Distribution patterns of interstitial polychaetes in sandy beaches of southern Brazil
Abstract
This study describes the distribution patterns of interstitial polychaetes along morphodynamic gradients on six exposed sandy beaches in Santa Catarina and Paraná (South Brazil). Three random transects were sampled at two points on each beach, one at the swash and another at the surf zone, in winter and summer conditions. Six sediment replicates were collected at each sampling point using a corer of 4.6 cm internal diameter that removed 10 cm into the sediment. Abundance and composition of interstitial polychaete were correlated to wave height, slope, grain size, CaCO3, chlorophyll a, omega indexes, temperature and relative tide range using a canonical correspondence analysis (CCA). A factorial ANOVA showed that taxa richness, mean density and Shannon’s diversity were significantly higher at the reflective beaches, but average values differ significantly between transects and these differences change according to the beach zones on both sampling dates. PERMANOVA showed that polychaete associations differ among transects according to the beach zones. The composition of interstitial polychaete associations was significantly correlated to beach morphodynamics and features (P < 0.01). Polychaete associations of reflective beaches were more diverse than in other morphodynamic states. Intermediate beaches may also sustain diverse associations due to temporal variability of the morphodynamic patterns. Beaches presenting extreme dissipative morphodynamics and compacted sediments appear to be unfavourable for the occurrence of interstitial polychaetes.
Problem
Beaches are highly dynamic environments that have their structure and topography determined by granulometric characteristics and hydrodynamic processes, such as wave regime, tides and currents. Beaches were classified by Komar (1998) and Short (1999), according to their morphodynamic features, as ‘reflective’ (with larger grain diameter, absence of surf zone, and ascending and frontal waves); ‘dissipative’ (with fine sediments, extensive surf zone, spilling breaker waves and circulation currents) and ‘intermediate’, beaches in the midst of these two extremes, presenting plunging and spilling breaking waves (Komar 1998; Short 1999).
The occurrence and distribution of dominant meiofaunal groups in sandy beaches, such as nematodes and copepods, are clearly correlated to beach morphodynamics, but also depend on biological interactions and environmental alterations generated by processes of urbanization and tourism (Moellmann & Corbisier 2003; Rodríguez et al. 2003; Gheskiere et al. 2005, 2006; Kotwick et al. 2005; Moreno et al. 2006). As a rule, interstitial fauna is more representative in reflective beaches than are macrofauna and bacteria. The environmental optimum for interstitial fauna, in terms of diversity and abundance, develops where an optimum balance between hydrodynamic energy and organic matter input exist (McLachlan & Brown 2006). Interstitial polychaetes are frequent and constant components of meiofaunal associations in sandy beaches (Westheide 1972, 1974, 1987, 1990; Villora-Moreno et al. 1991; Villora-Moreno 1997; Lee & Correa 2004; Lee et al. 2006).
In spite of a reasonable knowledge of the correlations between interstitial fauna and beach morphodynamics, it is not clear whether these correlations are also valid for meiofaunal polychaete associations. Higher diversity and abundance of meiofaunal polychaetes are known from medium and coarse sand bottoms (Villora-Moreno 1997). These animals establish associations and follow zonation patterns that are reasonably well defined on the mesolittoral and infralittoral (Westheide 1972; Villora-Moreno et al.1991). Villora-Moreno (1997) suggested that the heterogeneity of the interstitial environment, the number of microhabitats formed and the diversity of interstitial polychaetes are correlated. Furthermore, Lee & Correa (2004) and Lee et al. (2006) concluded from toxicity tests that the reduction of interstitial space caused by rejects from mining was more limiting to the survival of polychaetes than the chemical contamination by the metals.
Most of the studies on interstitial polychaetes from Brazil have a taxonomic focus (Marcus 1946, 1947, 1948, 1955; Siewing 1954; Westheide 1974; Santos & Silva 1992/93). Studies on beach morphodynamics and macrofaunal ecology have shown a great variety of morphologic, hydrodynamic and granulometric patterns of sandy beaches on the central and northern littoral of the Santa Catarina and Paraná states (Borzone et al. 1996, 2003; Barros et al. 2001; Klein & Menezes 2001; Klein et al. 2002; Hoefel 1998). Consequently, a corresponding diversity and heterogeneity would be expected for meiofaunal associations in general, and for interstitial polychaetes in particular.
This study describes spatial variations of interstitial polychaete associations along sandy beaches of Paraná and the northern littoral of Santa Catarina states in Southern Brazil. A detailed study of physical environmental factors that are likely to influence the structure of interstitial polychaete associations at macro- and meso-spatial scales, was also carried out. Data on sediment texture, topography features, hydrodynamic regimes and microphytobenthic production were obtained from field observations and laboratory routines. These environmental descriptors were also correlated with distribution, richness, and abundance of interstitial polychaetes to provide baseline information on marine biodiversity patterns in this area.
Study Area
Sampling was carried out at six sandy beaches along 160 km of the coast of Paraná (PR) and Santa Catarina (SC). Beaches were selected according to their morphodynamic states, based on studies from Barros et al. (2001), Borzone et al. (1996) and Klein & Menezes (2001). The selected sites comprised two reflective beaches, Mansa (Mns) and Estaleiro (Est), two intermediate beaches, Nereidas (Ner) and Ilhota (Ilh), and two dissipative beaches, Atami (Atm) and Navegantes (Nav) (Fig. 1). The local tidal regime consists of microtides of discontinuous semidiurnal periods with a mean amplitude of 0.8 m (Schettini et al. 1999), which may reach as much as 1.2 m during meteorological tides (Carvalho et al. 1996; Schettini et al. 1999).
Location of the six beaches studied: Mansa and Estaleiro (reflective beaches), Nereidas and Ilhota (intermediate beaches), and Atami and Navegantes (dissipative beaches).
Material and Methods
Field and lab routines
Sampling was carried out during austral winter (September 2005) and summer (February 2006) at three transects (1, 2 and 3) disposed at two hydrodynamic zones (swash and surf zone) per beach. Distance between transects varied from 50 to 100 m and two sampling points were established on each transect, one at the saturated section of the lower mesolittoral, an area under the influence of the swash (Sw), and another at the intermediate section of the surf zone (Sf) (Fig. 2).
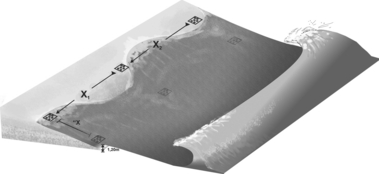
Sampling design used in the interstitial polychaete sampling on the six beaches studied. X1 and X2 indicate random distance between transects. ‘X represents the variation of distances between samples from the swash zone and surf zone, which was standardized by depth (1.2 m).
At each sampling point, six random replicates were collected inside an area of 3 × 3 m using a PVC tube of 4.6 cm diameter and 10 cm height. A total of 36 replicates per beach were collected at each sampling time (Fig. 3). Points located on the surf zone were taken at the standard depth of 1.2 m during low water spring tides.
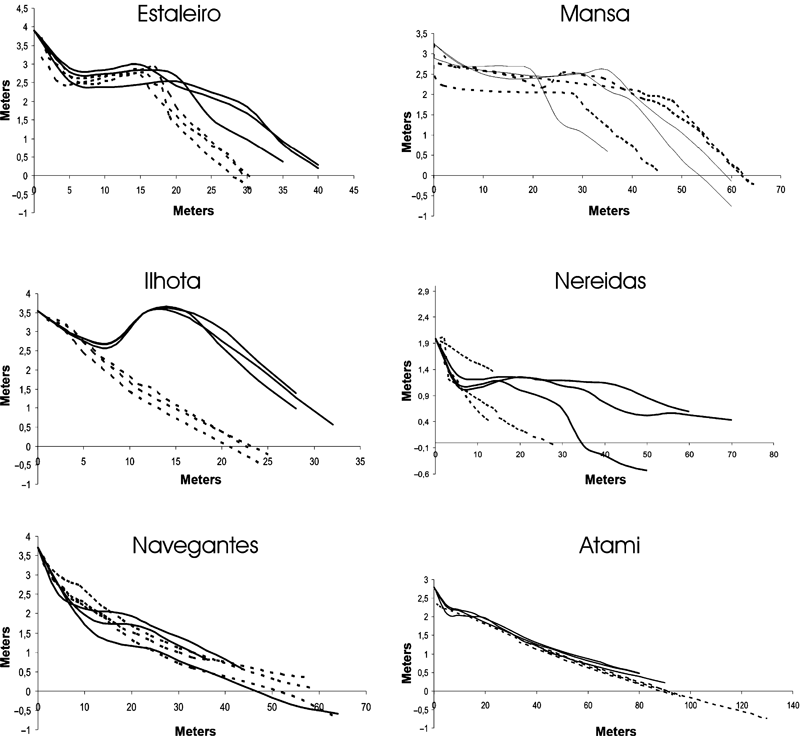
Diagrams of the beach profiles of the transects at the six beaches studied. Solid lines represent summer profiles and dotted lines represent winter profiles.
Samples were conserved in plastic containers, labelled and kept in an icebox to relax the animals. In the laboratory, samples were fixed in saline formol at 4% (Westheide 1990). For quantitative analyses and to extract the polychaetes from the sediment, samples were washed and meiofauna retained with a sieve of 0.062 mm mesh size. The flotation method with colloidal silica Ludox TM 50, adjusting the specific gravity to 1.15, was employed to retrieve animals (Higgins & Thiel 1988; Somerfield et al. 2005).
Meiofaunal specimens obtained were sorted on square, galvanized Petri dishes under the stereomicroscope. Only interstitial polychaetes were counted and identified to the lowest taxonomic level following the relevant literature (Marcus 1946, 1948, 1955; Gray 1969; Westheide 1974, 1990; Brown 1981; Jouin & Rao 1987; Capaccioni et al. 1989; Nordheim 1989; Jouin 1996).
Salinity, temperature, granulometry and samples of microphybenthos were measured or taken at two points on each profile (at the swash and surf zones) at the same depth used for faunal sampling. For each sampling point, temperature was determined with a thermometer buried in the sediment and salinity obtained with a refractometer. For the analyses of granulometry, organic matter and carbonate concentrations, two sediment samples were taken at each point with the same faunal sampler. Sediments were processed following the methodology described by Suguio (1973) Granulometric parameters were obtained using the software SYSGRAN, version 3.0 (Camargo 2006), following the method of McCammon (1962). To determine the carbonate concentrations, a fraction of the sediment was exposed to acid dissolution using hydrochloric acid (HCl) at 10% volume. The concentration of organic matter was determined after burning 5 g of sediment in a muffle furnace for 8 h at 800 °C. Permeability determinations were obtained from the upper mesolittoral region at each profile, from geotechnical tests in open PVC tubes (permeameter) according to the method described by Caputo (1980).
For microphytobenthic analyses, three samples of the top first centimetre of the sediment were collected using a 2.5-cm diameter plastic syringe at each sampling point of the six studied beaches. Samples were preserved in dark plastic containers and frozen for posterior pigment analyses. To extract microphytobenthic pigments (chlorophyll a and phaeopigments), 15 cm3 of acetone 90% was added to frozen samples, which were maintained in the freezer (−12 °C) for 24 h. The sediment was then filtered in cotton. Chlorophyll a and phaeopigment concentrations were determined before and after acidification with HCl 2 N, from absorbance readings at 665 and 759 μm in a spectrophotometer. Chlorophyll a and phaeopigments were calculated using Lorenzen’s equation (1967).
Beach declivity, wave height (Hb), wave period (T), swash period (Ts), swash slope and width, and surf zone width were measured in situ for each profile. Air temperature, rainfall, wind intensity and direction, and wind velocity data were obtained from meteorological stations at Pontal do Sul and Itajaí (Araújo 2005, 2006) for the studied seasons (winter and summer). Astronomical tide variation was followed using the software WXTIDE32 version 4.5 (Hopper 2006) available online.
Beaches were classified according to their morphodynamic stage by calculating the dimensionless parameter (omega index Ω) for sediment fall velocity (Dean 1973) as adapted for natural beaches by Wright & Short (1984): Ω = Hb/(WsT); where Hb is the significant breaking wave height; Ws the sediment fall velocity and T the wave period. The type of wave breaking and the energy dissipation characteristics in the surf zone were calculated using the surf scaling parameter (ε) (Guza & Inman 1975), where: ε = abω2/g tan2 β, ab is the breaking wave amplitude, ω is the wave radiation frequency, g is gravity acceleration and β is the beach gradient. The relative tidal influence in relation to the incident wave was calculated by the relative tide range (RTR) (Masselink & Short 1993), where RTR = TR/Hb, and TR is the tidal amplitude.
Data analyses
Two spatial scales were considered to describe the variation tendencies of interstitial polychaete associations. One scale in tens of kilometres was adopted to test the null hypothesis that ‘interstitial polychaete associations do not differ amongst the beaches, considering the winter and summer prevalent morphodynamics’. A second scale in tens of metres was used to test the null hypothesis that ‘interstitial polychaete associations do not differ between the swash and the surf zones within the beaches, considering their longitudinal morphodynamic variations and the studied seasons’.
Mean taxa number (S), mean polychaete numbers per 10 cm2 (Dt) and mean Shannon–Wiener diversity index (H) (nats· ind–1) were calculated for each sampling date (winter and summer). The design consisted of four factors: time (two level, random), beach (six levels, random and crossed with time), transect (three levels, random and nested in beach) and level (two levels fixed crossed with time, beach and transect). However, in the univariate and multivariate analyses, sampling dates were not treated as factors, and each data set was analysed separately.
Degrees of freedom, mean square estimates, F-ratios and P-value to univariate analyses were calculated according to Underwood (1997) with application of the software R 2.6.1 (R Development Core Team, 2007). Data was log (x + 1) transformed to decrease the heterogeneity of variance (Underwood 1997).
A non-parametric permutational multivariate analysis of variance, (PERMANOVA), version 1.6 (Anderson 2001, 2005) was also carried out. The analysis used Bray–Curtis distances calculated from the abundance matrix of the interstitial polychaetes [log (x + 1) transformed] added to dummy variable (Clarke et al. 2006). Tests for differences in the structure of interstitial polychaete associations were based on the same design applied to univariate analysis. For all tests, a subset of 9999 permutations was used. Non-metric multidimensional scaling (nMDS) was also employed (PRIMER 6.0, Clarke & Warwick 2001) to help interpret the results. Two nMDS plots were used to interpret the variation of polychaete associations in the scale of kilometres, taking into consideration the beaches in winter and summer. Ten nMDS plots were applied in the scale of metres, taking into consideration transects and beach level on each beach in winter and summer. Atami Beach was not considered in nMDS plots applied in the scale of meters due to the absence of polychaetes.
A canonical correspondence analysis (CCA) was applied to the mean abundance matrix of the interstitial polychaetes taxa to correlate the sampling points, and the environmental variables. Environmental variables were selected using the Monte Carlo permutation test (P < 0.05 for 999 permutations) (Ter-Braack 1985), non-colinearity among variables (P < 0.05), absence of outliers and univariated normality (Legendre & Legendre 1998; McGarigal et al. 2000).
A correspondence analysis (CA) was applied to the same matrix used for the CCA. This analysis was carried out to check the robustness of the correlations amongst the abundance of interstitial polychaete taxa and putative environmental gradients. When the first autovalue calculated by CA is much greater than that for CCA, it is most likely that the dominant environmental gradient has not been considered (Palmer 2007).
The statistical package CANOCO 3.12 was used to perform the CA and CCA. Biplot diagram was constructed to illustrate the axis 1 and 2 of CCA using the software MVSP 3.1. The biplot diagram for CA was not exhibited and only taxa with relative abundance above 1% were considered.
For the analyses, sampling points were coded according to the following: beach location, Estaleiro (Est), Ilhota (Ilh), Mansa (Mns), Nereidas (Ner) and Navegantes (Nav); transect, one (1), two (2) and three (3); beach level, swash zone (sw) and surf zone (sf); and season, winter (win) and summer (sum). Samples from Atami Beach were not considered in the analyses due to virtual non-existence of polychaetes.
Results
Morphodynamic characteristics of the beaches
Estaleiro and Ilhota were characterized by medium and coarse sediments, with high structural complexity, absence of redox layer and high microphytobenthic biomass. Similarly, high hydraulic conductivity, high declivity of the beach face, low values of omega index (Ω) and surfing scaling parameter also characterized these beaches as reflective. Diagrams of sub-aerial beach profiles indicated higher variation between seasons in the Ilhota, Estaleiro, Nereidas and Mansa beaches (Fig. 3). There was a higher sub-aerial sand volume in summer.
Mansa Beach showed in general poorly and moderately selected sediments that were composed primarily of medium sand on the swash zone and fine sand on the surf zone. A high degree of inclination of the beach face and narrow surf zone provide reflective morphodynamic characteristics of this beach. In addition, Mansa presented a mixed control by wave amplitude and tide.
Navegantes, Nereidas and Atami are exposed, dissipative beaches that present a well-developed surf zone composed of well-selected fine sediments. In addition, they show low hydraulic conductivity in a wide swash zone of gentle declivity and the presence of redox layer on the top centimetres.
Occurrence of interstitial polychaete associations
The morphotype level of taxonomic resolution at the genus level or grouped type-species was used for ecological analyses. Formal identification of most of the interstitial polychaete species requires the examination of reproductive organs, epidermal and salivary glands in live mature specimens (Nordheim 1989; Westheide 1990).
The genera Protodrilus, Polygordius, Saccocirrus, Hesionides and Hesionura have already been recorded for the coast of Paraná (Barros et al. 2001; Lana et al. 2006), whereas Protodriloides and Dinophilus have been recorded for the first time. In addition, specimens of Protodrilus, Polygordius, Saccocirrus, Hesionides, Protodriloides and Dinophilus were recorded for the first time in the central and northern coasts of Santa Catarina.
Taxa number per sample in the winter and summer varied significantly among Level*Transect(Beach) (Table 1). There are differences between transects within beaches and these differences change according to the levels. Mean richness values varied from zero to four taxa of polychaetes, and were lower at the surf zone or swash zone according to the transects of each beach (Fig. 4A,B). Richness was lowest at Navegantes; polychaetes were absent at Atami.
| Factors | df | Winter | Summer | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Richness | Density | Diversity | Richness | Density | Diversity | ||||||||||||||
| MS | F | P | MS | F | P | MS | F | P | MS | F | P | MS | F | P | MS | F | P | ||
| Beach | 5 | 7.854 | 48.51 | *** | 42.341 | 21.03 | *** | 1.8954 | 27.23 | *** | 9.551 | 21.07 | *** | 89.53 | 14.77 | *** | 1.7660 | 21.89 | *** |
| Level | 1 | 1.752 | 11.12 | *** | 13.693 | 23.24 | *** | 0.0331 | 0.67 | n.s. | 10.237 | 23.35 | *** | 44.91 | 3.60 | n.s. | 1.4473 | 22.44 | *** |
| Transect (Beach) | 12 | 0.162 | 3.00 | *** | 2.013 | 5.21 | *** | 0.0696 | 2.86 | *** | 0.453 | 6.13 | *** | 6.06 | 12.61 | *** | 0.0807 | 3.86 | *** |
| Level × Beach | 5 | 5.408 | 34.31 | *** | 27.997 | 47.52 | *** | 1.0625 | 21.55 | *** | 0.685 | 1.56 | n.s. | 7.41 | 0.59 | n.s. | 0.1127 | 1.75 | n.s. |
| Level × Transect (Beach) | 12 | 0.158 | 2.92 | *** | 0.589 | 1.52 | n.s. | 0.0493 | 2.02 | * | 0.438 | 5.93 | *** | 12.48 | 25.95 | *** | 0.0645 | 3.09 | *** |
| Residuals | 180 | 0.054 | 0.387 | 0.0244 | 0.074 | 0.48 | 0.0209 | ||||||||||||
- P > 0.05 (n.s.), P < 0.05 (*), P < 0.01(**), P < 0.001(***).
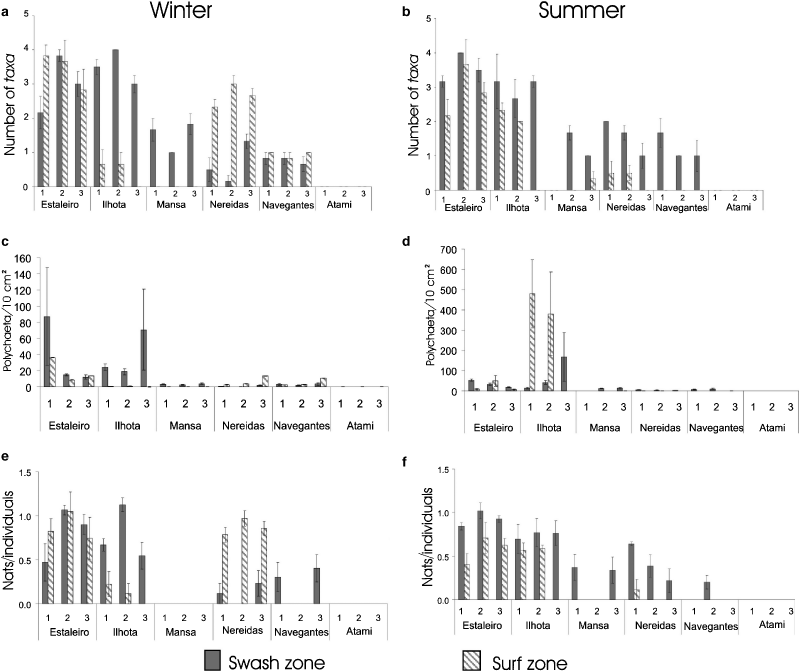
Number of taxa (A and B), density (C and D) and diversity (E and F) (mean ± SE) of interstitial polychaetes considering the swash and surf zones of each of the transects (1, 2 and 3) of the studied beaches during winter (A, C and E) and summer (B, D and F) periods.
Average polychaete density in winter varied significantly among Level*Beach and Transect(Beach) interactions (Table 1), but no significant variations were detected among Level*Transect(Beach). Polychaete density in summer varied significantly among Level*Transect(Beach). Mean densities ranged between 0 and 479 polychaetes per 10 cm2 and were lower at the surf zone and on the beaches of Navegantes, Atami and Mansa in the winter (Fig. 4C, D). The highest density was recorded at the surf zone on transects 1 and 2 on Ilhota Beach in summer, due to high abundances of Protodriloides.
The Shannon index varied significantly among Level*Transect(Beach) (Table 1) in winter and summer. There are differences between transects within beaches and these differences change according to the levels. The lowest values for diversity were observed at Navegantes and Mansa (Fig. 4E and F) and changed according to the transects and levels on the other beaches.
Estaleiro and Ilhota showed the highest polychaete abundance and species richness. Navegantes and Nereidas Beach showed similar spatial and seasonal patterns, with higher abundance during winter. No interstitial polychaetes were recorded at Atami Beach (Table 2).
| Ilhota | Estaleiro | Navegantes | Nereidas | Mansa | Atami | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| win | sum | win | sum | win | sum | win | sum | win | sum | win | sum | |||||||||||||
| sw | sf | sw | sf | sw | sf | sw | sf | sw | sf | sw | sf | sw | sf | sw | sf | sw | sf | sw | sf | sw | sf | sw | sf | |
| Saccocirrus spp. | 84.6 | 72.7 | 74.7 | – | 7.6 | 64 | 41.1 | 0.9 | – | – | – | – | – | – | – | – | 5.4 | – | – | – | – | – | – | – |
| Hesionides sp. | 4.8 | 9.1 | 0.1 | – | 1.1 | 6.9 | 1.3 | 1.8 | – | – | – | – | 19 | 56.1 | – | – | 7.5 | – | – | – | – | – | – | – |
| Protodrilus spp. | 7 | – | 12.5 | – | 7 | 13.2 | 43.5 | 4.7 | – | – | 4.2 | – | 71.4 | 23 | 48.2 | 42.9 | 87.1 | – | 96.3 | 100 | – | – | – | – |
| Hesionura sp. | 3.5 | – | 12.5 | – | 9.7 | 14.2 | 6.7 | 27.9 | – | – | – | – | – | 9.2 | – | – | – | – | 3.7 | – | – | – | – | – |
| Polygordius sp. | – | – | – | – | 74.2 | – | 6.8 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Protodriloides sp1 | – | – | – | 64.1 | – | – | 0.4 | 9.2 | 100 | 100 | 4.2 | – | 4.8 | 11.7 | – | – | – | – | – | – | – | – | – | – |
| Protodriloides sp2 | – | – | – | 35.9 | – | – | – | 54.1 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Dinophilus sp. | – | – | – | – | – | – | – | – | – | – | 91.6 | – | – | – | 51.8 | 57.1 | – | – | – | – | – | – | – | – |
| Syllidae | 0.1 | – | 0.1 | – | 0.4 | 1.7 | 0.2 | 1.2 | – | – | – | – | 4.8 | – | – | – | – | – | – | – | – | – | – | – |
| Pisione sp. | – | 18.2 | – | – | – | – | – | 0.2 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Total abundance | 1132 | 11 | 2198 | 8569 | 1130 | 577 | 1023 | 653 | 80 | 154 | 166 | – | 21 | 196 | 85 | 7 | 93 | – | 243 | 3 | – | – | – | – |
| Taxa number | 5 | 3 | 5 | 2 | 6 | 5 | 7 | 8 | 1 | 1 | 3 | – | 4 | 4 | 2 | 2 | 4 | – | 2 | 1 | – | – | – | – |
The three-factor PERMANOVA evidenced significant differences among Level*Transect(Beach) interaction in winter and summer (Table 3). Polychaetes associations varied between the surf and swash zone according to the transects of each beach, so the null hypothesis that polychaete associations occur independently of the morphodynamic types of the beaches was rejected.
| Factors | df | Winter | Summer | ||||
|---|---|---|---|---|---|---|---|
| MS | F | P(MC) | MS | F | P(MC) | ||
| Beach | 5 | 695,929,398 | 279.886 | 0.0001 | 361,923,470 | 111.783 | 0.0001 |
| Level | 1 | 51,713,227 | 0.2749 | 0.8820 | 481,089,692 | 38.875 | 0.0277 |
| Transect(Beach) | 12 | 24,864,704 | 30.258 | 0.0001 | 32,377,390 | 105.427 | 0.0001 |
| Beach × Level | 5 | 188,125,938 | 17.468 | 0.0814 | 123,751,540 | 39.917 | 0.0007 |
| Level × Transect(Beach) | 12 | 107,697,964 | 131.058 | 0.0001 | 31,002,347 | 100.950 | 0.0001 |
| Residual | 180 | 8,217,560 | 3,071,066 | ||||
- P(MC), P-value obtained with Monte Carlo permutation test.
The nMDS plots applied to a scale of kilometres showed a clear dependence of the interstitial polychaete associations on the morphodynamic gradient of the studied beaches (Fig. 5). This pattern was strongly associated with changes in species number and abundance of interstitial polychaetes. The higher wave height turns the homogeneous beach morphology in winter, causing a decreased dispersion of the interstitial polychaete associations among the beaches (Fig. 5).
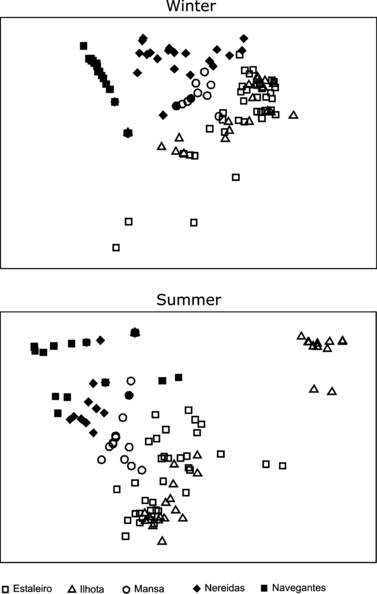
nMDS plots applied to abundance [log(x + 1) transformed] of the interstitial polychaete taxa collected at Ilhota, Estaleiro, Mansa, Nereidas, Navegantes, to winter (Stress = 0.09) and summer (Stress = 0.09). Open symbols represent beaches classified as reflective and filled symbols intermediate and dissipative beaches.
The nMDS carried out in the scale of metres for transects and levels within each beach showed zonation patterns related to beach levels (Fig. 6). Therefore, the null hypothesis of independence of polychaete associations taking into consideration different hydrodynamic zones (swash and surf zones) at each beach during winter and summer was also rejected.
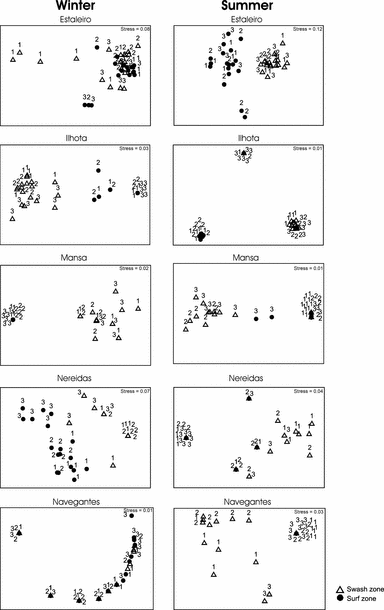
nMDS plots applied to the faunal composition of interstitial polychaete matrix [log(x + 1) transformed] collected at Estaleiro, Ilhota, Mansa, Nereidas and Navegantes, in winter and summer. Legend indicates the beach level. The transects were indicated with the numbers 1, 2 and 3.
Ilhota Beach showed the most marked zonation pattern amongst the beaches, which was particularly evident during summer when high abundances of Protodriloides sp. 1 and Protodriloides sp. 2 were observed at the surf zone in the transects 1 and 2. At Estaleiro, zonation patterns were more evident during summer, but this was not as distinct as in Ilhota. During winter zonation was masked by high breaking wave energy (Table 4), which is responsible for resuspending a large quantity of sediment on the surf zone and eroding sediments previously deposited on the beach face. In this hydrodynamic cycle, interstitial polychaetes that adhere to the sediment are possibly dislodged from their preferential zones. During summer zonation was defined by the occurrence of Protodrilus and Saccocirrus at the swash zone, and of Protodriloides sp. 2 and Hesionura at the surf zone.
| Beach | season | transect | Hb | Tw/Tsw | K | slope | SSP | RTR | Index Ω | temperature | salinity | grain size | CaCO3 | OM | Chl a | Phaeo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zsw | Zsf | Zsw | Zsf | Zsw | Zsf | Zsw | Zsf | Zsw | Zsf | Zsw | Zsf | Zsw | Zsf | ||||||||||
| Estaleiro | winter | 1 | 250 | 1.04 | 0.0044 | 16.37 | 1.03 | 0.4 | 2.81 | 18 | 19 | 31 | 30 | 0.4095 | 1.162 | 2.79 | 2.52 | 0.58 | 1.64 | 0.2767 | 0 | 0 | 0 |
| 2 | 250 | 0.95 | 0.0058 | 9.855 | 2.72 | 0.4 | 4 | 19 | 20 | 30 | 30 | 0.9371 | 0.6821 | 2.61 | 1.62 | 1.03 | 1 | 0.2013 | 0 | 0 | 0 | ||
| 3 | 250 | 0.85 | 0.0039 | 10.74 | 2.58 | 0.4 | 4.22 | 19 | 19 | 31 | 31 | 0.9294 | 0.8058 | 1.66 | 3.28 | 1.08 | 0.36 | 0.4528 | 0.352 | 0 | 0 | ||
| summer | 1 | 60 | 1 | 0.0227 | 8.174 | 0.82 | 1.67 | 0.61 | 26 | 26 | 35 | 35 | 0.323 | 1.094 | 5 | 4.33 | 2.17 | 1.09 | 0.3321 | 0.201 | 0.125 | 0.23 | |
| 2 | 60 | 1 | 0.0101 | 8.824 | 0.91 | 1.67 | 1.15 | 26 | 27 | 35 | 35 | 1.249 | 0.9958 | 4.46 | 4.57 | 1.72 | 1.81 | 0.3723 | 0.423 | 0.084 | 0 | ||
| 3 | 60 | 1 | 0.0115 | 8.976 | 0.77 | 1.67 | 1.01 | 27 | 28 | 35 | 35 | 0.9962 | 0.9852 | 3.63 | 3.48 | 1.28 | 1.21 | 0.4604 | 0.415 | 0.034 | 0.1 | ||
| Ilhota | winter | 1 | 150 | 0.97 | 0.0099 | 7.448 | 3.95 | 0.67 | 4.65 | 17 | 19 | 34 | 34 | 2.074 | 1.205 | 1.09 | 2.95 | 1.24 | 1.73 | 0.2767 | 0.377 | 0.048 | 0 |
| 2 | 150 | 0.99 | 0.0059 | 7.84 | 3.88 | 0.67 | 2.9 | 18 | 17 | 34 | 34 | 1.436 | 1.273 | 1.13 | 3.86 | 1.17 | 1.55 | 0.7296 | 0.478 | 0 | 0 | ||
| 3 | 150 | 0.74 | 0.0057 | 6.771 | 5.84 | 0.67 | 4.49 | 18 | 18 | 34 | 34 | 1.575 | 1.002 | 1.47 | 1.58 | 0.84 | 1.6 | 0.3019 | 0.126 | 0.028 | 0 | ||
| summer | 1 | 70 | 0.81 | 0.0209 | 10.46 | 0.43 | 1.43 | 1.58 | 28 | 29 | 34 | 34 | 1.244 | 2.331 | 9.37 | 9.55 | 3.59 | 4.48 | 0.717 | 2.981 | 0.014 | 0.03 | |
| 2 | 85 | 0.78 | 0.0172 | 11.6 | 0.4 | 1.18 | 1.65 | 28 | 29 | 35 | 35 | 0.9932 | 2.52 | 7.85 | 10.26 | 2.45 | 3.79 | 0.8201 | 1.286 | 0.017 | 0.09 | ||
| 3 | 50 | 0.85 | 0.0151 | 12.1 | 0.09 | 2 | 1.19 | 28 | 30 | 35 | 35 | 1.186 | 2.365 | 7.22 | 11.37 | 2.57 | 4.61 | 0.9434 | 1.95 | 0.033 | 0.15 | ||
| Navegantes | winter | 1 | 80 | 0.38 | 0.002 | 2.368 | 18.8 | 1.25 | 2.06 | 18 | 19 | 25 | 24 | 1.391 | 2.317 | 3.4 | 3.47 | 1.48 | 1.3 | 0.2264 | 0.151 | 0.035 | 0 |
| 2 | 100 | 0.38 | 0.0015 | 2.027 | 29.7 | 1 | 1.66 | 18 | 19 | 25 | 25 | 0.8816 | 2.298 | 2.36 | 2.6 | 0.5 | 0.69 | 0.2264 | 0.05 | 0 | 0 | ||
| 3 | 150 | 0.64 | 0.001 | 1.192 | 75.3 | 0.67 | 2.84 | 18 | 19 | 26 | 25 | 1.396 | 2.047 | 2.89 | 2.86 | 0.85 | 1.07 | 0.4277 | 0.075 | 0 | 0 | ||
| summer | 1 | 70 | 0.38 | 0.0028 | 1.604 | 28.8 | 1.43 | 4.29 | 28 | 29 | 34 | 32 | 2.397 | 2.36 | 3.77 | 4.27 | 1.36 | 1.9 | 0 | 0 | 0 | 0 | |
| 2 | 80 | 0.83 | 0.0021 | 1.49 | 26.4 | 1.25 | 4.32 | 28 | 31 | 34 | 32 | 2.346 | 2.655 | 3.63 | 3.69 | 1.28 | 1.27 | 0 | 0.073 | 0 | 0.52 | ||
| 3 | 70 | 0.76 | 0.0033 | 1.604 | 15.4 | 1.43 | 4.39 | 28 | 31 | 35 | 32 | 2.61 | 2.617 | 3.36 | 3.91 | 1.03 | 1.48 | 0.0428 | 0.375 | 0 | 0 | ||
| Nereidas | winter | 1 | 110 | 0.78 | 0.0012 | 2.349 | 22.1 | 1.36 | 4.45 | 19 | 18 | 35 | 35 | 1.507 | 1.516 | 0.45 | 0.45 | 0.89 | 0.69 | 0.0755 | 0.629 | 0.01 | 0 |
| 2 | 100 | 0.86 | 0.0012 | 2.972 | 15.7 | 1.5 | 7.24 | 19 | 19 | 35 | 35 | 2.393 | 1.944 | 1.86 | 2.02 | 0.38 | 1.29 | 0.2013 | 0.201 | 0 | 0 | ||
| 3 | 100 | 0.99 | 0.0012 | 2.753 | 18.8 | 1.5 | 6.11 | 19 | 21 | 35 | 34 | 1.391 | 2.218 | 1.76 | 0.73 | 0.34 | 0.51 | 0.2767 | 0.604 | 0 | 0 | ||
| summer | 1 | 100 | 0.53 | 0.0023 | 0.611 | 315 | 1.5 | 4.46 | 27 | 31 | 35 | 35 | 2.214 | 2.924 | 2.66 | 4.05 | 0.87 | 0.94 | 0.4654 | 0.591 | 0 | 0 | |
| 2 | 100 | 0.52 | 0.0022 | 1.289 | 39 | 1.5 | 3.55 | 27 | 30 | 35 | 35 | 1.837 | 2.712 | 4.01 | 3.66 | 1.03 | 0.88 | 0.4101 | 0.385 | 0 | 0 | ||
| 3 | 100 | 0.43 | 0.0023 | 1.346 | 36.5 | 1.5 | 5.64 | 27 | 31 | 35 | 35 | 2.369 | 2.224 | 3.83 | 5.45 | 0.51 | 1.93 | 01434 | 0.387 | 0.113 | 0 | ||
| Mansa | winter | 1 | 40 | 0.96 | 0.003 | 6.379 | 2.6 | 3.75 | 2.67 | 19 | 17 | 30 | 30 | 1.948 | 2.71 | 3.5 | 1.02 | 2.3 | 0.75 | 0 | 0.453 | 0 | 0 |
| 2 | 40 | 1.13 | 0.0024 | 8.563 | 0.89 | 3.75 | 1.21 | 19 | 19 | 27 | 25 | 2.378 | 2.464 | 3.77 | 2.3 | 1.52 | 1.01 | 0.327 | 0.302 | 0 | 0 | ||
| 3 | 50 | 1.07 | 0.0023 | 5.873 | 1.86 | 3 | 1.55 | 19 | 18 | 20 | 18 | 2.231 | 2.355 | 3.29 | 2.41 | 0.89 | 1.42 | 0.1761 | 0.252 | 0 | 0.05 | ||
| summer | 1 | 30 | 0.95 | 0.0036 | 7.219 | 0.68 | 5 | 2.61 | 26 | 27 | 24 | 24 | 2.442 | 2.171 | 3.31 | 7.77 | 0.52 | 1.4 | 0.1962 | 0.35 | 0.026 | 0.05 | |
| 2 | 50 | 0.91 | 0.0055 | 6.264 | 1.58 | 3 | 2.07 | 26 | 28 | 24 | 24 | 1.867 | 2.853 | 3.93 | 2.34 | 1.09 | 0.88 | 0.0528 | 0.189 | 0.041 | 0.08 | ||
| 3 | 60 | 0.94 | 0.0029 | 7.525 | 1.02 | 2.5 | 1.42 | 26.5 | 27 | 24 | 24 | 1.368 | 2.803 | 5.54 | 10.98 | 1.66 | 2.33 | 0.1811 | 0.299 | 3E-04 | 0 | ||
| Atami | winter | 1 | 80 | 0.87 | 0.0005 | 1.222 | 21.3 | 1.88 | 4.43 | 19 | 19 | 32 | 32 | 2.782 | 2.362 | 2.11 | 2.17 | 0.74 | 086 | 0.2642 | X | 0 | X |
| 2 | 80 | 0.77 | 0.0005 | 1.358 | 32.7 | 1.88 | 5.89 | 19 | 21 | 35 | 31 | 2.766 | 2.86 | 1.69 | 3.09 | 0.85 | 0.84 | 0.6541 | 0.453 | 0 | 0 | ||
| 3 | 60 | 0.49 | 0.0005 | 1.22 | 32.3 | 2.5 | 5.37 | 19 | 22 | 33 | 31 | 2.922 | 2.753 | 2.02 | 1.4 | 0.56 | 1.21 | X | 0.327 | X | 0 | ||
| summer | 1 | 70 | 0.51 | 0.0014 | 0.955 | 39.8 | 2.14 | 5.82 | 25 | 27 | 34 | 34 | 2.98 | 3.129 | 3.77 | 7.56 | 0.69 | 2.09 | 0.3321 | X | 0.557 | X | |
| 2 | 90 | 0.41 | 0.0014 | 1.07 | 75.6 | 1.67 | 7.29 | 26 | 29.5 | 34 | 34 | 2.958 | 3.134 | 3.32 | 5.08 | 1.08 | 1.25 | 0.7572 | 0.479 | 0.083 | 0 | ||
| 3 | 90 | 0.54 | 0.0014 | 1.108 | 44.9 | 1.67 | 8.13 | 27 | 30.5 | 34 | 34 | 2.995 | 3 | 3.16 | 6.2 | 0.68 | 1.9 | X | 0.294 | X | 0.14 | ||
In the surf zone at Mansa, few interstitial polychaetes were found, usually restricted to transect 3 during summer. Zonation patterns on Nereidas and Navegantes revealed the same pattern in summer, with Dinophilus occurring at the swash zone in two transects of Navegantes and in the three transects of Nereidas. The zonation pattern on Nereidas beach in winter was characterized by Hesionides on the surf zone and Protodrilus on the swash zone.
The distribution pattern of the interstitial polychaete populations within the different morphodynamic features (cusps, bars and canals) observed on each beach in each level could be associated with the significant variability among Level*Transect(Beach) to univariate and multivariate analyses.
Beach variables and interstitial polychaetes
The occurrence and abundance of taxa or the associations of interstitial polychaetes are conditioned by the environmental variables considered (Table 5), as supported by the similarity of the first autovalue calculated for the CA and the CCA.
| Axis | 1 | 2 | 3 | 4 | |
|---|---|---|---|---|---|
| CCA summary | |||||
| Eigenvalues | 0.693 | 0.481 | 0.124 | 0.076 | |
| Taxa–environment correlations | 0.899 | 0.829 | 0.587 | 0.666 | |
| Cumulative percentage variance | |||||
| of taxa | 24.8 | 42.1 | 46.6 | 49.3 | |
| of taxa -environment relation | 48.3 | 81.8 | 90.4 | 95.7 | |
| Total Inertia | 2.790 | ||||
| Sum of all eigenvalues | 2.790 | ||||
| Sum of all canonical eigenvalues | 1.437 | ||||
| CA summary | |||||
| Eigenvalues | 0.885 | 0.710 | 0.588 | 0.224 | |
| Cumulative percentage variance | |||||
| of taxa data | 31.7 | 57.2 | 78.3 | 86.3 | |
| Sum of all eigenvalues | 2.7902.790 | ||||
The CCA evidenced four significant ecological correlations amongst interstitial polychaete associations and environmental variables (Fig. 7). The occurrence and distribution of Hesionura, Hesionides, Protodrilus and Saccocirrus were correlated primarily to coarse sands, higher wave height and higher declivity. These factors have grouped the majority of the samples from Estaleiro, Ilhota and Mansa, and a few samples of Nereidas from the winter period. Protodriloides sp. 1 and Protodriloides sp. 2 were associated mainly to CaCO3 percentual, concentration of chlorophyll a, presence of medium and fine sands. These variables have grouped winter samples from Navegantes and Nereidas, and summer samples from the surf zone of Ilhota and one sample from Estaleiro.
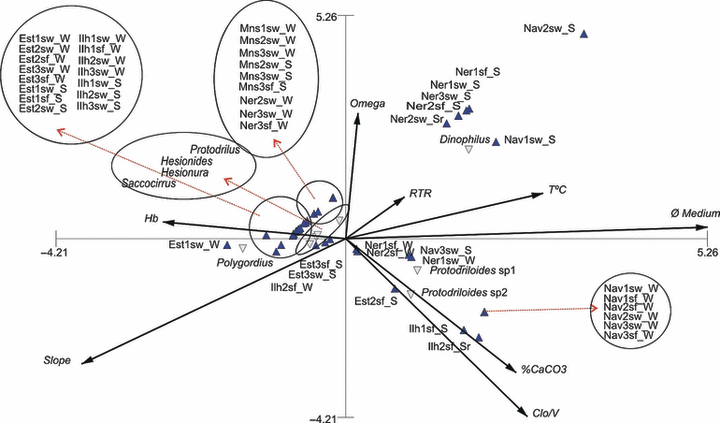
Diagram of axis 1 and 2 obtained from the CCA considering the environmental variables, taxa and samples, and labelled according to the beach, profile, beach level and season.
Occurrence of Dinophilus were related mainly to higher omega indexes, higher temperature and smaller grain size, which have grouped the majority of the summer samples from the swash zone of Nereidas and Navegantes. Polygordius was associated to higher declivity and higher wave height, and characterized only the surf zone of transect 1 from Estaleiro Beach in winter.
Discussion
Despite their small size, interstitial polychaetes present a variety of feeding habits and life histories (Westheide 1984), which are determinant for their occurrence in specific habitats in different beach types. Structural complexity of the interstitial environment is prevalent for the establishment of the associations of meiofaunal polychaetes. A higher diversity of these animals occurs in areas with poorly selected sediments with biogenic contributions (shell fragments) rather than in muddy or sandy-mud sediments (Villora-Moreno et al. 1991; Villora-Moreno 1997).
Zonation varies amongst transects within each beach, and is closely related to the dynamic equilibrium and seasonal variation of beach morphology. In general terms, reflective beaches are characterized by the presence and dominance of Protodrilus and Saccocirrus at the swash zone, and Hesionura, Hesionides and Protodriloides at the surf zone. The dissipative and intermediate beaches (Navegantes and Nereidas) were characterized by the presence of Protodriloides sp. 1 in winter and Dinophilus sp. in summer. It is known that these animals form associations and follow zonation patterns that are reasonably well defined for sandy beaches (Westheide 1972; Villora-Moreno et al. 1991). Westheide (1972) described patterns of vertical and horizontal distribution of interstitial polychaete populations on a beach in Tunisia that are determined by hydrodynamic and morphological seasonal variations.
Swash zones of reflective beaches are characterized by larger grain size, higher inclination and higher hydraulic flow, which, associated with the wave pattern of exposed beaches, provide an ideal environment for the occurrence of Protodrilus and Saccocirrus species. Most of the species in these genera are typical for intertidal beach environments composed of medium and coarse sediments (Marcus 1946, 1948; Nordheim 1989; Westheide 1990; Villora-Moreno et al. 1991; Villora-Moreno 1997). These polychaetes cling to sand grains or shells using their caudal appendages and sticky skin due to mucous produced by adhesive glands along the body and tail. The presence of coarse sediments and shell fragments is essential for the functional and reproductive maintenance in this species, particularly in high hydrodynamic environments (Marcus 1946; Westheide 1990).
Protodrilids are mobile and jawless surface deposit feeders or herbivores, and saccocirrids are mobile and jawless burrowers (Marcus 1946; Fauchald & Jumars 1979). Such feeding behaviours support the view that both morphotypes may feed on phytoplankton and other food sources brought by the rising water at the swash zone, which is filtered by a broad layer of sediment. Moreover, the microphytobenthic primary production, which may exceed the first millimetres of the sediment, is an additional source of energy (McLachlan & Brown 2006).
The life history of protodrilids and saccocirrids may also be the determinant of their wide occurrence in the studied beaches. Both present internal fertilization, indirect development with planktotrophic larvae, a lifespan of a year, and seasonal reproductive events (Westheide 1990; Giangrande 1997). Dispersal of these animals may be determined by their characteristics of grouping abundantly in a narrow strip of the mesolittoral (facilitating internal fertilization) and by the presence of planktotrophic larvae that are transported by littoral drift currents.
The two known species of Protodriloides are found on medium and coarse sediments, but rarely on fine ones, and are restricted to sublittoral and lower mesolittoral regions where abundances ranging from 100 to 1000 individuals per 100 cm3 occur (Westheide 1990).
There are few studies on the feeding habit of protodriloids. However, the phylogenetic proximity to protodrilids and saccocirrids, which are included in the order Protodrilida (Purschke & Jouin 1988; Westheide 1990; Purschke & Müller 1996; Worsaae & Kristensen 2005), and the co-occurrence in the same point on the beach that presented a high chlorophyll a concentration, strongly suggest that these animals are surface deposit feeders or herbivores. Patches of microphytobenthos may influence meiofaunal distribution due to higher availability of food, as recorded for copepods and nematodes (Santos et al. 1995). Thus, high concentrations of chlorophyll a, particularly at the surf zone of Ilhota beach, may influence the occurrence and distribution of Protodriloides. In addition, the patchy distribution of this animal may be explained by its life history. It reproduces by pseudo-copulation, with production of ‘cocoons’ by epidermal glands in a female region fertilized directly by males. These ‘cocoons’ are deposited on the sand grains, and may remain there for 10–20 days before they hatch a juvenile form (Westheide 1990).
Populations of Protodriloides find ideal habitats at the surf zone from intermediate beaches such as Ilhota. The low incidence and breaking height of waves on the rhythmic bars during summer cause waves to lose energy over the intermediate region of the surf zone. This is due to the presence of runnels and trough, which determine the sedimentation of finer grains and generate environmental stability that enables the proliferation of microphytobenthos. Physical characteristics of the surf zone such as depth are fundamental to the establishment of zonation patterns in benthic animals (Fleischack & Freitas 1989). The pattern indicates a spatial-temporal variability of polychaete associations that is clearly related to shifts in levels of hydrodynamic energy, topography and granulometry during winter and summer periods.
The phyllodocids, to which the genus Hesionura belongs, are recognized as carnivorous, mobile and jawless. Hesionids, to which the genus Hesionides belongs, are classified as mobile mandibulate carnivores or possibly surface deposit feeders (Fauchald & Jumars 1979). There is evidence that zonation in coastal marine environments is determined primarily by physical factors on the mesolittoral region and by biological interactions on the infralittoral (Connell 1961). Because these animals are carnivores, this may be a determinant factor for their presence on the infralittoral, where they are better competitors for a feeding niche whilst avoiding the turbulence of the swash zone.
The cosmopolitan Hesionides and Hesionura are found under similar morphodynamic beach conditions. They are typical of coarse, medium and fine sediments of the intertidal and, particularly, infralittoral environments (Westheide 1974, 1987; Capaccioni et al. 1989; Jing & Baoling 1991; Villora-Moreno et al. 1991; Baoling & Jing 1992; Villora-Moreno 1997). In Brazil, Hesionides gohari is known from Bahia State and shows wide distribution in warm marine zones, and Hesionura laubieri is known from both São Paulo and Bahia States (Westheide 1974). The infralittoral of exposed sandy beaches presents symmetrical sedimentary structures formed by wave action, which are characterized by crest and trough (ripple marks). This results in microregions of great granulometric heterogeneity consisting of coarse, medium and fine grains (Komar 1998; Short 1999), which may favour the occurrence of these animals.
Polygordius occurred only at the swash zone of transect 1 from Estaleiro Beach and was more representative during winter, suggesting a strong correlation with higher wave heights and higher declivity of the swash zone. Polygordiids are reported in coarse, clean sands with low concentrations of detritus and the presence of shell fragments (Westheide 1990). In Brazil, Marcus (1948) has described Polygordius eschaturus on the same beach in São Sebastião Island (SP) where he had described Protodrilus corderoi and Saccocirrus pussicus. These taxa were collected in the lower mesolittoral with medium and coarse sediments. P.J.P. Santos (unpublished observations) identified the same species of Polygordius at Vermelha Beach (RJ), which shows reflective morphodynamic characteristics and coarse sands as well.
The distribution of Dinophilus was highly correlated to dissipative beach conditions and higher temperatures, and was a characteristic taxon in tidal pools formed on the swash zone on Navegantes and Nereidas during summer. This result corroborates earlier records of their occurrence in various interstitial habitats, particularly in sheltered pools (Westheide 1990). In Brazil, Marcus (1948) reported the occurrence of Dinophilus gyrociliatus in fine sands with gravel in Santos Bay.
The high correlation of Dinophilus with temperature may be associated with its life history, which is characterized by a strong sexual dimorphism, continuous reproduction (several generations in the same year) and direct development (Simonini & Prevedelli 2003a,b). Simonini & Prevedelli (2003a,b) have shown that age at first maturation, as well the lifespan, decreases with an increase in temperature. However, fecundity rates may increase owing to production of eggs of a larger size and in higher numbers. This would result in a higher juvenile survival rate as reproductive compensation for a lower somatic growth. Overall, these characteristics could explain the higher representativity of this taxon during summer, although they are not sufficient to explain the absence of the group during winter. It is possible that local populations are intimately connected to the morphodynamic equilibrium of intermediate and dissipative beaches, and occupy deeper regions during winter, beyond the wave-shoaling zone. Moreover, these beaches undergo erosive processes during this time of the year, when the sediment removed from the subaerial portion of the beach is deposited beyond the surf zone. The wave pattern during summer, comprising local waves originated by local winds (Alves & Melo 2001; Araujo et al. 2003), favours the accretion of sediment to the subaerial portion. Laminar flow of low hydraulic energy on swash zones, in association with infragravity waves, will result in the formation of bars and runnels (Hughes & Turner 1999). As a result, a large saturation zone is formed, with ‘trough’ occurring on the mesolittoral. When these environments are associated with higher temperatures, they become ideal habitats for the survival and reproduction of Dinophilus.
The sampling of three transects per beach allowed for the verification of clear morphodynamic variations along a same beach (Short 1999), which may determine small-scale variations in the occurrence and distribution of interstitial polychaetes. Such a pattern was also recorded by Schoeman et al. (2003) for macrofaunal taxa. The variability of the associations observed amongst transects on Estaleiro and Ilhota, on either the swash zone or the surf zone, is an example of a marked morphodynamic and biological heterogeneity at local beach scale.
Conclusions
Interstitial polychaetes are more diversified and abundant at reflective beaches, as already recognized by Westheide (1972), and Villora-Moreno et al. (1991). However, intermediate and dissipative beaches may also sustain diversified associations, owing primarily to the seasonality of morphodynamic patterns. Dissipative extremes such as the prevailing conditions of Atami beach, with low permeability and well-selected fine sediments, cause a high level of sediment compression that diminishes infiltration, aeration and oxygenation of the sediment, and limits the occurrence and the vertical distribution of meiofauna and interstitial polychaetes. The exception was Mansa beach, which was characterized as reflective and presented low abundance compared to Estaleiro and Ilhota.
Our analysis evidenced a large variability in morphodynamic states and diversity of interstitial habitats both between and within beaches. The occurrence and distribution of interstitial polychaete associations were closely correlated to such environmental variability. Owing to this strict dependency on prevailing beach morphodynamics and features, adequate sampling designs for meiofaunal polychaetes are mandatory for adequate environmental monitoring and for the evaluation of impacts associated with pollution or structural modifications of the coastline (Kennedy & Jacoby 1999; Mauri et al. 2003; Nipper & Carr 2003; Lee & Correa 2004).
Acknowledgements
We thank the Graduate Programme in Biological Sciences – Zoology of Universidade Federal do Paraná and CAPES for the logistics and financial support to the first author. Cinthya S.G. Santos and Verônica M. de Oliveira helped with polychaete identification. Sérgio Netto, Carlos A. Borzone and Mauricio Camargo provided critical comments on earlier versions of the manuscript. The helpful suggestions of two anonymous reviewers greatly improved the manuscript. We also thank Katrine Worsaae for her critical comments on a related poster presented at the 9th International Polychaete Conference. Tito C.M. de Almeida provided incentive and support throughout the project, and the staff of the Benthos Laboratory (UFPR-CEM), Aquatic Community Ecology Laboratory (UNIVALI) and Laboratory of Marine Sciences (UNISUL) helped with lab material and discussions.




