Genetic diversity and population structure of the golden cuttlefish, Sepia esculenta (Cephalopoda: Sepiidae) indicated by microsatellite DNA variations
Abstract
The golden cuttlefish, Sepia esculenta Hoyle, 1885 (Cephalopoda: Sepiidae) is a valuable and important fishery resource for China, Japan and Korea. This fishery has experienced severe population decline largely due to overexploitation in past decades. To provide guidelines for fisheries management, we estimated genetic diversity and population structure across four locations along the coast of Japan and one location in China (a total 281 individuals) using nine microsatellite DNA loci. Sepia esculenta showed high genetic variability with mean allele richness ranging from 15.8 to 21.4, and mean heterozygosity from 0.80 to 0.90. Weak but significant genetic differentiation was present and the FST value was 0.020 across all five populations. The useful information obtained in this study will offer insights into how to fine-tune conservation and fishery management measures for this species and resource in the future.
Problem
The golden cuttlefish, Sepia esculenta Hoyle, 1885, is a nektobenthic cephalopod inhabiting shallow coastal waters between 10 and 100 m depth, from central Japan to the Philippine Islands in the Northwestern Pacific (Okutani 1995). Members of the species grow to about 20 cm in mantle length during a short lifespan of 1 year or less (Natsukari & Tashiro 1991; Natsukari et al. 1991). Mature male and female S. esculenta migrate inshore to mate and spawn during the breeding period from December to May in Southern Japan (Watanuki & Kawamura 1999). The female lays individual egg capsules each with one egg on the branches of macroalgae or the sea floor and produces 23–65 egg capsules during a single egg-laying period (Watanuki et al. 2000). Newly hatched juveniles are miniature adults that already have schooling and nektobenthic tendencies (Jereb & Roper 2005).
Sepia esculenta is of great interest as a commercial resource for China, Japan and Korea, and has experienced severe population declines largely due to overexploitation in past decades (Hao et al. 2007). The significant decline of S. esculenta stocks has not only alerted people to its endangered status, but has also drawn attention to the necessity of evaluating the genetic population structure, which is important for delineating management units and maintaining a sustainable fishery (Thorpe et al. 2000). Prior to this study, the only information concerning the stock structure of this species was a preliminary allozyme study by Zheng et al. (2004), which found no evidence of genetic differentiation among the samples from the coastal waters of the Province of Shandong, China. No verified genetic information existed for this species along the coastal waters of Japan.
In cephalopods, recent work using microsatellite markers showed genetic homogeneity in several species such as the veined squid Loligo forbesi across the population occupying the European shelf seas of the Northeast Atlantic (Shaw et al. 1999), the California squid Loligo opalescens collected along the North America Pacific coast (Reichow & Smith 2001), and the European squid Loligo vulgaris and cuttlefish Sepia officinalis in the Adriatic (Garoia et al. 2004). However, significant population structuring was also found in Sepia officinalis (Pérez-Losada et al. 2002) and Octopus vulgaris (Cabranes et al. 2008) collected around the Iberian Peninsula. Sepia officinalis and O. vulgaris exhibited the same genetic pattern of isolation-by-distance (IBD), where populations showed increasing genetic differentiation with geographical distance, indicating locally restricted gene flow over small scales.
Given its mode of spawning, the nektobenthic habit of adults, and lacking the pelagic larval phase, it is presumed that S. esculenta should display restricted dispersal ability, and consequently distinct genetic differentiation between samples might be expected. Therefore, our study was designed to investigate whether population structure occurred in S. esculenta along coastal waters of Japan and China to determine if there was a genetic population subdivision between Chinese and Japanese populations.
In the present study, we investigated five sample populations of S. esculenta, with four sample populations from the coast of Japan and one from Shandong Province, China, using microsatellite markers. Due to their codominant, multiallelic and highly polymorphic nature, microsatellite markers are an ideal tool to investigate genetic variation and population structure. Moreover, the high mutation rate of these markers allows us to trace back recent events and is much less influenced by historical features, compared to allozymes or mtDNA markers (Jarne & Lagoda 1996).
Material and Methods
Sample collection and DNA extraction
Sepia esculenta samples were collected by trawling from four localities from Japan: Fukui (135º34′ E, 35º34′ N; n = 43), Nagasaki (129º21′ E, 32º30′ N; n = 68), Ehime (132º39′ E, 33º45′ N; n = 49), and Aichi (137º03′ E, 34º31′ N; n = 62), and one site in China: Rizhao (119º21′ E, 35º05′ N; n = 54) (Fig. 1). Samples from the coastal waters of Japan were collected from October 2005 to May 2006, and the Rizhao sample from the Province of Shandong, China, was collected in May 2004. From each animal, a small piece of muscular tissue from the tip of the arm was excised and preserved at −70 °C until further processing. DNA was extracted following the TNES–urea SDS–phenol–chloroform procedures described by Asahida et al. (1996).
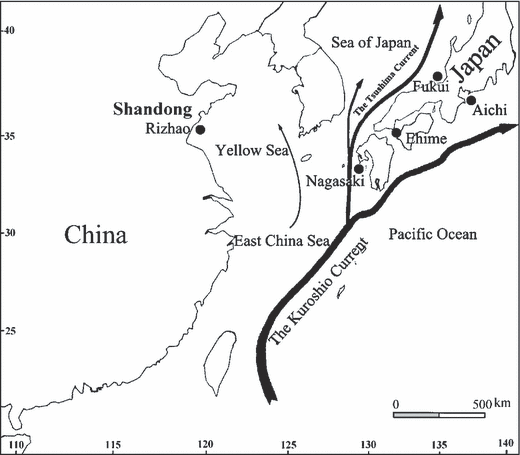
Sampling locations of the golden cuttlefish Sepia esculenta.
Microsatellite analysis
We assessed variation at nine polymorphic Sepia esculenta microsatellite loci: Secu6, Secu75, Secu84, Secu101, Secu113, Secu117, Secu146, Secu164 and SecuC10 (Zheng et al. 2007). PCR primers and amplification conditions of these nine loci were as described previously (Zheng et al. 2007). Amplification products were size-separated on an ABI PRISM 377 DNA automatic sequencer. All genotyping was performed using the software GENOTYPER 3.1.2 (Applied Biosystems).
Data analysis
Microsatellite diversity within samples was estimated using allelic richness (A: El Mousadik & Petit 1996) in FSTAT 2.9.3.2 (Goudet 2001), and observed (HO) and expected (HE) heterozygosities in GENEPOP 3.4 (Raymond & Rousset 1995) as implemented for online use (http://genepop.curtin.edu.au/). Significant deviations from Hardy–Weinberg equilibrium (HWE) (Fisher’s exact test using Markov chain method) were also calculated using GENEPOP. To examine the independence of the microsatellite loci, linkage disequilibrium between all pairs of loci was tested. Significance levels for multiple comparisons of loci across samples were adjusted using a standard Bonferroni correction (Rice 1989).
To estimate genetic differentiation among samples, two methods were used. First, null alleles can inflate estimates of genetic differentiation by reducing heterozygosity within populations (Chapuis & Estoup 2007). Therefore, the program FREENA (Chapuis & Estoup 2007) was used to calculate the frequency of null alleles at different loci and FST values were re-calculated after correcting for the presence of null alleles. FST were calculated with the Weir & Cockerham (1984) method for pairs of populations with GENETIX 4.05 software package (Belkhir et al. 2004). The significance of FST was tested using 1000 permutations. Secondly, we tested for simple frequency differentiation between pairs of samples with Fisher’s exact test implemented in genepop. Sequential Bonferroni corrections (Rice 1989) for multiple comparisons were applied. Population structure was further investigated with principle components analysis (PCA) performed on allelic frequency data using PCAGEN 1.2.1 software (Goudet 1999).
An isolation-by-distance (IBD) model has been suggested as appropriate for investigating marine dispersal (Palumbi 2003). IBD was tested using the Mantel test implemented in the software package TFPGA (Miller 1997) by correlating geographical distance (the shortest distance in the marine environment without crossing land) to FST/1−FST as suggested by Rousset (1997). Significance of the IBD pattern was tested on 10,000 permutations of the data.
Results
Genetic variation
Allelic richness (A) and estimates of variability at the nine microsatellite loci within all samples are displayed in Table 1. Among all samples, locus Secu101 exhibited the lowest variation (12 alleles in total), whereas Secu164 had the largest number of alleles (51). Observed heterozygosities ranged from 0.73 (in Aichi) to 0.84 (in Ehime) and expected heterozygosities ranged from 0.80 (in Aichi) to 0.90 (in Rizhao). No linkage disequilibrium was detected among loci (no P-values were significant after Bonferroni correction) and all loci were therefore considered genetically independent. Sepia esculenta samples showed considerable variation in their allelic richness (A) over all nine microsatellite loci, ranging from 15.8 for Ehime to 21.4 for Rizhao. Significant deviations from Hardy–Weinberg equilibrium occurred at 12 of the 45 locus-population combinations after Bonferroni correction (Table 1). All significant FIS values were positive, indicating a deficit of heterozygotes in the populations.
| Fukui (n = 43) | Nagasaki (n = 68) | Ehime (n = 49) | Aichi (n = 62) | Rizhao (n = 54) | |
|---|---|---|---|---|---|
| Secu6 | |||||
| N | 43 | 68 | 49 | 62 | 54 |
| A | 20.0 | 17.7 | 16.6 | 16.5 | 23.9 |
| HO | 0.95 | 0.81 | 0.92 | 0.66 | 0.74 |
| HE | 0.92 | 0.91 | 0.92 | 0.84 | 0.94 |
| FIS | −0.04 | 0.11 | 0.00 | 0.22 | 0.21 |
| R | 0.00 | 0.05 | 0.00 | 0.08 | 0.09 |
| Secu75 | |||||
| N | 43 | 68 | 49 | 61 | 54 |
| A | 25.0 | 23.7 | 26.8 | 23.3 | 24.6 |
| HO | 0.84 | 0.87 | 0.94 | 0.89 | 0.78 |
| HE | 0.94 | 0.94 | 0.96 | 0.94 | 0.94 |
| FIS | 0.11 | 0.08 | 0.02 | 0.06 | 0.17 |
| R | 0.04 | 0.03 | 0.01 | 0.02 | 0.08 |
| Secu84 | |||||
| N | 43 | 68 | 49 | 62 | 54 |
| A | 20.0 | 22.7 | 22.2 | 15.0 | 21.4 |
| HO | 0.98 | 0.94 | 0.88 | 0.74 | 0.87 |
| HE | 0.93 | 0.92 | 0.93 | 0.81 | 0.92 |
| FIS | −0.05 | 0.02 | 0.06 | 0.08 | 0.05 |
| R | 0.00 | 0.00 | 0.02 | 0.02 | 0.00 |
| Secu101 | |||||
| N | 43 | 68 | 49 | 62 | 54 |
| A | 5.0 | 7.1 | 5.0 | 5.0 | 10.3 |
| HO | 0.35 | 0.49 | 0.53 | 0.42 | 0.43 |
| HE | 0.63 | 0.64 | 0.66 | 0.50 | 0.67 |
| FIS | 0.45 | 0.25 | 0.2 | 0.17 | 0.37 |
| R | 0.17 | 0.11 | 0.07 | 0.07 | 0.13 |
| Secu113 | |||||
| N | 43 | 68 | 49 | 62 | 54 |
| A | 14.0 | 16.0 | 15.2 | 11.8 | 22.8 |
| HO | 0.88 | 0.78 | 0.78 | 0.86 | 0.82 |
| HE | 0.86 | 0.87 | 0.87 | 0.84 | 0.92 |
| FIS | −0.03 | 0.10 | 0.10 | −0.01 | 0.11 |
| R | 0.00 | 0.04 | 0.02 | 0.01 | 0.04 |
| Secu117 | |||||
| N | 43 | 68 | 49 | 62 | 54 |
| A | 22.0 | 20.8 | 22.2 | 21.5 | 23.5 |
| HO | 0.88 | 0.78 | 1.00 | 0.86 | 0.74 |
| HE | 0.92 | 0.93 | 0.94 | 0.93 | 0.95 |
| FIS | 0.04 | 0.17 | −0.07 | 0.08 | 0.22 |
| R | 0.02 | 0.07 | 0.00 | 0.03 | 0.10 |
| Secu146 | |||||
| N | 43 | 68 | 49 | 62 | 54 |
| A | 18.0 | 21.3 | 12.6 | 15.2 | 15.5 |
| HO | 0.91 | 0.87 | 0.92 | 0.86 | 0.83 |
| HE | 0.92 | 0.94 | 0.89 | 0.89 | 0.90 |
| FIS | 0.01 | 0.08 | −0.04 | 0.03 | 0.08 |
| R | 0.00 | 0.02 | 0.00 | 0.02 | 0.02 |
| Secu164 | |||||
| N | 43 | 68 | 49 | 62 | 54 |
| A | 28.0 | 27.7 | 26.5 | 17.3 | 28.3 |
| HO | 0.79 | 0.87 | 0.82 | 0.60 | 0.59 |
| HE | 0.85 | 0.91 | 0.87 | 0.59 | 0.91 |
| FIS | 0.07 | 0.05 | 0.06 | −0.02 | 0.35 |
| R | 0.03 | 0.01 | 0.03 | 0.02 | 0.16 |
| SecuC10 | |||||
| N | 43 | 68 | 46 | 62 | 54 |
| A | 25.0 | 22.7 | 21.7 | 16.6 | 22.8 |
| HO | 0.88 | 0.74 | 0.83 | 0.71 | 0.83 |
| HE | 0.92 | 0.93 | 0.94 | 0.89 | 0.94 |
| FIS | 0.04 | 0.21 | 0.12 | 0.20 | 0.12 |
| R | 0.00 | 0.09 | 0.05 | 0.09 | 0.06 |
| Multilocus | |||||
| A | 19.7 | 20.0 | 18.8 | 15.8 | 21.4 |
| HO | 0.83 | 0.79 | 0.84 | 0.73 | 0.74 |
| HE | 0.88 | 0.89 | 0.89 | 0.80 | 0.90 |
| FIS | 0.05 | 0.11 | 0.05 | 0.09 | 0.18 |
- N, number of genotypes per loci; A, allelic richness based on 43 individuals; HO, observed heterozygosity; HE, expected heterozygosity; FIS, Weir & Cockerham (1984) estimate of Wright’s (1951) fixation index (bold type indicates significant deviations from HWE after standard Bonferroni correction); R, null allele frequency based on Dempster et al. (1977) estimator.
Population differentiation and relationships
Global FST among samples was 0.020 (95% CI: 0.012–0.029) and ranged from 0.005 (Secu75) to 0.040 (Secu164) among loci. Pairwise FST varied from 0.009 to 0.037 (Table 2), and all comparisons between samples were significantly different from zero (P < 0.01). The largest values of FST were found between the Aichi site and the other four sites. The exact test for homogeneity of allelic frequencies showed significant heterogeneity for all Sepia esculenta samples. The results of the PCA were similar to the FST analysis (Fig. 2). The first three axes of the PCA explained 90% of the overall variation, with Axes 1 and 2 explaining 45% and 30%, respectively. There was no significant correlation between genetic differentiation and geographical distance in the five S. esculenta samples (r = 0.56, P = 0.082) or the four Japanese S. esculenta samples (r = 0.71, P = 0.17).
| Fukui | Nagasaki | Ehime | Aichi | Rizhao | |
|---|---|---|---|---|---|
| Fukui | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| Nagasaki | 0.010* | < 0.001 | < 0.001 | 0.001 | |
| Ehime | 0.023* | 0.009* | < 0.001 | < 0.001 | |
| Aichi | 0.035* | 0.024* | 0.024* | < 0.001 | |
| Rizhao | 0.024* | 0.015* | 0.009* | 0.037* |
- *P < 0.05 after sequential Bonferroni correction.
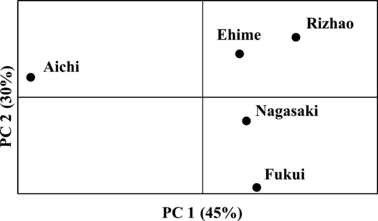
Plot of the first two axes resulting from a principle component analysis on multilocus genotypes of the cuttlefish Sepia esculenta.
Discussion
To our knowledge, this is the first study attempting to assess genetic diversity and population structure in Sepia esculenta using microsatellite markers. Polymorphism levels found in S. esculenta samples using microsatellite DNA loci (HE = 0.80–0.90) are comparable with those observed in the squid Loligo forbesi (HE = 0.55–0.91, Shaw et al. 1999) and the common octopus Octopus vulgaris (HE = 0.835–0.909, Cabranes et al. 2008), but a little higher than those observed with the cuttlefish Sepia officinalis (HE = 0.614–0.784, Pérez-Losada et al. 2002; HE = 0.581–0.664, Garoia et al. 2004; HE = 0.556–0.795, Wolfram et al. 2006). The genetic variability within S. esculenta samples is much higher than that obtained previously using allozymes for the same species (HE = 0.017–0.025, Zheng et al. 2004). This is not surprising given the higher mutation rates and polymorphism of microsatellite loci compared with allozyme loci (Estoup et al. 1998).
In this study, 12 significant deviations from Hardy–Weinberg equilibrium were found in seven of the nine microsatellite DNA loci, and these occurred among four of the five populations. All cases that departed from Hardy–Weinberg expectations were caused by heterozygote deficit, indicated by the high positive FIS. Heterozygote deficit relative to Hardy–Weinberg expectations in microsatellite loci has been commonly reported in marine invertebrate (e.g.Launey et al. 2002; Duran et al. 2004; Kenchington et al. 2006) including cephalopod populations (Garoia et al. 2004; Cabranes et al. 2008). The heterozygote deficit observed may result from technical factors, such as the presence of null alleles, or biological factors, such as inbreeding, Wahlund effect or selection. In our study, inbreeding is not a plausible cause as inbreeding should affect all loci equally, generating uniform heterozygote deficiencies across loci, which is not the case. Another possible reason for heterozygote deficits, and one that has been most widely cited as the cause of deficits at allozyme loci, is selection. Microsatellites are generally considered to be neutral markers (noncoding DNA regions), which means that selection cannot be invoked for observed deficits of heterozygotes, unless the microsatellite loci analysed are themselves linked to loci that are under selection. Because there was no evidence in our data indicating that any pair of microsatellite loci was linked, this hypothesis would require that each microsatellite locus be linked to a specific locus (or loci) that is under selection. As has been observed in previous microsatellite studies in cephalopods, heterozygote deficits could be largely caused by null alleles (Pérez-Losada et al. 2002; Wolfram et al. 2006). In the present study, null alleles (mean frequencies per locus up to 0.14) were indeed detected. In addition, the seasonal coastalward migration of mature cuttlefish for reproduction could cause the admixture of individuals from different cohorts and might contribute to generating the observed departures from HWE in the collected samples (e.g. Wahlund effect, Garoia et al. 2004).
Our microsatellite data provide the first evidence of genetic differentiation among populations of S. esculenta along the coast of Japan and suggest genetic population subdivision between Chinese and Japanese populations of S. esculenta. Genetic divergence estimated by pairwise FST (0.009–0.037) in the present study is similar to that reported in a congeneric species Sepia officinalis along the Iberian Atlantic coast (θ = 0.014–0.022, Pérez-Losada et al. 2002) and that in the Adriatic Sea population (FST = 0.018–0.022, Garoia et al. 2004). All pairs of samples, even those separated by distances as short as ∼450 km (Ehime-Nagasaki), showed significant differentiation, indicating restricted dispersal of S. esculenta. The nektobenthic habit of the adults in combination with the direct hatching of benthic eggs to nektobenthic juveniles might be the cause of such limited genetic exchange and ultimately of the significant genetic differentiation of local Sepia populations (Perez-Losada et al. 2007). Low levels of gene flow among populations along continuously inhabited coasts was also indicated by the studies in S. officinalis using allozymes (Perez-Losada et al. 1999), microsatellites (Pérez-Losada et al. 2002) and DNA sequences (Perez-Losada et al. 2007). Gene flow resulting from restricted dispersal is expected to lead to greater similarity among sedentary groups living closer together (‘isolation-by-distance’, Wright 1943). In our study, however, Mantel tests indicate no significant correlation between geographic and genetic distances. Based on PCA, the northeastern Japanese population (Aichi) appears to be the most distinct. Oceanographic variability has been shown to alter the patterns of the migrations of some coastal cephalopod species, such as octopods, loliginids and sepioids (review by Semmens et al. 2007). Therefore, we suggested that local oceanographic processes (Fig. 1) might act as dispersal barriers between the Aichi population and the other three Japanese populations of S. esculenta. The Pacific coastlines of Japan are under the influence of the Kuroshio current, whereas the Sea of Japan is under the influence of the Tsushima current, a branch of the Kuroshio current. The movement of sea water may well explain the geographical subdivision between the Aichi population and the other three Japanese populations of S. esculenta. In addition, we expected that the largest level of differentiation would be between the Rizhao population and the Japanese populations, but this was not the case. The low FST between the Rizhao population and the Japanese populations might be due to a certain proportion of dispersal events occurring over large distances caused by water from the Yellow Sea skirting the East China Sea and flowing into the Sea of Japan. Sampling more populations in the East China Sea would help to address this issue in the future.
In conclusion, high genetic diversity in terms of allelic diversity and mean heterozygosity at microsatellite loci was found in the S. esculenta populations. This could suggest the S. esculenta populations might have high adaptability to changing environments. The evidence that there was significant genetic differentiation among S. esculenta populations provides useful information for fishery management when considering increasing commercial exploitation of the species.
Acknowledgements
We thank Mr T. Shimizu (Ehime Prefectural Chuyo Fisheries Experimental Station), Mr K. Hattori (Aichi Prefectural Fisheries Experimental Station), Mr H. Hatanaka (Fukui Prefectural Fisheries Experimental Station), Dr M. Nakagawa (Seikai National Fisheries Research Institute, Fisheries Research Agency) and Dr T.X. Gao (Fisheries College, Ocean University of China) for collecting samples. We also thank Dr K. Okutani (Japan Agency for Marine-Earth Science and Technology) for his advice in identifying Sepia esculenta, and Dr C.C. Lu couple for proofreading. This study was supported in part by a Grant-in-Aid for Scientific Research (A-2-15208018) from the Japan Society for the Promotion of Science, the National Natural Science Foundation of China (no. 30600463) and the National High Technology Research and Development Program (2007AA09Z433).




