Loggerhead sea turtles (Caretta caretta) as bioturbators in neritic habitats: an insight through the analysis of benthic molluscs in the diet
Abstract
Molluscs are a diverse and ubiquitous group of organisms which contribute to the formation of biogenic sediments and are one of the major prey taxa for the neritic-stage loggerhead sea turtles (Caretta caretta) worldwide. Here we investigated to what degree molluscs contribute to the diet of individual turtles, and what role the feeding strategy of loggerheads might play in bioturbation, one of the key processes in nutrient transport in marine ecosystems. We performed a detailed analysis of benthic molluscs from the digestive tracts of 62 loggerhead sea turtles (curved carapace length: 25.0–85.4 cm) found in the Northern Adriatic Sea. From 50 of the turtles that contained benthic molluscs, we identified 87 species representing 40 families and three classes (Gastropoda, Bivalvia and Scaphopoda), including 72 new dietary records for loggerhead turtle. Most of the identified molluscs were small-sized species (shell length ≤ 3 cm) and were often found in a subfossil condition. Their intake may be considered a byproduct of infaunal mining, while larger molluscs were mainly found crushed into smaller fragments. Through such foraging behaviour loggerheads actively rework sediments, increase the surface area of shells and the rate of shells disintegration, acting as bioturbators in this system. We conservatively estimate that loggerheads in the neritic zone of the Adriatic Sea bioturbate about 33 tonnes of mollusc shells per year, and hypothesize about the possible effects of bioturbation reduction on environmental changes in the Northern Adriatic ecosystem.
Problem
The issue of the present and past roles of sea turtles in ecosystems is underlined as one of the global research priorities for sea turtle management and conservation in the 21st century (Hamann et al. 2010). Sea turtles act at multiple levels, as predators, prey, competitors, substrate for epibionts, hosts of parasites and pathogens, nutrient transporters and modifiers of habitats (Bjorndal 2003; Bjorndal & Jackson 2003). Knowledge on the role of sea turtles in the ecosystems they utilize is necessary for our ability to predict how natural and anthropogenic-driven environmental changes can affect their populations in order to make informed management decisions (Bjorndal 2003).
Loggerhead sea turtle, Caretta caretta (Linnaeus, 1758) is an endangered (IUCN 2009), large, long-lived top predator in marine ecosystems, with a complex life history characterised by switching between different habitats (Bolten 2003; Casale et al. 2008a) and shifts in trophic status and ecological roles (Bjorndal 2003). Although the species is carnivorous generalist, exhibiting differences in the diet composition between populations and regions (Bjorndal 1997), molluscs represent one of the major prey groups for the neritic-stage loggerheads worldwide (Dodd 1988; Laurent & Lescure 1994; Limpus & Limpus 2003; Lazar et al. 2006; Casale et al. 2008a).
Molluscs are diverse and ubiquitous organisms with important roles in ecosystems, such as biodeposition, predation, boring, grazing, suspension and deposit feeding. Nearly all molluscs produce shells which mainly consist of calcium carbonate (Gutiérrez et al. 2003). The shell remains stable on the sea floor after the animal dies, forming biogenic sediments together with the skeletal remains of other marine species (Kidwell 1985; de Bruyne 2004). Biological reworking of sediments by different organisms, from microbes and rooting plants to burrowing animals, is termed bioturbation (Meysman et al. 2006). These organisms–sediment interactions, which structure the subsurface of both terrestrial and marine ecosystems and play a major role in biogeochemical processes, are considered to be at least as important as the trophic interactions classically studied by ecologists (Reise 2002). Modification of the sediment texture, bio-irrigation and dispersal of solid particles are major biogeochemical implications of bioturbation, which affect transport of nutrients in the marine ecosystems and formation of seascapes; however, the actual mechanisms behind bioturbation are less established (Meysman et al. 2006).
The present study focuses on the bioturbating role of loggerhead turtles in the neritic foraging habitats of the Northern Adriatic Sea based upon detailed qualitative and quantitative analysis of benthic molluscs in the diet of this sea turtle species. We selected benthic molluscs for two specific reasons. First, the majority of carbonates in Adriatic sediments originate from shell and skeletal fragments (Vdović & Juračić 1993). Secondly, a recent study on the feeding ecology of loggerhead turtles in the Adriatic Sea emphasized molluscs as the major prey group in their diet, accounting for 41.1% of dry mass (Lazar 2009). The aim of this study was therefore to investigate to what degree a dietary regime largely based upon molluscs, together with loggerhead feeding behaviour in neritic areas (Preen 1996; Houghton et al. 2000; Schofield et al. 2006), may contribute to the individual intake (energy gain by feeding) and what is the possible role of such feeding strategy in bioturbation.
Study Area
The Adriatic Sea is a relatively shallow (mean depth: 239 m), temperate, semiclosed sea, with the continental shelf (<200 m in depth) covering about 74% of the surface area (Fig. 1). Its northern part is <100 m in depth and under permanent influx of fresh water, mainly arriving from the Po River (Cushman-Roisin et al. 2001). We carried out the present study in the Northern Adriatic Sea, which hosts one of the largest neritic foraging habitats for loggerhead turtles in the Mediterranean (Lazar & Tvrtković 2003; Margaritoulis et al. 2003; Lazar et al. 2004). The Northern Adriatic bottom consists of three sediment types: sand (mean grain size: 360 μm), mud (mean grain size: 70 μm) and areas with a mixture of these two sediment types (Vatova 1949; Fedra et al. 1976). The majority of carbonates in the sediments are of biogenic origin (Vdović & Juračić 1993).
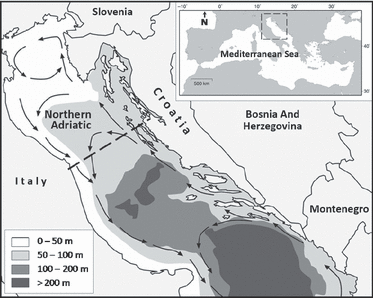
Study area of the Northern Adriatic Sea (northward from the dashed line), with bathymetry and direction of major sea currents.
So far, no complete list of marine mollusc species exists for the Adriatic Sea. However, several studies which partially described the distribution of benthic marine flora and fauna (e.g.Vatova 1935, 1949; Zavodnik 1971; Hrs-Brenko et al. 1994; Jaklin & Arko-Pijevac 1997) defined the Northern Adriatic Sea as an area with high macrofaunal density, including a high diversity of molluscs (Vatova 1949; Gamulin-Brida 1967; Scaccini 1967; Fedra et al. 1976; Zavodnik & Vidaković 1987; McKinney 2007).
Material and Methods
We performed general necropsies of 62 loggerhead sea turtles with notch-tip curved carapace length (CCL) ranging between 25.0 and 85.4 cm (mean CCL = 45.1 ± 14.3 cm), found stranded or incidentally captured dead by fisheries in the Northern Adriatic Sea (Slovenia and Croatia) in 1995–2004. We isolated the oesophagus, stomach and intestinal tracts, rinsed gut contents in clear water through a 1-mm mesh sieve, and preserved samples in 4% buffered formaldehyde. All benthic mollusc shells were isolated and identified under a stereomicroscope to the lowest taxon possible. The taxonomic nomenclature used follows the European Register of Marine Species (ERMS, Costello et al. 2008). We counted ingested specimens when possible, either based upon the number of whole shells or upon the number of apertures (in the case of crushed shells of gastropods) and calculated the percent of occurrence for each species.
To differentiate possibly targeted (primary) prey from incidentally eaten items, we compared the shell size of identified molluscs. Due to the low percentage of organic content present in their shells (0.1–5%; Marin et al. 2008), we argue that ingestion of small-sized molluscs would, consequently, have a very small effect on the individual energy gain. Hence, we arbitrarily grouped molluscs in two groups, the larger-sized species (shell length > 3 cm) and the small-sized species (shell length ≤ 3 cm), and compared the occurrence of these two groups in the loggerhead diet. Approximate shell sizes, distribution depths and species habitats have been taken from the literature (Nordsieck 1968, 1969; Riedl 1970; Milišić 1991; Poppe & Goto 1993a,b; Zavodnik & Šimunović 1997). We also checked for new dietary records in loggerheads by comparing the identified species from this study with data from the literature (Dodd 1988; Laurent & Lescure 1994; Godley et al. 1997; Houghton et al. 2000; Frick et al. 2001; Tomás et al. 2001; Parker et al. 2005; Revelles et al. 2007; Seney & Musick 2007; Casale et al. 2008a), bearing in mind synonyms and non valid species names.
The bioturbating role of loggerhead turtles was quantified by estimating the total mass of ingested (bioturbated) mollusc shells per year within 102,000 km2 of neritic area of the Adriatic Sea. We based our calculations on a mean density of 16.15 turtles per 100 km2 as recorded in the Northern Adriatic waters (Casale et al. 2004), a mean dry mass of 32.9 g of mollusc shells found per turtle (Lazar 2009), and a digestion time for benthic prey of 3 days (Casale et al. 2008b), assuming (i) uniform density of turtles across the whole neritic area of Adriatic, (ii) replacement of the whole gut content every 3 days and (iii) a foraging period of only six warm months of the year when favourable sea temperatures enable active feeding (Lazar et al. 2003).
Results
The digestive tracts of seven of the 62 loggerheads analysed were empty or contained only small amounts of completely digested and unidentifiable organic items. Of the remaining 55 turtles, 50 (91%) had ingested benthic molluscs. In total, we found 726 mollusc shells of three classes: Gastropoda (601 specimens), Bivalvia (123 specimens) and Scaphopoda (2 specimens). The average number of shells per individual turtle was 14.5 ± 24.1, and the maximum number of molluscs was 132, isolated from a turtle of 70.0 cm CCL.
We have identified 91 taxa of benthic molluscs, including 87 species representing 40 families, and recorded 72 new species in the diet of loggerhead turtles: 37 gastropods, 34 bivalves and one scaphopod. Most of the identified species belonged to gastropods (50 species, 57.5%), followed by bivalves (36 species, 41.4%). Scaphopods were represented by Antalis dentalis only. Identified taxa, their frequency of occurrence and the total number of individuals are listed in Table 1. In terms of numbers, Gibulla magus was the most abundant mollusc in the diet, followed by Nassarius incrassatus, Corbula gibba and Natica stercusmuscarum, while the remaining 83 species were detected with small numbers of individuals. When the frequency of occurrence is considered, C. gibba, Bittium reticulatum, Turritella communis and G. magus were the species most frequently found (Table 1). Overall, 10 gastropod species and four bivalves were found with an occurrence frequency >10%.
| Family/taxa | Shell length (mm) | Depth range (m) | Substrate type | Occurrence (%) | Total no. (min–max per turtle) |
|---|---|---|---|---|---|
| Gastropoda | |||||
| Acteonidae | |||||
| Acteon tornatilis (Linnaeus, 1758)* | 25 | wd | s | 2.0 | Frag |
| Aporrhaidae | |||||
| Aporrhais pespelecani (Linnaeus, 1758) | 65 | 10–55 | m, s | 12.0 | 18 (Frag-16) |
| Buccinidae | |||||
| Pollia dorbignyi (Payraudeau, 1826)* | 20 | wd | r | 2.0 | Frag |
| Calliostomatidae | |||||
| Calliostoma granulatum (von Born, 1778)* | 19 | 15–80 | m, s | 2.0 | Frag |
| Calliostoma sp. | 8.0 | Frag | |||
| Calyptraeidae | |||||
| Calyptraea chinensis (Linnaeus, 1758)* | 22 | 0–70 | m, s | 2.0 | Frag |
| Crepidula gibbosa Defrance, 1818 * | 50 | wd | m | 6.0 | 7 (1–4) |
| Crepidula unguiformis Lamarck, 1822 | 27 | wd | m, r | 2.0 | 1 |
| Cerithiidae | |||||
| Bittium reticulatum (da Costa, 1778)* | 8–15 | wd | p | 54.0 | 38 (Frag-12) |
| Cerithiopsidae | |||||
| Cerithiopsis tubercularis (Montagu, 1803)* | 9 | wd | r | 6.0 | 11 (2–6) |
| Conidae | |||||
| Comarmondia gracilis (Montagu, 1803) | 25 | 7–150 | g, r | 2.0 | 1 |
| Mangelia attenuata (Montagu, 1803)* | 14 | wd | r | 2.0 | 1 |
| Mangelia multilineolata (Deshayes, 1835)* | 10 | wd | r | 4.0 | 2 |
| Mangelia paciniana (Calcara, 1839)* | 8 | wd | r | 8.0 | 4 (Frag-2) |
| Mangelia unifasciata (Deshayes, 1835)* | 6 | – | m | 2.0 | Frag |
| Mangelia sp. | 10.0 | 1 (Frag-1) | |||
| Mangeliidae gen. sp. | 2.0 | 1 | |||
| Raphitoma histrix Bellardi, 1847* | – | – | – | 2.0 | 1 |
| Raphitoma sp. | 2.0 | 1 | |||
| Coralliophilidae | |||||
| Coralliophila sp.* | 6.0 | 3 (1) | |||
| Cylichnidae | |||||
| Cylichna cylindracea (Pennant,1777)* | 6 | wd | s | 2.0 | 1 |
| Epitoniidae | |||||
| Epitonium clathrus (Linnaeus, 1758)* | 40 | 5–70 | s, m | 6.0 | 3 (1) |
| Epitonium sp. | 8.0 | 2 (Frag-1) | |||
| Eulimidae | |||||
| Eulima glabra (da Costa, 1778)* | 10 | wd | m | 4.0 | 3 (3) |
| Melanella polita (Linnaeus, 1758)* | 10 | 0–10 | m, s | 4.0 | 1 |
| Vitreolina incurva (Bucquoy, Dautzenberg & Dollfus, 1883)* | 2.5 | – | m | 2.0 | Frag |
| Fasciolariidae | |||||
| Fasciolaria lignaria (Linné, 1758) | 70 | Shallow | r | 2.0 | Frag |
| Fusinus rostratus (Olivi, 1792) | 35–65 | >40 | m, s | 10.0 | 4 (frag-1) |
| Fusinus syracusanus (Linné, 1758) | 80 | 1–50 | m, s | 2.0 | Frag |
| Fusinus sp. | 4.0 | 1 (Frag-1) | |||
| Fissurellidae | |||||
| Diodora graeca (Linnaeus, 1758)* | 50 | wd | r, p | 2.0 | 1 |
| Muricidae | |||||
| Bolinus brandaris (Linné, 1758) | 100 | 3–100 | s, m | 4.0 | Frag |
| Muricidae gen. sp. | 8.0 | 2 (2) | |||
| Muricopsis cristata (Brocchi, 1814)* | 40 | 0–100 | p, r | 2.0 | Frag |
| Ocinebrina edwardsi (Payraudeau, 1826)* | 8–15 | – | p, s | 6.0 | 2 (Frag-1) |
| Trophonopsis muricatus (Montagu, 1803)* | 12 | 0–15 | – | 6.0 | 12 (1–10) |
| Nassariidae | |||||
| Nassarius cuvierii (Payraudeau,1826)* | 17 | >30 | r, s | 2.0 | Frag |
| Nassarius incrassatus (Ström, 1768)* | 8–15 | wd | s, m | 40.0 | 78 (Frag-17) |
| Nassarius reticulatus (Linnaeus, 1758) | 20–30 | 0–40 | s, m | 4.0 | 2 (2) |
| Naticidae | |||||
| Euspira guillemini (Payraudeau, 1826)* | 24 | – | m, s | 24.0 | 19 (Frag-4) |
| Euspira pulchella (Risso, 1826)* | 15 | – | m | 26.0 | 39 (Frag-15) |
| Euspira sp. | 4.0 | 21(3–18) | |||
| Natica hebraea (Martyn, 1784) | 55 | 0–10 | s, m | 2.0 | 1 |
| Natica stercusmuscarum (Gmelin, 1791) | 30–38 | 20–100 | s, m | 18.0 | 56 (Frag-27) |
| Natica sp. | 6.0 | Frag | |||
| Pyramidellidae | |||||
| Chrysallida sp.* | 2.0 | Frag | |||
| Odostomia turriculata Monterosato, 1869* | – | – | – | 6.0 | 2 (Frag-1) |
| Odostomia sp. | 4.0 | 3 (1–2) | |||
| Turbonilla lactea (Linnaeus, 1758)* | 14 | – | m | 6.0 | 4 (1–2) |
| Turbonilla pusilla (Philippi, 1844)* | 3 | – | m | 4.0 | 2 (1) |
| Rissoidae | |||||
| Alvania cimex (Linné, 1758)* | 4–5 | Shallow | p | 4.0 | 1 |
| Rissoa paradoxa (Monterosato, 1884)* | – | – | p | 2.0 | 1 |
| Rissoa variabilis (von Mühlfeldt, 1824)* | 8 | 15–80 | p, s | 2.0 | 3 (3) |
| Rissoidae gen. sp. | 22.0 | 8 (Frag-5) | |||
| Trochidae | |||||
| Clanculus cruciatus (Linné, 1758)* | 7–10 | 0–60 | r | 2.0 | Frag |
| Gibbula adriatica (Philippi, 1844)* | 9–11 | Shallow | p | 4.0 | Frag |
| Gibbula albida (Gmelin, 1791)* | 10–24 | 0–20 | s, m, r, p | 2.0 | 1 |
| Gibbula guttadauri (Philippi, 1836)* | 6–11 | Shallow | – | 2.0 | 1 |
| Gibbula magus (Linnaeus, 1758) | 40 | 0–70 | m, s, r | 48.0 | 183 (Frag-99) |
| Gibbula sp. | 4.0 | 1(Frag-1) | |||
| Jujubinus striatus (Linnaeus, 1758)* | 10 | wd | p, s, r | 18.0 | 8 (Frag-2) |
| Trochidae gen. sp. | 2.0 | 3 (3) | |||
| Turritellidae | |||||
| Turritella communis Risso, 1826 | 30–45 | 10–200 | s, m | 50.0 | 27 (Frag-5) |
| Turritella turbona Monterosato,1877* | 71 | >30 | s, g | 12.0 | 14 (Frag-5) |
| Turbinidae | |||||
| Bolma rugosa (Linnaeus, 1767) | 60 | 10–85 | r, s, m | 2.0 | 1 |
| Gastropoda unident | 30.0 | Frag | |||
| Bivalvia | |||||
| Anomiidae | |||||
| Anomia sp. | 2.0 | Frag | |||
| Pododesmus patelliformis (Linnaeus, 1761)* | 15–40 | 0–50 | r | 2.0 | Frag |
| Arcidae | |||||
| Arca tetragona Poli, 1795* | 25 | 0–120 | r | 4.0 | 1 (Frag-1) |
| Astratidae | |||||
| Astarte fusca (Poli, 1795)* | 15–25 | 30–80 | g, m | 4.0 | 1 (Frag-1) |
| Astarte sp. | 2.0 | Frag | |||
| Cardiidae | |||||
| Acanthocardia aculeata (Linnaeus, 1758)* | 40–101 | 0–125 | m, s, g | 2.0 | Frag |
| Acanthocardia paucicostata (Sowerby G. B. II, 1841)* | 20–40 | wd | s, m | 2.0 | 1 |
| Acanthocardia tuberculata (Linnaeus, 1758)* | 30–90 | 5–15 | s, m | 2.0 | Frag |
| Acanthocardia sp. | 2.0 | Frag | |||
| Cardiidae gen. sp. | 8.0 | 3 (Frag-2) | |||
| Parvicardium ovale (Sowerby G.B. II, 1840)* | 10 | >30 | m | 2.0 | 1 |
| Plagiocardium papillosum (Poli, 1795)* | 6–15 | 1–60 | s, g | 22.0 | 6 (Frag-2) |
| Corbulidae | |||||
| Corbula gibba (Olivi, 1792)* | 3–15 | wd | s, m | 56.0 | 63 (Frag-16) |
| Lentidium mediterraneum (Costa O.G., 1829)* | 3–10 | Shallow | s, m | 2.0 | 1 |
| Lentidium sp. | 2.0 | 1 | |||
| Glycymerididae | |||||
| Glycymeris sp. | 2.0 | 1 | |||
| Hiatellidae | |||||
| Hiatella arctica (Linnaeus, 1767)* | 50 | wd | s, m, r, p | 24.0 | 13 (frag-9) |
| Hiatella rugosa (Linnaeus, 1767)* | 50 | – | r | 2.0 | Frag |
| Lucinidae | |||||
| Lucinella divaricata (Linnaeus, 1758)* | 5–10 | 0–60 | s, m | 2.0 | 1 |
| Mytilidae | |||||
| Modiolus barbatus (Linnaeus, 1758) | 30–65 | Shallow | p, r | 2.0 | 1 |
| Musculus costulatus (Risso, 1826)* | 6–13 | 0–50 | r, p | 4.0 | 1 (Frag-1) |
| Mytilidae gen. sp. | 2.0 | 1 | |||
| Noetiidae | |||||
| Striarca lactea (Linnaeus, 1758)* | 4–15 | 0–130 | r | 4.0 | 2 (Frag-2) |
| Nuculanidae | |||||
| Nuculana commutata (Philippi, 1844)* | 6–10 | 7–65 | s, m | 2.0 | Frag |
| Nuculana pella (Linné, 1767)* | 9–15 | 4–180 | s, m | 2.0 | Frag |
| Nuculidae | |||||
| Nucula nitidosa Winckworth, 1930* | 13 | 0–20 | s, m | 2.0 | Frag |
| Nucula nucleus (Linnaeus, 1758)* | 12 | 10–50 | s, m, g | 4.0 | 4 (1–3) |
| Nucula sp. | 2.0 | Frag | |||
| Pectinidae | |||||
| Aequipecten opercularis (Linnaeus, 1758)* | 110 | 0–15 | s, m, p | 10.0 | 2 (Frag-1) |
| Chlamys flexuosa (Poli, 1795)* | 26–45 | >30 | s, m | 2.0 | Frag |
| Chlamys glabra (Linné, 1758)* | 35–75 | >6 | s, m, r, p | 6.0 | 2 (Frag-1) |
| Chlamys varia (Linné, 1758)* | 70 | 0–50 | r | 32.0 | 11 (Frag-3) |
| Chlamys sp. | 2.0 | 1 | |||
| Crassadoma multistriata (Poli, 1795)* | 25–45 | 10–180 | r | 2.0 | Frag |
| Pecten jacobeus (Linné, 1758)* | 80–150 | 25–50 | s, m | 2.0 | Frag |
| Pectinidae gen. sp. | 2.0 | 1 | |||
| Psammobiidae | |||||
| Gari fervensis (Gmelin, 1791)* | 24 | 0–110 | s, m | 2.0 | 1 |
| Tellinidae | |||||
| Tellina pulchella Lamarck, 1818* | 15–30 | Shallow | s | 2.0 | 1 |
| Tellina sp. | 4.0 | 1 (Frag-1) | |||
| Thyasiridae | |||||
| Thyasira flexuosa (Montagu, 1803)* | 5–13 | 10–100 | s, m | 2.0 | Frag |
| Veneridae | |||||
| Clausinella fasciata (da Costa,1778)* | 10–30 | 4–110 | s, m, r | 2.0 | Frag |
| Dosinia lupinus (Linnaeus, 1758)* | 15–40 | wd | s | 4.0 | Frag |
| Gouldia minima (Montagu, 1803)* | 10–16 | wd | s, m, g | 2.0 | Frag |
| Paphia aurea (Gmelin, 1791)* | 10–45 | 0–36 | s, m | 2.0 | Frag |
| Pitar rudis (Poli, 1795)* | 18–26 | 0–80 | s, m | 2.0 | Frag |
| Venus casina Linnaeus, 1758* | 25–51 | 5–200 | s, m | 2.0 | Frag |
| Venus verrucosa Linnaeus, 1758 | 20–72 | 0–15 | s, m, r, p | 4.0 | 1 (Frag-1) |
| Bivalvia unident | 18.0 | Frag | |||
| Scapophoda | |||||
| Dentaliidae | |||||
| Antalis dentalis (Linnaeus, 1758)* | 5–30 | 1–164 | s, m | 10.0 | 2 (Frag-1) |
- s = sand; m = mud; p = phytal; r = rock; g = gravel; frag = fragments; unident = unidentifiable shell fragments; wd = species with wide distribution; – = data not available; *newly recorded taxa in the diet of loggerhead sea turtles.
Although we identified a great diversity of molluscs in the loggerhead diet, most of them (52 species, 59.8%) belonged to small-sized species, with shells ≤ 3 cm in length. The majority of these small shells were found in a subfossil condition, characterised by damaged and battered shells filled with sediments (mud and sand) and often biofouled with tubicolous polychaetes and bryozoans. The group of larger-sized molluscs included 32 of 87 identified species, some of which were frequently recorded (e.g. T. communis, G. magus, Chlamys varia). Most of the other species from this group (e.g. N. stercusmuscarum, Bolinus brandaris, Aporrhais pespelecani, Aequipecten opercularis, Chlamys sp., Hiatella arctica, Acanthocardia sp.) were usually found crushed into smaller fragments, which in some cases made counting of individual specimens impossible.
The depth distribution of recorded species is given in Table 1. Some species (e.g. Trophonopsis muricatus, Natica hebraea, Gibbula albida) occur only in shallow areas, at a maximum depth of about 20 m. Other species (e.g. Nassarius cuvierii, Turritella turbona, Astarte fusca) live at depths >30 m, while most species, however, have a wide vertical distribution and can be found in the Northern Adriatic across all depths. We estimated that loggerheads in the neritic habitats of the Adriatic Sea yearly ingest a minimum of 32,979 kg of mollusc shells during the ‘summer foraging period’ alone (six warm months of the year).
Discussion
Our results showed that turtles were predominantly feeding in neritic habitats. A high number of newly recorded species in the diet and a great diversity of recorded mollusc species support the opportunistic feeding nature of loggerheads (Dodd 1988; Bjorndal 1997, 2003; Tomás et al. 2001; Casale et al. 2008a).
Several studies have emphasised the importance of molluscs in the diet of loggerheads in different neritic feeding areas (Laurent & Lescure 1994; Godley et al. 1997; Houghton et al. 2000; Limpus & Limpus 2003; Casale et al. 2008a). In these cases, targeted prey were mostly large-sized species, such as maxima clam Tridacna maxima Röding, 1798 (Limpus & Limpus 2003), Mediterranean mussel Mytilus galloprovincialis Lamarck, 1819 (Houghton et al. 2000) and larger gastropods from families Muricidae, Buccinidae, Fasciolariidae, Cerithiidae and Turritellidae (Laurent & Lescure 1994; Godley et al. 1997; Casale et al. 2008a), which were also found in our study. Empty shells of these gastropods are often used by hermit crabs and sea anemones (Pax & Müller 1962; Stachowitsch 1980; Williams & McDermott 2004). In terms of energetic budgets (Steimle & Terranova 1985), hermit crabs and sea anemones are more valuable prey for large predators such as sea turtles compared with shells of gastropods, which have an extremely low organic content (Marin et al. 2008). Moreover, observation of foraging behaviour showed that loggerheads select their diet by expulsion of crushed mollusc shells from the nares and oral cavity (Schofield et al. 2006), minimising the intake of poor quality items such as shells. As most of the large species were found crushed, this suggests a similar pattern of feeding behaviour in loggerheads from the Northern Adriatic. The presence of opercula of larger gastropods (e.g. Bolinus brandaris) and remains of large bivalves (e.g. Chlamys spp., Acanthocardia sp.) show that larger molluscs are the primary prey, in agreement with the findings of other authors (Laurent & Lescure 1994; Godley et al. 1997; Houghton et al. 2000; Limpus & Limpus 2003; Casale et al. 2008a). However, most mollusc species in the present study (about 60%) belonged to the small-sized group, mostly recorded with a low frequency of occurrence, with low or no nutritive value.
With the exception of some epifaunal species of the genera Arca, Modiolus, Chlamys, and Anomia, most recorded bivalves live buried in the substrate (Zavodnik 1971). While searching for prey, loggerheads dig shallow meandering trenches and harvest infaunal species in areas where epifaunal communities are not well developed; this behaviour is termed infaunal mining (Preen 1996). Houghton et al. (2000) observed two loggerheads digging out bivalve molluscs from soft bottom using flippers, whereas Schofield et al. (2006) reported frequent digging activities with both the beak and the flippers. Based upon the composition of molluscan fauna in our study and the presence of both endofaunal and epifaunal species, it is likely that loggerheads actively foraged on the surface of sandy and muddy bottoms, with occasional bites into substrate.
There are two major implications of such foraging behaviour. First, it results in the opportunistic intake of a variety of molluscan species, depending on the composition of local benthic communities. Most of these species (small-sized species) have a very limited or no contribution to the individual energy gain and probably are not selected prey. Their intake could be considered a byproduct of the search for energetically more valuable prey, such as larger molluscs or hermit crabs and sea anemones inhabiting empty gastropod shells, the latter two being confirmed as a frequent prey of loggerheads (Lazar et al. 2006). Secondly, a diet largely based upon molluscs and the foraging behaviour itself has an impact on neritic foraging habitats. When feeding upon large shells, loggerheads crush them with their jaws into small fragments. These fragments can be expelled from the oral cavity or deposited with feces in the same or remote marine habitats (Bjorndal 2003; Schofield et al. 2006). By crushing shells into fragments, turtles increase the surface area of shells and the rate of shell disintegration, and the abundance of support surfaces in the habitat for burrowing invertebrates. Through this process, turtles are directly involved in natural recycling in benthic environments. In addition, by infaunal mining, loggerheads mix sediment layers and influence sediment texture and compaction, bio-irrigation and dispersal of solid particles. Through such foraging behaviour, loggerhead turtles actively rework sediment and act as bioturbators in neritic feeding habitats, influencing the transport of nutrients in the marine ecosystems similar to other large marine vertebrates such as grey whales (Nerini 1984), walruses (Oliver et al. 1983) and bottlenose dolphins (Rossbach & Herzing 1999).
Quantification of the bioturbating role of loggerheads is one of the key issues for predicting their impact on marine communities and quantitative ecological modelling. Our conservative estimate showed that 33 tonnes of mollusc shells are ingested by loggerheads per year. This is just a rough estimate of the possible current extent of the bioturbating effect of loggerheads in the neritic habitats of the Adriatic. Although it is clear that assumptions about the uniform density of turtles across the whole neritic area and the replacement of the whole gut content every 3 days can hardly be met, it is likely that loggerheads forage longer than just during the warm 6-month period (as, due to low sea temperature in the winter months, turtles may be lethargic and not feeding; Lazar et al. 2003). Moreover, the amount of mixed, and not ingested, sediment due to infaunal mining is not included in our calculation, so that the mass of bioturbated shells and sediment is probably higher than estimated.
Quantification of the roles of sea turtles in the evolution and maintenance of the structure and dynamics of marine ecosystems is a huge challenge, mainly because their populations were seriously depleted long ago, so that present populations may already be ecologically extinct. This is the case with green turtles and hawksbill turtles in the Caribbean, where the current populations are only a small percentage of pre-exploited pristine population sizes (Jackson et al. 2001; Bjorndal & Jackson 2003; Moran & Bjorndal 2007). Declines of top predators may have numerous cascading effects in marine ecosystems, extending far beyond simple predator–prey interactions (Heithaus et al. 2008). The shallow Northern Adriatic is among the most heavily fished regions in the Mediterranean, resulting in the bycatch of several thousand loggerhead sea turtles per year (Lazar & Tvrtković 1995; Casale et al. 2004). Although we cannot reconstruct past population numbers, according to the perceptions of fishermen, the number of loggerheads in the Adriatic seems to be lower than in previous decades (Lazar & Tvrtković 1995). Commercial fishing, mainly bottom trawling, coupled with land-sourced pollution, and possibly climatic change, has resulted in repeated episodes of bottom anoxia, benthic mortalities and marine snow development in the Northern Adriatic over the last three decades (Rosenberg 1985; Stachowitsch 1991; Degobbis et al. 1995; Kollmann & Stachowitsch 2001). These human-caused disturbances have led to the collapse and disappearance of native benthic filter-feeding communities (the ‘O-R-M community’; Kollmann & Stachowitsch 2001), which were capable of removing large amounts of seston and plankton from the water column by storing it in the form of benthic biomass (Ölscher & Fedra 1977). If the decrease in the number of neritic-foraging loggerhead sea turtles was substantial, this might also have reduced their bioturbation role and present a contributing factor to the environmental changes observed in the Northern Adriatic. Certainly, historic numbers of loggerheads foraging in the small Adriatic Sea were not in the range of tens or hundreds of millions, as has been estimated for green turtles in the Caribbean (Jackson et al. 2001). Nonetheless, estimates of the carrying capacity for sea turtles in the neritic habitats of the Adriatic might be a crucial piece of information for setting recovery goals in order to restore ecologically functional populations of loggerhead turtles in this marine ecosystem.
Acknowledgements
This study was carried out within the research project Nos. 119-1193080-3171 and 183-1193080-0831 of the Ministry of Science, Education and Sports of Croatia, under the permits Nos. 612-07/97-31/67 and 531-06/1-02-2 of the Ministry of Environmental Protection and Physical Planning of Croatia, and the permits Nos. 354-09-66/00 and 35714-165/01 of the Ministry of the Environment, Spatial Planning and Energy of Slovenia. For help in the collection of turtles we are thankful to Valter Žiža (Aquarium Piran) and to collaborating fishermen in Croatia and Slovenia. We also thank S. Galetović and D. Miše for their help with laboratory analysis, and Dr. M. Hrs-Brenko for identification of bivalves. We acknowledge the use of the MAPTOOL program for graphics in this paper. MAPTOOL is a product of seaturtle.org. The manuscript was improved thanks to the constructive comments of Dr. Scott Heppell (Oregon State University) and two anonymous reviewers.




