changes from sandy in the shallow areas to muddy towards the deeper areas.
Sampling methods
Sampling was conducted for 2 months in each season, between October 2007 and August 2008. A total of 151 samples, evenly distributed among seasons, were performed in three estuarine zones: 45 in the upper, 61 in the middle and 45 in the lower estuary. Some samplings were not conducted due to inclement weather. To minimise the confounding effects caused by variations in tidal stage and environmental conditions between each sampling period, as well as to standardise the sampling regime, all sites were sampled at flood tide during the full or new moon because the tidal gradient is better defined in such conditions. To ascertain the sampling time and flooding period, we measured salinity increase before the fishing procedure. All environmental measurements were taken at each occasion after fish sampling. Two consecutive days were required to sample all sites.
Fishes were collected at seven sites: two in the upper – U1 and U2, three in the middle – M1, M2 and M3, and two in the lower estuary – L1 and L2. There was no single method to sample the three zones with similar efficiency. Thus, the three zones were sampled separately, in each case using the most suitable, active fishing method. In the upper estuary, fish were collected with 75 × 75 cm rectangular sieves with 1-mm mesh and a small bag to prevent fish from escaping. Three series of 30 sieve samples were collected near vegetated river margins, covering different areas in the site. The swept area estimated from 30 sieves was approximately 17 m2. Site U1 was 3 km away from the mouth of the estuary, with a shoreline dominated by trees and grass, while site U2 was 2.6 km from the mouth with a margin covered predominantly by grass.
In the middle estuary, fishes were collected by a seine 40 m long, 5 m high and 6 m at the cod end. The net has 10-mm mesh between adjacent knots at the wings, 5-mm mesh at the central part and 2.5 mm at the cod end. The net was set up with the help of a small boat and hauls were performed perpendicularly to the shoreline at a standardised distance of 15 m. Each seine covered an area of approximately 450 m2, according to the following equation: A = D × L, where D is the distance from the margin (15 m) and L is the net length effectively used in the haul (30 m). Two sites (M2 and M3) were located in the main channel, while the third site (M1) was in a protected lagoon adjacent to the main channel in the upper part of the middle estuary. This site was 2 km away from the estuary mouth. Site M2 was located in the main channel next to a mangrove formation between tidal channels, 500 m away from the river mouth with sandy substrate. Site M3 was a sandy beach along a sandbank by the sea connection, with high dynamism and low physical structure. Two seines were performed at M1 due to the smaller lagoon area and three seines at M2 and M3.
In the lower estuary, fishes were collected by bottom trawl with a 7-m-long net with 20-mm mesh at the wings and 12-mm mesh at the cod end. The ground rope was 8 m long and the head rope 7 m. The distance travelled was obtained using the coordinates registered at the beginning and at the end of each trawl with a global positioning system (GPS, Garmin III) used to determine the swept area. For each sample, the swept area (A) was estimated: A = D × h × X2, where D is the length of the path, h is the length of the head rope, and X2 is that fraction of the head rope which encompasses the width of the path swept by the trawl (Sparre & Venema 1995). In this study, the samples were taken at speeds of between 2 and 2.5 knots and it was assumed that X2 = 0.6, with the swept area corresponding to approximately 3780 m2. Two sites (L1 and L2) were sampled in the lower estuary. Site L1 was 900 m from the river mouth where there was a greater influence of the estuarine plume, 10 m depth and featured a substrate composed of sand and plant organic debris brought by the river. Site L2 was 2.3 km away from the river mouth, 17 m deep and had a muddy substrate. All the three fishing methods are directly related to the area sampled and thus fish densities were comparable. The catch per unit area was used for the estimation of density and was calculated by dividing the catch by the sampled area (individuals m−2 × 102). However, caution is needed when interpreting the results because of possible influences of different techniques. Wolter & Bischoff (2001) and Leeuw et al. (2007) also used different fishing methods to assess different habitats.
A series of environmental variables was measured at each fish sampling occasion. Temperature, salinity and dissolved oxygen were determined using a multiprobe YSI 85. Turbidity was calculated using a Policontrol model AP2000 turbidimeter. Depth was measured with a Speedtech model SM-5 digital sounder. Three measurements of each environmental variable were taken from water collected near to the bottom in a Van Dorn bottle.
Data analysis
One-way analysis of variance (ANOVA, P < 0.05) was used to compare environmental variables among seasons for each site followed by an a posteriori Tukey HSD test (Zar 1999). Environmental data were previously log transformed using log10(x + 1), to address the assumptions of normality and homoscedasticity of the parametric analyses. The coefficient of variation was calculated for all environmental variables by each season for each estuarine zone. A two-tailed Chi-squared test (**P < 0.01) was used to compare for significant departure from 1.
Species richness was calculated with the first and second Jackknife estimators. This procedure was performed using the software PC-ORD for Windows (McCune & Mefford 1997). The median of total length (TL) of fish species was compared between the sites of each zone by using the median test. In the middle estuary, sites M2 and M3 corresponded to main channel and were grouped and compared with M1 (the adjacent lagoon).
Fish density data were square root-transformed and converted into a triangular matrix of similarities, using the Bray–Curtis similarity coefficient. The results of this procedure were displayed on an ordination plot, generated by a non-metric multidimensional scaling procedure to assess spatial variation. We used a non-parametric permutation-based one-way analysis of similarity (ANOSIM) to test for differences in the fish assemblage structure among the estuarine zones (upper, middle and lower) and to compare assemblages among seasons within each zone. The principal species responsible for the sample groupings, and for the discrimination between specified groupings in these analyses, were identified using the SIMPER routine (Clarke 1993). These analyses were performed using the statistical package PRIMER version 5.2.4 (Plymouth Routines in Multivariate Ecological Research Package, Clarke & Warwick 1994).
Environmental influences on the dominant species of each zone, defined as having a frequency of occurrence >30 and a total number of individuals >1%, were assessed with a canonical correspondence analysis (CCA) on log-transformed [log10(x + 1)] data (ter Braak 1986). The statistical significance of each environmental variable was assessed with a Monte Carlo permutation test, using 1000 sample permutations. The CCA was performed using CANOCO software for Windows (version 4.5) on fourth root-transformed data (Plant Research International, Wageningen, the Netherlands).
Results
Environmental variables
The temperature ranged from 16.1 to 27.6 °C in the upper estuary, from 20.9 to 29.3 °C in the middle estuary, and from 19.4 to 26.3 °C in the lower estuary. The upper estuary had values comparatively lower than the middle and lower estuaries during all seasons except spring. In the upper and middle estuaries the temperature was higher in spring, while in the lower section no significant seasonal difference in temperature was found (Table 1). Salinity ranged from 0.1 to 1.9 psu in the upper estuary (oligohaline), from 0.1 to 33.1 psu in the middle estuary (mixohaline), and from 17.9 to 35.1 psu in the lower estuary (mixo-polyhaline). Seasonal changes in salinity were recorded only for the upper estuary and for the protected adjacent lagoon (M1) in the middle estuary, with higher values in winter and spring and lower values in summer and autumn (Table 1). Turbidity ranged from 0.02 to 28.50 nephelometric turbidity units (NTU) in the upper estuary, from 0.02 to 23.2 NTU in the middle estuary, and from 0.02 to 20.4 NTU in the lower estuary. The lowest turbidity values were primarily recorded in the lower estuary. Seasonally, the highest values were recorded in spring and summer, except at M3, which had the highest values in summer and the lowest values in spring (Table 1). Saturation of dissolved oxygen ranged from 67.2 to 94.5% in the upper estuary, from 52.6 to 102.8% in the middle estuary, and from 38.9 to 93.6% in the lower estuary. Although some significant differences existed in dissolved oxygen between seasons, mean saturation values were always higher than 60% (Table 1).
| Seasons | Upper estuary | Middle estuary | Lower estuary | ||||
|---|---|---|---|---|---|---|---|
| U1 | U2 | M1 | M2 | M3 | L1 | L2 | |
| Temperature (ºC) | |||||||
| Spring | 27.3 (0.1)a | 25.8 (0.7)a | 26.6 (0.5)a | 27.1 (0.9)a | 28.6 (0.3)a | 23.8 (0.7)a | 23.2 (0.4)a |
| Summer | 22.0 (0.4)b | 21.6 (0.1)b | 23.6 (0.1)b | 24.3 (0.7)b | 24.3 (0.6)b | 24.2 (0.6)a | 23.2 (1.0)a |
| Autumn | 20.0 (0.6)bc | 20.1 (0.6)bc | 21.7 (0.4)b | 23.7 (0.4)b | 24.1 (0.3)b | 24.7 (0.3)a | 24.5 (0.4)a |
| Winter | 18.4 (0.8)c | 18.3 (0.9)c | 23.2 (0.5)b | 23.6 (0.4)b | 23.6 (0.4)b | 23.2 (0.3)a | 23.1 (0.3)a |
| F-ANOVA | 20.76** | 21.84** | 25.79** | 6.82** | 12.81** | n.s. | n.s. |
| Salinity (psu) | |||||||
| Spring | 0.3 (0.1)b | 0.8 (0.2)a | 10.5 (4.1)ab | 11.3 (4.0)a | 26.8 (0.4)a | 34.1 (0.2)a | 34.2 (0.1)a |
| Summer | 0.0 (0.0)b | 0.0 (0.0)b | 0.2 (0.0)c | 13.0 (5.5)a | 23.3 (0.6)a | 33.4 (0.5)a | 33.6 (0.2)a |
| Autumn | 0.0 (0.0)b | 0.0 (0.0)b | 2.4 (1.4)bc | 19.1 (5.2)a | 21.3 (4.4)a | 33.5 (0.1)a | 31.1 (2.6)a |
| Winter | 1.2 (0.2)a | 1.1 (0.2)a | 26.2 (1.1)a | 31.6 (0.7)a | 29.4 (0.5)a | 34.0 (0.2)a | 34.3 (0.3)a |
| F-ANOVA | 38.78** | 38.63** | 18.12** | n.s. | n.s. | n.s. | n.s. |
| Turbidity (NTU) | |||||||
| Spring | 11.4 (0.3)a | 14.6 (4.7)a | 10.1 (1.0)a | 15.3 (2.2)a | 0.02 (0.0)c | 0.4 (0.2)a | 0.6 (0.5)a |
| Summer | 9.1 (2.0)a | 16.1 (3.0)a | 12.0 (1.5)a | 15.7 (3.1)a | 13.2 (1.7)a | 6.2 (3.3)a | 1.0 (0.6)a |
| Autumn | 5.0 (1.0)ab | 10.2 (1.8)a | 4.1 (1.0)b | 1.7 (0.3)b | 1.9 (0.3)b | 6.0 (1.4)a | 2.1 (0.5)a |
| Winter | 2.1 (0.7)b | 7.3 (3.1)a | 0.8 (0.3)b | 1.6 (0.6)b | 1.5 (0.7)bc | 3.4 (2.1)a | 1.3 (0.2)a |
| F-Anova | 8.15** | n.s. | 39.94** | 31.16** | 27.44** | n.s. | n.s. |
| Dissolved oxygen (% saturation) | |||||||
| Spring | 76.2 (0.1)b | 70.7 (1.0)b | 80.3 (7.8)a | 77.8 (7.1)a | 82.5 (1.6)bc | 83.1 (3.6)a | 75.8 (4.4)a |
| Summer | 85.5 (1.3)ab | 82.1 (1.2)a | 100.0 (1.6)a | 85.1 (2.2)a | 88.2 (0.3)ac | 69.4 (3.1)ab | 71.1 (7.7)a |
| Autumn | 86.9 (0.6)a | 85.1 (0.7)a | 78.4 (5.7)a | 78.6 (4.1)a | 86.4 (2.0)bc | 60.6 (5.3)b | 64.2 (3.1)a |
| Winter | 85.8 (3.4)b | 86.4 (3.3)a | 71.3 (9.1)a | 91.5 (2.4)a | 92.6 (1.5)a | 75.2 (2.2)a | 75.1 (1.7)a |
| F-ANOVA | 3.7* | 16.4** | n.s. | n.s. | 6.0** | 5.6** | n.s. |
- Superscripts indicate significant differences levels from ANOVA. Significant levels *P < 0.05; **P < 0.01.
The within-zone variability in environmental variables, as indicated by the coefficient of variation, had the highest values for salinity in the middle estuary during spring, summer and autumn (Table 2). High variability was also found for turbidity throughout all zones in most seasons.
| Seasons | Upper | Middle | Lower |
|---|---|---|---|
| Temperature (ºC) | |||
| Spring | 0.09* | 0.10* | 0.08* |
| Summer | 0.03* | 0.07* | 0.17* |
| Autumn | 0.10* | 0.07* | 0.03* |
| Winter | 0.21* | 0.04* | 0.02* |
| Salinity (psu) | |||
| Spring | 0.25* | 7.08* | 0.00* |
| Summer | 0.00* | 10.75* | 0.02* |
| Autumn | 0.00* | 10.11* | 0.64 |
| Winter | 0.20* | 0.23* | 0.01* |
| Turbidity (NTU) | |||
| Spring | 6.28* | 5.12* | 1.30* |
| Summer | 3.90* | 1.99* | 10.60* |
| Autumn | 2.35* | 0.93 | 2.58* |
| Winter | 7.54* | 1.45 | 5.27* |
| Dissolved oxygen (% saturation) | |||
| Spring | 0.15* | 2.43* | 0.95 |
| Summer | 0.13* | 0.55 | 2.69* |
| Autumn | 0.04* | 1.04 | 1.72 |
| Winter | 0.73 | 1.92* | 0.29* |
- Values significantly different from 1 (*P < 0.01) according to Chi-squared test were shown.
Species composition
We collected 111 species of fishes (40 families) from the Mambucaba River estuary in a total of 151 samples with absolute mean density and biomass values of 100.5 ind·m−2× 102 and 234.0 g·m−2 × 102, respectively (Table 3). The margins of the upper estuary had the highest mean density and biomass (296.2 ind·m−2 × 102 and 417.5 g·m−2 × 102), the middle estuary had intermediate values (28.6 ind·m−2 × 102 and 237.63 g·m−2 × 102), and the lowest values were found in the lower estuary (2.4 individuals·m−2 × 102 and 45.62 g·m−2 × 102). Species richness was higher in the lower estuary (66 species), decreased in the middle (50 species), and had the lowest values in the upper estuary (18 species). These values corresponded to about 75% of the first estimate of Jackknife for each estuarine zone (upper, 22.9 species; middle, 63.8 species; lower, 86.5 species). Considered separately, the highest mean species richness (ANOVA, P < 0.05) was found at site M1 and the lowest at M3, with intermediate values recorded for the sites in the lower and upper zones.
| Species | Density (%) | Biomass (%) | Frequence of occurence (%) | Average total length ± SD (mm) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upper | Middle | Lower | Upper | Middle | Lower | Upper | Middle | Lower | Upper | Middle | Lower | |
| Dormitator maculatus | 73.20 | 77.36 | 97.78 | 43 ± 18.6 | ||||||||
| Astyanax sp. | 10.42 | 2.35 | 62.22 | 31.4 ± 7.7 | ||||||||
| Microphis brachyurus lineatus | 9.76 | 0.04 | 5.47 | 0.00 | 80.00 | 4.92 | 119.8 ± 14.1 | 111.7 ± 22.1 | ||||
| Geophagus brasiliensis | 1.81 | 1.82 | 3.46 | 10.00 | 37.78 | 45.90 | 44.7 ± 25.0 | 125.3 ± 47.9 | ||||
| Eleotris pisonis | 1.28 | 0.03 | 4.50 | 0.00 | 44.44 | 1.64 | 73 ± 23.3 | 43.5 ± 2.1 | ||||
| Evorthodus lyricus | 0.79 | 0.22 | 0.45 | 0.05 | 22.22 | 14.75 | 43.2 ± 13.6 | 58.6 ± 7.9 | ||||
| Gobionellus shufeldti | 0.53 | 3.47 | 0.10 | 0.34 | 15.56 | 27.87 | 33.2 ± 7.1 | 46.5 ± 12.3 | ||||
| Pseudophallus mindi | 0.53 | 0.05 | 26.67 | 68.9 ± 16.3 | ||||||||
| Centropomus parallelus | 0.40 | 1.69 | 0.10 | 2.04 | 17.78 | 22.95 | 34 ± 6.8 | 79.4 ± 49.5 | ||||
| Hoplias malabaricus | 0.31 | 3.48 | 11.11 | 112.6 ± 31.0 | ||||||||
| Phalloceros caudimaculatus | 0.26 | 0.03 | 8.89 | 21 ± 6.8 | ||||||||
| Poecilia vivipara | 0.22 | 0.45 | 0.05 | 0.02 | 6.67 | 8.20 | 29.4 ± 4.5 | 28.7 ± 5.1 | ||||
| Gymnotus carapo | 0.22 | 2.32 | 11.11 | 160.7 ± 24.2 | ||||||||
| Trinectes microphthalmus | 0.09 | 0.05 | 0.05 | 0.04 | 2.22 | 2.22 | 36.5 ± 12.0 | 90.0 | ||||
| Trinectes paulistanus | 0.04 | 6.07 | 0.38 | 0.00 | 1.41 | 0.75 | 2.22 | 32.79 | 22.22 | 22.0 | 44.7 ± 13.2 | 120.3 ± 25.1 |
| Australoheros facetus | 0.04 | 0.14 | 2.22 | 61.0 | ||||||||
| Myrophis punctatus | 0.04 | 0.06 | 2.22 | 164.0 | ||||||||
| Oligosarcus hepsetus | 0.04 | 0.02 | 2.22 | 51.0 | ||||||||
| Anchoa tricolor | 19.54 | 0.52 | 1.64 | 42.1 ± 8.7 | ||||||||
| Eugerres brasilianus | 16.51 | 0.02 | 13.36 | 0.02 | 55.74 | 2.22 | 67.4 ± 33.5 | 119.0 | ||||
| Atherinella brasiliensis | 12.07 | 6.52 | 55.74 | 79.3 ± 36.6 | ||||||||
| Eucinostomus melanopterus | 8.80 | 3.37 | 31.15 | 59.1 ± 15.3 | ||||||||
| Eucinostomus argenteus | 8.71 | 3.91 | 15.26 | 4.04 | 60.66 | 35.56 | 104.4 ± 29.1 | 115.6 ± 24.3 | ||||
| Hyporhamphus unifasciatus | 5.81 | 28.81 | 26.23 | 248.6 ± 29.4 | ||||||||
| Anchoa januaria | 4.44 | 0.95 | 6.56 | 62.4 ± 9.8 | ||||||||
| Gobionellus oceanicus | 3.18 | 3.60 | 22.95 | 117.9 ± 40.2 | ||||||||
| Citharichthys arenaceus | 1.27 | 0.02 | 0.30 | 0.00 | 29.51 | 2.22 | 61.8 ± 14.4 | 89.0 | ||||
| Achirus lineatus | 0.97 | 0.55 | 0.41 | 0.68 | 42.62 | 17.78 | 54.7 ± 12.9 | 100.7 ± 23.4 | ||||
| Strongylura timucu | 0.94 | 5.29 | 39.34 | 323.3 ± 89.1 | ||||||||
| Caranx latus | 0.64 | 1.93 | 14.75 | 125.2 ± 15.8 | ||||||||
| Anchoa lyolepis | 0.48 | 7.59 | 0.07 | 1.22 | 6.56 | 26.67 | 56.1 ± 9.8 | 80.6 ± 11.4 | ||||
| Citharichthys spilopterus | 0.38 | 0.02 | 0.47 | 0.01 | 24.59 | 2.22 | 107.6 ± 21.5 | 102.0 | ||||
| Gobionellus boleosoma | 0.38 | 0.02 | 19.67 | 42 ± 5.6 | ||||||||
| Strongylura marina | 0.32 | 0.87 | 4.92 | 282.3 ± 48.9 | ||||||||
| Bathygobius soporator | 0.28 | 0.46 | 21.31 | 125.7 ± 83.2 | ||||||||
| Mugil curema | 0.25 | 0.72 | 9.84 | 107.3 ± 56.2 | ||||||||
| Genidens genidens | 0.17 | 0.17 | 0.17 | 0.83 | 4.92 | 13.33 | 88.9 ± 37.1 | 202 ± 48.6 | ||||
| Hyporhamphus roberti | 0.14 | 0.22 | 3.28 | 184.3 ± 15.6 | ||||||||
| Mugil sp. | 0.14 | 0.00 | 8.20 | 27.8 ± 3.7 | ||||||||
| Sphoeroides greeleyi | 0.13 | 0.17 | 6.56 | 88.4 ± 78.8 | ||||||||
| Mugil liza | 0.10 | 0.46 | 3.28 | 120.3 ± 79.1 | ||||||||
| Citharichthys macrops | 0.08 | 0.02 | 1.64 | 65.3 ± 19.7 | ||||||||
| Oligoplites saurus | 0.08 | 0.24 | 4.92 | 142.3 ± 80.3 | ||||||||
| Micropogonias furnieri | 0.05 | 3.22 | 0.03 | 1.93 | 4.92 | 57.78 | 87.3 ± 20.0 | 95.8 ± 34.5 | ||||
| Gobionellus stomatus | 0.05 | 0.02 | 6.56 | 98.8 ± 19.7 | ||||||||
| Oreochomis niloticus niloticus | 0.05 | 0.02 | 1.64 | 54.3 ± 2.5 | ||||||||
| Paralichthys orbignyanus | 0.05 | 0.03 | 4.92 | 79.8 ± 16.4 | ||||||||
| Paralichthys brasiliensis | 0.04 | 0.13 | 4.92 | 138.7 ± 36.1 | ||||||||
| Catathyridium garmani | 0.03 | 0.12 | 3.28 | 126 ± 21.2 | ||||||||
| Oligoplites saliens | 0.03 | 0.52 | 1.64 | 286.5 ± 33.2 | ||||||||
| Syngnathus folletti | 0.03 | 0.00 | 1.64 | 120.0 | ||||||||
| Eucinostomus gula | 0.01 | 2.22 | 0.05 | 3.71 | 1.64 | 31.11 | 146.0 | 130.5 ± 14.43 | ||||
| Chilomycterus spinosus spinosus | 0.01 | 2.00 | 0.31 | 5.41 | 1.64 | 37.78 | 157.0 | 76.2 ± 25.7 | ||||
| Symphurus tesselatus | 0.01 | 0.45 | 0.00 | 0.70 | 1.64 | 26.67 | 65.0 | 158.6 ± 29.4 | ||||
| Sphoeroides spengleri | 0.01 | 0.05 | 0.00 | 0.03 | 1.64 | 4.44 | 20.0 | 74.5 ± 0.7 | ||||
| Sphoeroides testudineus | 0.01 | 0.05 | 0.01 | 0.99 | 1.64 | 4.44 | 61.0 | 247.5 ± 20.5 | ||||
| Acanthistus brasilianus | 0.01 | 0.00 | 1.64 | 40.0 | ||||||||
| Archosargus probatocephalus | 0.01 | 0.25 | 1.64 | 203.0 | ||||||||
| Awaous tajasica | 0.01 | 0.00 | 1.64 | 79.0 | ||||||||
| Centropomus undecimalis | 0.01 | 0.43 | 1.64 | 345.0 | ||||||||
| Ctenosciaena gracillicirrhus | 18.95 | 8.46 | 46.67 | 88.9 ± 16.8 | ||||||||
| Paralonchurus brasiliensis | 18.56 | 22.79 | 77.78 | 134.6 ± 38.1 | ||||||||
| Larimus breviceps | 7.40 | 4.94 | 33.33 | 101.4 ± 19.2 | ||||||||
| Stellifer brasiliensis | 6.49 | 6.40 | 44.44 | 114.2 ± 30.6 | ||||||||
| Stellifer rastrifer | 5.08 | 5.11 | 55.56 | 107.4 ± 32.6 | ||||||||
| Pellona harroweri | 3.79 | 1.89 | 28.89 | 100.9 ± 9.7 | ||||||||
| Diapterus rhombeus | 3.41 | 7.43 | 44.44 | 141.8 ± 20.2 | ||||||||
| Cynoscion jamaicensis | 1.91 | 0.59 | 15.56 | 85.8 ± 15.2 | ||||||||
| Etropus crossotus | 1.79 | 1.56 | 46.67 | 117 ± 19.5 | ||||||||
| Prionotus punctatus | 1.60 | 0.49 | 60.00 | 73.7 ± 20.8 | ||||||||
| Selene setapinis | 1.24 | 0.39 | 33.33 | 75.7 ± 20.7 | ||||||||
| Haemulon steidacneri | 0.91 | 5.40 | 11.11 | 196.1 ± 24.3 | ||||||||
| Ophioscion punctatissimus | 0.88 | 1.64 | 15.56 | 141.1 ± 31.3 | ||||||||
| Trichiurus lepturus | 0.86 | 0.16 | 22.22 | 204.4 ± 58.8 | ||||||||
| Stellifer stellifer | 0.74 | 0.14 | 15.56 | 66.4 ± 18.3 | ||||||||
| Peprilus paru | 0.72 | 0.35 | 13.33 | 78 ± 21.9 | ||||||||
| Harengula clupeola | 0.62 | 0.99 | 24.44 | 140.1 ± 30.4 | ||||||||
| Menticirrhus americanus | 0.62 | 1.61 | 13.33 | 114.7 ± 38.7 | ||||||||
| Chirocentrodon bleekerianus | 0.60 | 0.13 | 22.22 | 89 ± 10.9 | ||||||||
| Isopisthus parvipinnis | 0.55 | 0.58 | 13.33 | 110.9 ± 4.2 | ||||||||
| Genidens barbus | 0.52 | 1.52 | 13.33 | 169.1 ± 34.2 | ||||||||
| Orthopristis rubber | 0.33 | 0.80 | 13.33 | 121.4 ± 64.4 | ||||||||
| Cynoscion striatus | 0.26 | 0.10 | 6.67 | 85.2 ± 13.38 | ||||||||
| Diplectrum radiale | 0.21 | 1.19 | 11.11 | 194.6 ± 18.5 | ||||||||
| Stellifer sp. | 0.19 | 0.07 | 4.44 | 89.8 ± 11.7 | ||||||||
| Polydactylus virginicus | 0.12 | 0.21 | 11.11 | 150.2 ± 37.2 | ||||||||
| Conodon nobilis | 0.10 | 0.16 | 8.89 | 134.8 ± 7.7 | ||||||||
| Dactylopterus volitans | 0.10 | 0.71 | 4.44 | 235 ± 52.5 | ||||||||
| Bardiella ronchus | 0.05 | 0.02 | 4.44 | 88 ± 25.45 | ||||||||
| Dasyatis say | 0.05 | 0.71 | 4.44 | 289 ± 206.4 | ||||||||
| Lagocephalus laevigatus | 0.05 | 0.03 | 4.44 | 101.0 | ||||||||
| Paralichthys patagonicus | 0.05 | 0.01 | 4.44 | 89 ± 21.2 | ||||||||
| Rhinobatos percellens | 0.05 | 0.76 | 4.44 | 451 ± 161.2 | ||||||||
| Syacium papillosum | 0.05 | 0.22 | 2.22 | 179 ± 52.3 | ||||||||
| Synodus foetens | 0.05 | 0.17 | 4.44 | 228 ± 36.7 | ||||||||
| Zapteryx brevirostris | 0.05 | 1.12 | 2.22 | 400.5 ± 7.7 | ||||||||
| Anisotremus surinamensis | 0.02 | 0.01 | 2.22 | 95.0 | ||||||||
| Chloroscombrus chrysurus | 0.02 | 0.05 | 2.22 | 165.0 | ||||||||
| Cynoscion acoupa | 0.02 | 0.01 | 2.22 | 113.0 | ||||||||
| Cynoscion microlepidotus | 0.02 | 0.00 | 2.22 | 47.0 | ||||||||
| Etropus longimanus | 0.02 | 0.00 | 2.22 | 68.0 | ||||||||
| Narcine brasiliensis | 0.02 | 0.16 | 2.22 | 207.0 | ||||||||
| Odontoscion dentex | 0.02 | 0.09 | 2.22 | 175.0 | ||||||||
| Porichthys porosissimus | 0.02 | 0.09 | 2.22 | 171.0 | ||||||||
| Rhinobatos horkelli | 0.02 | 0.07 | 2.22 | 259.0 | ||||||||
| Rypticus randali | 0.02 | 0.08 | 2.22 | 158.0 | ||||||||
| Sardinella brasiliensis | 0.02 | 0.02 | 2.22 | 133.0 | ||||||||
| Scorpaena isthmensis | 0.02 | 0.07 | 2.22 | 129.0 | ||||||||
| Selene vomer | 0.02 | 0.02 | 2.22 | 98.0 | ||||||||
| Symphurus plagusia | 0.02 | 0.01 | 2.22 | 119.0 | ||||||||
| Umbrina coroides | 0.02 | 0.08 | 2.22 | 175.0 | ||||||||
| Total | 2265 | 7866 | 4191 | 3193.45 | 65,231.68 | 77,614.74 | ||||||
| Number of species | 18 | 50 | 66 | |||||||||
The family Sciaenidae had the largest number of species (n = 18), followed by Paralichthyidae (9), Gobiidae (7), Carangidae (6) and Gerreidae (5). The families with the highest number of individuals in the upper estuary were Eleotridae (74.5% of the total number of individuals), Characidae (10.4%), Syngnathidae (10.3%) and Cichlidae (1.8%). In the middle estuary, Gerreidae (34% of the total number), Engraulidae (24.5%), Atherinopsidae (12.1%), Gobiidae (7.6%) and Achiridae (7.1%) were the most abundant families. Sciaenidae (64.9%), Gerreidae (9.6%) and Engraulidae (7.6%) were the most abundant families in the lower estuary.
The species that accounted for the majority (>75%) of the total number of individuals in each estuarine zone were: Dormitator maculatus, Astyanax sp. and Microphis brachyurus lineatus in the upper estuary, comprising 93.4% of the total number of individuals; Anchoa tricolor, Eugerres brasilianus, Atherinella brasiliensis, Eucinostomus melanopterus, Eucinostomus argenteus, Trinectes paulistanus and Hyporhamphus unifasciatus in the middle estuary (77.5%); and Ctenosciaena gracillicirrhus, Paralonchurus brasiliensis, Anchoa lyolepis, Larimus breviceps, Stellifer brasiliensis, Stellifer rastrifer, Eucinostomus argenteus, Pellona harroweri and Diapterus rhombeus in the lower estuary (75.2%). The most abundant species common to two estuarine zones were E. argenteus [lower (3.9%) and middle estuaries (8.7%)] and Geophagus brasiliensis [middle (1.8%) and upper estuaries (1.8%)]. There was a low abundance of remaining species (<1% of the total number) in at least a single estuarine zone.
The average total length of the most abundant species was 31.4 (Astyanax sp.) to 119.8 mm (Microphis brachyurus lineatus) in the upper estuary, 42.1 (A. tricolor) to 248.6 mm (Hyporhamphus unifasciatus) in the middle estuary, and 73.7 (Prionotus punctatus) to 141.8 mm (D. rhombeus) in the lower estuary (Table 3). Overall, the largest individuals were generally found in the lower estuary (median = 102 mm) followed by the middle (median = 66 mm), with the lowest sizes recorded in the upper estuary (median = 43 mm).
Significant differences existed for the total length of all measured fish, according to the median test (P < 0.01). In the upper estuary, higher median values were found at U2 (47 mm) than at U1 (32 mm). Significantly higher median values were found in M2 and M3 of the middle estuary (106 mm) compared with M1 (58 mm). No significant differences for total length were found in the lower estuary between L1 (102 mm) and L2 (101 mm).
Temporal and spatial patterns
Distinct fish assemblages were found for each estuarine zone based on the densities of all collected fish, according to MDS ordination (Fig. 2). Samples from sites in the middle estuary are separated in the ordination diagram. Sites for both the upper and the middle estuaries are clustered in opposite parts of the diagram.
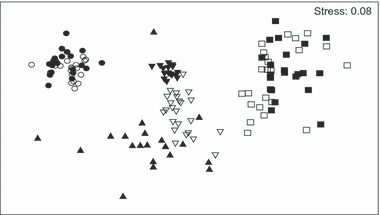
MDS ordination plot of the relationship between fish community and estuarine sites: empty circle = U2; full circle = U1; full inverted triangle = M1; empty inverted triangle = M2; full triangle = M3; empty square = L1; full square = L2.
Significant differences existed for assemblages between the three estuarine zones according to ANOSIM results (R global 0.825; P < 0.001), with each pair of comparisons differing significantly (lower versus middle R = 0.735; lower versus upper, R = 0.962 and middle versus upper, R = 0.789). The average similarity was higher (>50%) for U1, U2 and M1, suggesting a great number of constant species (Table 4). Dormitator maculatus, M. brachyurus lineatus and Astyanax sp. were identified as the discriminating species for U1 and U2, P. brasiliensis, S. rastrifer and D. rhombeus, for L1 and P. brasiliensis and M. furnieri for L2. The assemblage was more diverse in M1 and L1, with 11 and 13 discriminate species, respectively, as identified by SIMPER analysis. At M2 and M3, the assemblages were highly dominated by E. argenteus and A. brasiliensis, which were responsible for 41.1 and 64.2% of the similarity within each site, respectively. Trinectes paulistanus and E. brasilianus contributed the most to the similarity at M1 (Table 4).
| Average similarity (%) | L2 (33.20) | L1 (26.39) | M3 (19.66) | M2 (38.70) | M1 (60.34) | U2 (56.08) | U1 (53.83) |
|---|---|---|---|---|---|---|---|
| Contribution (%) | |||||||
| Paralonchurus brasiliensis | 28.28 | 12.15 | |||||
| Stellifer rastrifer | 4.49 | 16.16 | |||||
| Micropogonias furnieri | 11.30 | 5.93 | |||||
| Prionotus punctatus | 8.49 | 8.24 | |||||
| Ctenosciaena gracillicirrhus | 8.10 | 3.30 | |||||
| Etropus crossotus | 4.69 | 4.98 | |||||
| Sardinella brasiliensis | 5.90 | 3.60 | |||||
| Pellona harroweri | 3.94 | ||||||
| Chilomycterus spinosus spinosus | 3.13 | 2.71 | |||||
| Diapterus rhombeus | 1.43 | 9.28 | |||||
| Eucinostomus argenteus | 5.84 | 41.12 | 7.76 | ||||
| Eucinostomus gula | 4.60 | ||||||
| Larimus breviceps | 3.51 | ||||||
| Trinectes paulistanus | 3.10 | 13.91 | |||||
| Atherinella brasiliensis | 64.17 | 17.72 | |||||
| Bathygobius soporator | 7.19 | ||||||
| Hyporhamphus unifasciatus | 6.46 | 7.34 | |||||
| Strongylura timucu | 4.50 | 6.75 | |||||
| Mugil curema | 4.07 | ||||||
| Eugerres brasilianus | 3.07 | 11.90 | 14.67 | ||||
| Geophagus brasiliensis | 6.87 | 9.62 | 5.76 | ||||
| Gobionellus shufeldti | 9.94 | ||||||
| Gobionellus oceanicus | 7.74 | ||||||
| Citharichthys arenaceus | 7.42 | ||||||
| Citharichthys spilopterus | 6.21 | ||||||
| Achirus lineatus | 5.78 | ||||||
| Eucinostomus melanopterus | 4.85 | ||||||
| Centropomus parallelus | 3.58 | ||||||
| Dormitator maculatus | 56.38 | 39.65 | |||||
| Microphis brachyurus lineatus | 20.35 | 26.39 | |||||
| Astyanax sp. | 8.33 | 21.41 | |||||
| Eleotris pisonis | 4.62 | ||||||
Significant differences occurred in assemblage structure between seasons (P < 0.05) in the lower and upper estuaries, although groups were not clearly separated (R < 0.500). In the upper estuary, the highest differences were found between spring and the remaining seasons (R > 0.348) due to the absence of Astyanax sp. in spring and its presence in the other seasons, with a contribution to similarity >15%. Significant differences also existed in the lower estuary between spring and the other seasons (R > 0.404) due to highest contribution to similarity by E. gula (18.0%). Ctenosciaena gracillicirrhus (10.9%) and E. argenteus (10.2%) in spring, P. brasiliensis (16.1%) in summer, M. furnieri (18.2%) and P. brasiliensis (13.8%) in autumn, and P. brasiliensis (28.8%) and S. rastrifer (13.8%) in winter represented the greatest contribution to within-estuary similarities. Differences in fish assemblage were also found between summer and winter (R = 0.442) due to the high similarity contribution by Etropus crossotus (10.6%) and P. punctatus (11.9%) in summer and the contribution of less frequent species such as Cynoscion jamaicensis, Trichiurus lepturus and Menticirrhus americanus in winter. No seasonal differences were found for the middle estuary in the structure of fish assemblage (P > 0.05), with E. argenteus, E. brasilianus, A. brasiliensis and A. lineatus present throughout the year.
The density of dominant species differed among the three estuarine zones (Fig. 3). The upper estuary was dominated by Dormitator maculatus, Astyanax sp. and M. brachyurus lineatus, which were limited to this zone. Species with the highest densities in the middle estuary were members of the Gerreidae (E. melanopterus, E. brasilianus and E. argenteus) and the Achiridae (A. lineatus and Trinectes paulistanus). Paralonchurus brasiliensis, C. gracillicirrhus and L. breviceps were dominant in the lower estuary and collected exclusively in this estuarine zone (Fig. 3).
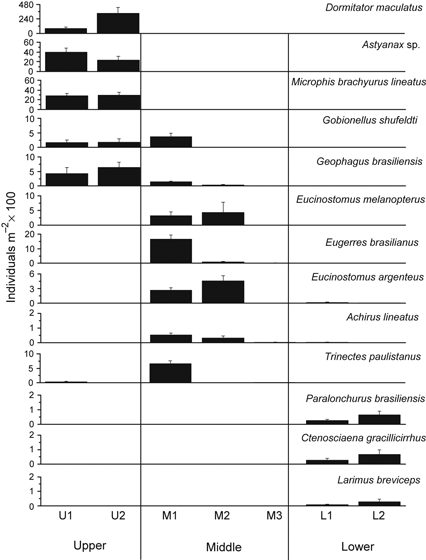
Density of selected species along the upper middle and lower estuarine zones.
Influence of environmental variables
The first two axes from canonical correspondence analysis accounted for 93.7% of the cumulative percentage of variance for the environmental–species relationship. Monte Carlo analysis revealed that salinity contributed most to species distribution, followed by temperature. Axis 1 was positively correlated with salinity and, to a lesser degree, with temperature, as well as inversely correlated with turbidity and dissolved oxygen. Axis 2 showed a negative correlation with turbidity and dissolved oxygen. Salinity drove most of the observed variation in assemblage structure and was directly associated with species from the lower estuary, such as E. gula, D. rhombeus, C. gracillicirrhus, E. crossotus, P. brasiliensis and S. rastrifer, in contrast to species from the upper estuary, such as D. maculatus, G. brasiliensis, Astyanax sp., E. pisonis and M. brachyurus lineatus. The sites M2 and M3 in the middle estuary were characterised by the highest temperatures and directly related to A. brasiliensis, E. argenteus and E. brasilianus, while M1, the protected adjacent lagoon in the middle estuary where these three species were abundant, was characterised by the highest turbidity and dissolved oxygen (Fig. 4).
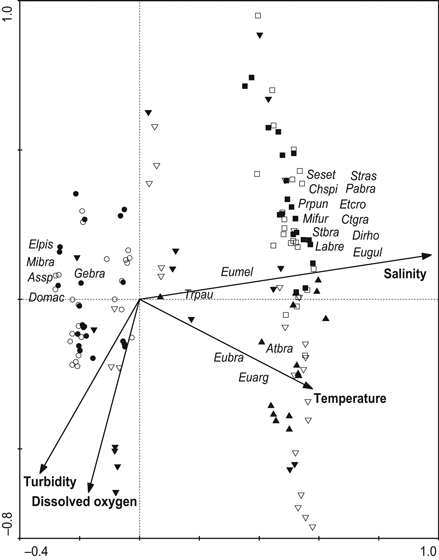
Ordination diagram from canonical correspondence analysis on density of fish species and environmental variables. Samples coded by sites as follows: empty circle = U2; full circle = U1; full inverted triangle = M1; empty inverted triangle = M2; full triangle = M3; empty square = L1; full square = L2. Species code: Domac = Dormitator maculatus; Assp = Astyanax sp.; Mibra = Microphis brachyurus lineatus; Gebra = Geophagus brasiliensis; Elpis = Eleotris pisonis; Eubra = Eugerres brasilianus; Atbra = Atherinella brasiliensis; Eumel = Eucinostomus melanopterus; Euarg = Eucinostomus argenteus; Trpau = Trinectes paulistanus; Ctgra = Ctenosciaena gracillicirrhus; Pabra = Paralonchurus brasiliensis; Labre = Larimus breviceps; Stbra = Stellifer brasiliensis; Stras = Stellifer rastrifer; Dirho = Diapterus rhombeus; Mifur = Micropogonias furnieri; Eugul = Eucinostomus gula; Chspi = Chilomycterus spinosus spinosus; Etcro = Etropus crossotus; Prpun = Prionotus punctatus and Seset = Selene setapinis.
Discussion
Three distinct fish assemblages were detected, each corresponding to an estuarine zone, indicating a lack of or reduced connectivity among the zones in this small open tropical estuary. As expected, the highest differences in assemblage were found between the upper and the lower estuaries. Despite its distinct fish assemblage, the middle estuary had 14 species in common with the lower estuary and eight species in common with the upper estuary. Only Trinectes paulistanus occurred in all the three zones. Of the most common species, only Eucinostomus argenteus (middle and lower estuaries) and Geophagus brasiliensis (middle and upper estuaries) contributed to more than 1% of total number of individuals in each zone. Differences in fish composition and structure among the estuarine zones can be attributed, at least partially, to the large variability in environmental conditions in the middle estuary. In particular, salinity acts as a barrier for both freshwater and marine species. During the sampling period, we observed regular changes in salinity in the middle estuary as a result of flood tides ranging from freshwater (salinity ≅ 0.1 psu) to approximately 25 psu in <6 h. Such large shifts in salinity can limit species distribution, resulting in different assemblages.
Different species composition among estuarine zones has been reported for large tropical estuaries (Barletta et al. 2005; Paiva et al. 2008). In these systems, a comparatively higher number of species share different estuarine zones compared with the Mambucaba estuary, where few species use more than one zone. Unfortunately, there is a lack of information on assemblage structure along the longitudinal salinity gradient for small tropical estuaries. Assemblages varied in the present study even in summer, when differences in the average salinity were lowest between the upper estuary (<0.1 psu) and the protected lagoon (0.2 psu) in the middle estuary, and in winter, when differences between the middle (26.2–29.4 psu) and lower estuaries (34.0–34.3 psu) were smallest.
Changes in species distribution between the estuarine zones are more evident in open estuaries than in other coastal areas, such as bays. The dynamics of environmental conditions in estuaries are greater than those observed in bays, where the salinity gradient is low. Within estuaries, the substrate is predominantly muddy and turbidity is increased. In bays, the wide connection to the sea enables species to distribute across a comparatively larger area, with changes in assemblage structure occurring less frequently. However, the narrow (20–40 m) and shallow (1–2 m) boundary between the middle and lower zone of the Mambucaba estuary contributes to differences in fish assemblages between these two zones. Even at the highest tides, the estuary mouth width on the main channel does not exceed 10 m and could act as a spatial filter for fish species, limiting their distribution. According to Horn & Allen (1976), estuary mouth width is the only significant predictor for the number of species, whereas Monaco et al. (1992) reported that the mouth depth is the best predictor. However, Pease (1999) found that both mouth depth and width are good predictors for estuarine fish richness. Besides the mouth width and depth, other physical features such as sandbanks can limit fish species distribution. Barletta-Bergan et al. (2002) reported that sandbanks formed in the estuarine mouth may impair egg production and larvae recruitment. In the Mambucaba estuary, sandbanks are common and can restrict juveniles from distributing, as seen with the Mugilidae and Sciaenidae. These families are dominant in middle zones of tropical estuaries (Chao et al. 1985; Pessanha & Araújo 2003; Vieira et al. 2008) but rare in the middle Mambucaba estuary.
An increased richness was observed along the estuarine gradient from the upper estuary (18 species) to the middle (50 species) and the lower estuary (66 species) and this coincided with increased salinity (upper <1.5, middle 0.2–31, lower >30 psu). Estuaries tend to have more species at the lower reaches than those at the upper reaches (Whitfield 1999; Akin et al. 2003; Martino & Able 2003). Greater numbers of species in the lower reaches have been linked to the prevalent marine conditions (Maes et al. 1998; Martino & Able 2003; Vega-Cendejas & de Santillana 2004). The majority of freshwater species are restricted to areas with mean annual salinities of <5 psu (Bulger et al. 1993; Wagner 1999). As in many other studies (Thiel et al. 1995; Maes et al. 1998; Marshall & Elliott 1998; Selleslagh & Amara 2008) salinity is the dominant factor influencing the distribution of fish. The influence of salinity on fish is often due to the tolerance and preference of species for this variable (Elliott et al. 1990). We also believe that salinity is the driving force determining the observed patterns in increased species richness in the lower estuary. On the other hand, the highest mean density and biomass of fish was recorded in the upper estuary. However, the use of different fishing techniques employed in each estuarine zone may have an effect on fish densities and biomass, even after standardisation, increasing the risk of confounding results.
Fish assemblage in the lower estuary was dominated by species of Sciaenidae such as Ctenosciaena gracillicirrhus and Paralonchurus brasiliensis, which are associated with shallow areas of the inner coastal shelf (Muto et al. 2000; Chaves et al. 2003) and outer bay zones (Araújo et al. 2006; Azevedo et al. 2007), where environmental conditions are more stable with slight changes observed across seasons (mean salinity >31 psu; mean turbidity <6.2 NTU). The middle estuary was dominated by Eugerres brasilianus, Eucinostomus melanopterus and E. argenteus (Gerreidae), followed by T. paulistanus (Achiridae), species that are associated with the relatively harsh and varying environmental conditions of middle estuarine zones. This pattern suggests that a few changes in the environmental conditions in deep water layers in the lower estuary generate similar characteristics of the inner coastal shelf. As a result, species found in the Ariidae family, a group of dominant fishes in estuarine systems associated with muddy substrate, occur in low abundance in the lower estuary. Although muddy substrate can be found in the middle estuary, the scarcity of these semi-anadromous marine catfishes can be explained by the lack of influence of the estuarine plume on the adjacent coastal area not attracting this species to use the area as spawning grounds.
Seasonal changes in salinity in estuaries are a main predictor of fish movements toward the inner and outer estuaries (Whitfield & Kok 1992; Valesini et al. 1997). During the wet season, floods decrease salinity and enable freshwater species to visit the estuarine areas, while marine stragglers leave the area to search for more stable salinity levels (Garcia & Vieira 2001). In the present study, only small changes in salinity occurred in the upper estuary (0.1–1.9 psu), characterising this zone as the upper limit of the tidal influence. It is therefore reasonable to suppose that small seasonal changes in salinity minimally influence changes in fish assemblage. Seasonal differences in salinity within the middle estuary were restricted to the protected adjacent lagoon and were irrelevant in the lower estuary. Seasonal changes in fish assemblage were limited to changes in occurrence of a few species, such as the absence of Astyanax sp. during spring in the upper zone, the dominance of Eucinostomus gula and C. gracillicirrhus during spring and Micropogonias furnieri in autumn in the lower zone. Variables other than salinity may influence seasonal variation in these fish species. Such shifts could be linked to processes of fish life history associated with reproductive seasons and recruitment (Robert et al. 2007; Mendoza-Carranza & Vieira 2008; Sánchez-Gil et al. 2008). In the middle estuary, the lack of seasonal change in fish assemblages can be related to resident species E. argenteus, E. brasilianus, Atherinella brasiliensis and Achirus lineatus, which are dominant, have long recruitment seasons with batch spawning, and tolerate a broad range of environmental conditions.
The protected sidewater lagoon (M1) seems to be a preferred habitat for the majority of the dominant species in the middle estuary (E. brasilianus, E. melanopterus, T. paulistanus, Gobionellus shufeldti, Gobionellus oceanicus, G. brasiliensis, Centropomus parallelus and Citharichthys arenaceus) and featured higher levels of similarity than M2 and M3. The lagoon also had the highest mean richness compared to all the other examined sites and was colonised by the smallest fish (median = 58 mm) in the middle estuary, suggesting the importance of this kind of habitat as nursery grounds. Overall, sidewater lagoons in estuarine areas were found to have abundant juvenile fishes and to serve as rearing grounds (Sindilariu et al. 2006). Continuous recruitment for fishes is caused by the presence of shallow sheltered areas, their permanent connection to the main channel and association with muddy substrate and high habitat heterogeneity. Marginal lagoons and artificial secondary channels have been built as mechanisms to help the system re-establish lateral connectivity and to create areas with more stable conditions and resources as a way to mitigate the effects of margin degradation and previous channelisation (Van Den Brink et al. 1996; Buijse et al. 2002). While these steps are important, the preservation of natural riparian vegetation such as mangroves and marshes must also be considered.
The fish assemblage that uses the upper estuary margins was characterised by species adapted to tropical areas between the lower river reaches and upper estuarine zones, as indicated by the highest densities of the families Eleotridae (Dormitator maculatus and Eleotris pisonis) and Syngnathidae (Microphis brachyurus lineatus) that are common to this transition environment (Teixeira 1994; Miranda-Marure et al. 2004). The low salinities (<1.5 psu) year round seem to be preferred by these species. Furthermore, riparian cover and vegetated margins, common in this part of the estuary, enable the occurrence of fishes in the high densities, observed in this study. While studying the ecology of the Eleotridae family in Central American coastal streams, Winemiller & Ponwith (1998) reported that D. maculatus and E. pisonis were most commonly captured from the root masses of dense beds of floating aquatic macrophytes and leaf litter packs. Vegetated margins in upper estuaries seem to play an important role in structuring typical assemblages within this zone. Although the vegetation cover in the upper Mambucaba estuary mainly comprised grass, as the original cover has been removed by anthropogenic activities, the typical assemblage in this part of the estuary indicates its importance in structuring the community. According to França et al. (2009), vegetated areas typically support high densities of fish and invertebrates regardless of the type of vegetation.
Canonical correspondence analysis revealed that temperature, turbidity and dissolved oxygen also drove part of the observed variation in assemblage structure. Lower temperatures in the upper zone contrasted with higher temperatures in the middle and lower zones seem to affect the typical assemblages of these zones. The lowest values of turbidity and dissolved oxygen appear to be associated with lower zones, whereas the highest values occur in the upper and middle zones. This trend confirms the expectation of higher turbidity in the upper and middle estuaries and coincides with the turbulence of these areas, particularly the middle estuary due to high nutrient loads from both continental drainage and flooding tides. The high turbidity levels are generally considered important in supporting nursery grounds for fishes and mobile invertebrates (Robertson & Blaber 1992) because visual predators are less effective in turbid waters (Blaber & Blaber 1980). Changes in turbidity levels, however, were also frequently correlated with changes in other abiotic factors such as season, substratum and salinity, making it difficult to distinguish causal factors. Nonetheless, the narrow turbidity range for the Mambucaba estuary (0.02–15.7 NTU) characterises this system as having predominantly clear waters where turbidity plays a minor role in fish distribution.
The observations reported in this study appear to indicate that fish assemblages along the longitudinal gradient (upper – lower estuary) were determined by the response of individual species to the dominant environmental gradient, mainly salinity. According to Martino & Able (2003), large-scale (10 km) patterns in the structure of estuarine fish assemblages are primarily a result of species responses to environmental gradients, whereas smaller scale (1 km) patterns appear to be the result of habitat associations that are most likely driven by habitat selection, competition, and/or predator avoidance strategies.
In the present study, we found distinct fish assemblages for each of three estuarine zones of the Mambucaba estuary and the overall species richness was relatively high (111 species). Differences in physical characteristics among the estuarine zones, such as embedded shelters, depth and width of the sites prevented the use of a single sampling method to search the whole estuarine gradient. Because trawling was limited to areas free of obstacles such as large wood debris, snags, emerged macrophytes and stones, seine and sieves were used as alternative sampling methods in the middle and upper estuaries, respectively. These methods were suitable to catch a wide size range of individuals. In the middle and lower estuaries, we sampled individuals of similar size range (middle 12–521 mm total length, lower 33–565 mm). In the upper estuary, the smaller range (14–188 mm) may reflect an assemblage comprising comparatively smaller sized individuals mainly found among the river margins. The use of different sampling methods to obtain more comprehensive information on the ichthyofauna has been recommended to overcome problems associated with habitat heterogeneity (Whitfield & Marais 1999; Sindilariu et al. 2006; Selleslagh & Amara 2008). This small open estuary had different fish assemblages in each zone, with differences between lower and middle zones being attributed to the high dynamics of the latter associated to the narrow between-zone connection. A more distinct assemblage was found in the upper estuary adapted to the upper limit of tidal influence and using the vegetated margins as shelter. Although we sampled the whole salinity gradient in this estuarine system, further investigation is needed to obtain a holistic picture of such dynamic environments.
Acknowledgements
We thank Alexandre Araújo, André Luiz Balbino dos Santos, Hamilton Hissa Pereira and Taynara Pontes Franco for their help in the field work. We are particularly grateful to Aurea Maria de Oliveira Teixeira for invaluable laboratory activities. This study was partially financed by CNPq – Brazilian National Counsel for Research Development (Proc. 474813-03-7).




