Temporal variability of disturbances: is this important for diversity and structure of marine fouling assemblages?
Abstract
Natural communities are constantly changing due to a variety of interacting external processes and the temporal occurrence and intensity of these processes can have important implications for the diversity and structure of marine sessile assemblages. In this study, we investigated the effects of temporal variation in a disturbance regime, as well as the specific timing of events within different regimes, on the composition and diversity of marine subtidal fouling assemblages. We did this in a multi-factorial experiment using artificial settlement tiles deployed at two sites on the North East coast of England. We found that although there were significant effects of disturbances on the composition of assemblages, there were no effects of either the variation in the disturbance regime or the specific timing of events on the diversity or assemblage composition at either site. In contrast to recent implications we conclude that in marine fouling assemblages, the variability in disturbance regimes (as a driving force) is unimportant, while disturbance itself is an important force for structuring robust ecosystems.
Problem
In the face of global climate change there is increasing pressure to understand and ultimately predict under what conditions species diversity can be maintained or even increased within different ecosystems (Chapin et al. 2000; Straton 2006). Recent research highlighted the importance of biodiversity in ecosystem function and stability (Tilman 1996) and it is strongly suggested that this biological diversity is maintained through a variety of external processes, such as disturbances (Connell 1978).
As a result of these external processes natural communities are characteristically variable, fluctuating in both space and time (Landres et al. 1999; Fraschetti et al. 2005) and an understanding of this variability is essential for the management of species assemblages within ecosystems (Wu & Luocks 1995; Benedetti-Cecchi et al. 2000). It is crucial to understand how these processes influenced ecological systems in the past and how they might affect communities in the present and in the future with a view to managing systems and sustaining biodiversity (Landres et al. 1999). Understanding natural variability draws on a number of disciplines but it is disturbance ecology which provides an understanding about both the spatial and temporal dynamics of communities and how different species assemblages respond to these driving forces over temporal periods (Landres et al. 1999).
Biotic and abiotic disturbances are widely accepted as playing critical roles in influencing the patterns of distribution, abundance and diversity of species (Shea et al. 2004). A disturbance can be defined as a temporally discrete event which abruptly kills or displaces individuals, or that directly results in a loss in biomass from a system (Grime 1977). It therefore not only increases mortality within a community assemblage, but it may also change the availability of resources creating new opportunities for species, that would otherwise be out competed, to exploit (Connell 1978; Roxburgh et al. 2004).
The response of a species to a disturbance is a trade off between its susceptibility/resistance to a disturbance and its ability to utilise newly opened resources, e.g., space for colonisation, either by in-growth from surrounding areas or the recruitment of propagules (Connell 1978). If a disturbance is repeated then this can be considered to be a regime, i.e., a sequence of events at regular or variable intervals. Temporal variability in a disturbance regime can be vital in affecting the outcome of this trade off. For example, highly variable disturbance regimes are expected to be more concentrated with a clustering of disturbance events and greater periods of recovery. This could have severe implications for species with very short recruitment periods that coincide with the clustered disturbances, they are potentially excluded from the assemblage, and the same could be true for species with specific growth rates, thereby increasing competitive exclusion. When disturbances are less variable, i.e., spaced more evenly over time, we could expect a reduction in competitive exclusion allowing the existence of both life strategies commonly present in benthic assemblages, i.e., opportunists and strong competitors (Benedetti-Cecchi 2003).
The majority of studies in experimental ecology have focussed on the variance in the response of a community to a driving force, e.g., a disturbance, and have largely ignored any of the variance inherent within this force. Therefore, little is known about the consequences of changing the variance in a driving force over explicit spatial or temporal scales, a process that is thought to become more variable with increasing global change (Smith & Buddermeier 1992; Benedetti-Cecchi 2003). However, theoretical models suggest that the spatial and temporal variability of disturbance regimes are important and that they actually increase species diversity (Abugov 1982; Benedetti-Cecchi 2003). These aspects have been largely under explored in natural systems (Navarrete 1996). Few studies that have been carried out in this area have validated this concept, as well as suggested that variability may also influence patch dynamics (Butler 1989; Collins 2000).
Sessile benthic assemblages mostly lack inter-specific trophic interactions (Wootton 1998), for example they do not prey on one another (but see, Boero et al. 2005), space is a limiting resource (Connell 1978) and two possible methods of colonisation occur either from the water column, in the form of larvae, or as lateral growth from surrounding species (Underwood & Chapman 1996; Sousa 2000). Macro benthic fouling assemblages were used in this study because they are short lived, therefore suitable to experimental manipulation conducted on relatively short-time scales compared with some other systems (Dayton 1971; Sousa 1979). In this study, we investigate the effects of a temporally variable disturbance regime (i.e., the distribution of disturbance events over time) and the specific timing of disturbance events within each of these regimes (i.e., its sequence), on the diversity of marine macro benthic fouling assemblages on the North East coast of England.
Material and Methods
Site description
This experiment was carried out from March 2005 to October 2005 at two sites on the North East coast of England. Two sites were used to provide a contrast encompassing the extreme range of biotopes on the North East coast. It was logistically impossible to include more sites to provide a formal within-region spatial analysis. Each site is therefore considered as a separate experiment and analysed separately, but the informal comparisons between the two experiments are still very informative. The first site, Hartlepool Marina (54°41′31.68′′ N, 001°12′00.13′′ W), is a non-tidal, fully enclosed marina with access through a lock system. The second site, Sunderland marina (54°55′05.47′′ N, 1°22′02.10′′ W), is fully marine with salinity always >30 PSU although it is located at the mouth of the River Wear. Hartlepool marina has a fouling assemblage dominated by the solitary ascidians Ciona intestinalis and Ascidiella aspersa, and the erect growing bryozoan Bugula flabellata. Sunderland marina has a benthic assemblage consisting of green and brown seaweeds such as Fucus spp. and Ectocarpus siliculosus, barnacles, Balanus crenatus and tube worms, Pomatoceros triqueter. Recruitment occurs throughout the summer in both marinas.
Experimental set-up
Roughened PVC panels (15 × 15 × 0.3 cm, Bay Plastics Ltd) were used as artificial settlement substrata (Thomason et al. 2002). Roughening was standardised using an electric sander (Metabo, SXE 425) with sand paper (P60) for 10 s/panel.
Settlement panels were arranged in a single row on PVC strips (205 × 25 × 0.3 cm). Panels were fixed reversibly to the strips with cable ties (100 × 2.5 mm) to allow the return of panels after sampling.
The experiment consisted of a two-factor nested design with temporal variability of disturbance as a fixed factor and sequence of disturbance events over time as a nested factor. All panels were submerged at a depth of 50 cm in the two study sites 2 months before the beginning of the experimental manipulation to allow natural colonisation.
Disturbance treatment
The disturbance treatment applied to the assemblages corresponds to the definition given by Grime (1977) of a loss in biomass. The area to be disturbed was selected randomly and all organisms in this area were crushed, using a solid PVC cylinder (diameter 4.6 cm). Each panel was disturbed in two separate areas, each covering 10% of the area of the panel, with the area to be disturbed assigned by random number generation to produce co-ordinates on a grid which was then overlaid on the panel.
Temporal variation in disturbance was quantified by the standard deviation of the interval between disturbance events from the mean interval of 15 days. The frequency of disturbance events totalled 10 through out the experimental period of 150 days and treatments included a control of no disturbances (control) and three levels of temporal variation: constant variation (constant; every 15 days), low variation (low) and high variation (high), each level of variation was calculated using a standard deviation around the constant variance level and produced disturbance regimes with at least 5 days between each disturbance event (Fig. 1). Within the low and high levels of temporal variation, three different sequences of disturbance events were nested (Fig. 1). To avoid confounding the mean effect size with temporal variability in the disturbance regime both the intensity (20% of the panel area) and the frequency (10 events) were maintained constant in the experimental design. Moreover the time since the last disturbance before sampling the communities was kept constant (15 days, Fig. 1) for all treatment levels (Benedetti-Cecchi 2003). For each treatment five replicate panels were used, giving a total of 60 panels per experiment.
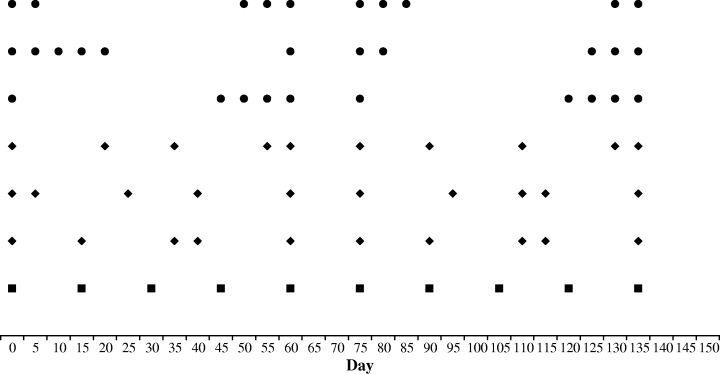
Disturbance calendar over the experimental period: the timing of each disturbance regime with its intrinsic sequence is shown. - Constant treatment, ◆- Low variability, •- High variability.
Sampling
Each panel was photographed at the beginning of the treatment phase and again after 150 days (Canon G3 Powershot, 4 × 106 pixels). Pictures were downloaded in Canon RAW format to maintain resolution using Canon Zoombrowser and analysed as 8 bit TIF files. Per cent cover of species was estimated in ImageJ V. 1.32j using overlaid points (Dethier et al. 1993; Abramoff et al. 2004) and in the event of multi-strata growth a value >100% was recorded. A 1-cm edge was left unsampled to avoid edge effects (Underwood 1997). Species identification was verified in the field. The wet weight of each panel was measured ( ± 1 g) after water was allowed to drain from the panels for 1 min and the dry weight was obtained at the end of the experimental period.
Data analysis
Dry weight was used as a proxy for community biomass, while species diversity (Shannon index, H’), species richness, evenness and total abundance were calculated from the abundances of single species (Magurran 1988). Multi-dimensional scaling ordination (MDS) plots were run to compare differences in community composition under different treatments. MDS plots were based on the Bray Curtis similarity coefficient calculated from non-standardised, square root transformed data, the latter was carried out to reduce the importance of abundant relative to rare species. To detect differences between the compositions of community assemblages experiencing different treatments, a one-way analysis of similarity (hereafter ANOSIM) was performed; using the non-standardised, square root transformed data. This approach was used to provide a conservative comparison of assemblage composition. Similarity percentage analysis (SIMPER), using square root transformed data, was used to identify which species contributed most to the observed differences.
To test for significant effects of temporal variability of disturbance (V), as well as the effects of the sequence of disturbance events (S) nested within this factor, a mixed model ANOVA was undertaken. Pairwise comparisons with post hoc tests were performed in the presence of significant effects: t-tests on the estimated marginal means adjusted for multiple comparisons with the Bonferroni procedure (Day & Quinn 1989).
Results
The fouling communities of the two experimental sites differed markedly in the species richness as well as in the relative abundances of common species. A total of 14 species comprised the benthic assemblage in Hartlepool marina whereas only three species were present in Sunderland. In non-manipulated assemblages the solitary tunicate Ascidiella aspersa (O.F. Müller, 1776) was the most common organism on the surface of the settlement panels in Hartlepool marina, with an average abundance of 58%, while in Sunderland marina the brown filamentous algae Ectocarpus siliculosus (Dillwyn) (Lyngbye, 1819) covered the largest area of the panel, with an average abundance of 89% (Fig. 2). Assemblage compositions were significantly different between undisturbed and disturbed communities in both Hartlepool and Sunderland marina (Fig. 3, Table 1).
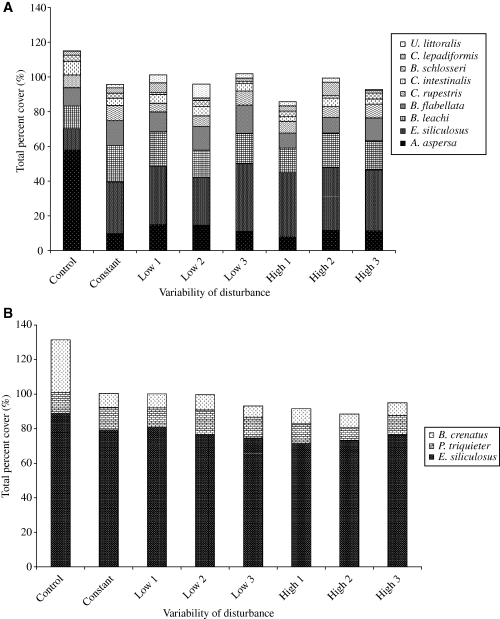
Dominant species (based on SIMPER analysis) in (A) Hartlepool marina, (B) Sunderland marina under different levels of variability of disturbance.
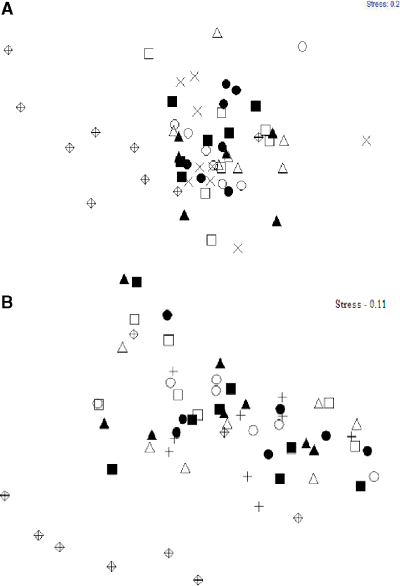
Multi-dimensional scaling ordination of communities in (A) Hartlepool marina and (B) Sunderland marina under different levels of variability of disturbance. Based on Bray Curtis similarity coefficient, non-standardised data and square root transformed abundances. Levels of variability of disturbance;  - Control, +- Constant, - Low 1, Low 2, •- Low 3, - High 1, □- High 2, - High 3.
- Control, +- Constant, - Low 1, Low 2, •- Low 3, - High 1, □- High 2, - High 3.
| Disturbance level | Hartlepool marina | Sunderland marina | ||
|---|---|---|---|---|
| R statistic | Sig. level | R statistic | Sig. level | |
| Control – constant | 0.331 | 0.002 | 0.369 | 0.044 |
| Control – low 1 | 0.264 | 0.018 | 0.261 | 0.033 |
| Control – low 2 | 0.265 | 0.019 | 0.242 | 0.029 |
| Control – low 3 | 0.457 | 0.004 | 0.323 | 0.019 |
| Control – high 1 | 0.390 | 0.005 | 0.276 | 0.018 |
| Control – high 2 | 0.388 | 0.004 | 0.312 | 0.027 |
| Control – high 3 | 0.307 | 0.015 | 0.294 | 0.020 |
In Hartlepool marina the only differences occurred between the undisturbed and disturbed assemblages (there were no effects within variability treatments) were due to monopolisation of the free space by A. aspersa in the undisturbed assemblages and E. siliculosus in the disturbed assemblages. E. siliculosus occupied less of the available space in the undisturbed assemblages, while in the presence of a disturbance its relative abundance increased from 5% to 34%, while the percentage of A. aspersa decreased from 38% to 15% (Fig. 4a and b). The abundances of all other species in the undisturbed assemblages remained unchanged, while in the disturbed assemblages the colonial ascidian Botrylloides leachi (Savigny, 1816) also increased in abundance after a disturbance. In the presence of disturbances the total percentage cover of species was reduced by approximately 20% (Fig. 2a).

Contributions to the fouling community of (A) Ascidiella aspersa and (B) Ectocarpus siliculosus in Hartlepool marina (stress value – 0.2) and (C) Balanus crenatus and (D) Pomatoceros triqueter in Sunderland marina (stress value – 0.11) Bubble value represents the absolute abundance of the species, while bubble diameter is scaled to the maximum abundance for the species.
In Sunderland marina there were no effects of the temporal variability of disturbances. The undisturbed assemblages were composed primarily of E. siliculosus (78%), and in lower abundance by the acorn barnacle Balanus crenatus (Brugiere, 1789) (17%) and the tube worm Pomatoceros triqueter (Linnaeus, 1718) (5%). E. siliculosus retained its monopolisation of the assemblage after disturbances while the contribution of B. crenatus was reduced to 6% and P. triqueter increased to 9% (Fig. 4c and d). Once again disturbed assemblages were reduced in total percentage cover by a mean of 25% (Fig. 2b).
There was a significant effect of treatment on the total abundance and biomass of assemblages in Hartlepool marina, while in Sunderland there were significant effects on all dependant variables tested apart from species richness. Significant differences were found between disturbed and undisturbed assemblages, but never amongst the different levels of variability of disturbances. There was no effect of the sequence of disturbance events at either site (Table 2). In both, Hartlepool and Sunderland marina, biomass (dry weight, g) and total abundance were reduced in the disturbed assemblages, not surprising as the disturbance was chosen to result in a loss in biomass (Fig. 5a and c; P < 0.001, Table 2). There were also significant differences between the evenness and diversity of undisturbed and disturbed assemblages in Sunderland but not Hartlepool (Table 2), and significant differences among the variability treatments were absent at both sites (Fig. 5b and d; Table 2).
| Dependant variable | Source of variation | Numerator df | Denominator df | F-value | P-value |
|---|---|---|---|---|---|
| (a) | |||||
| Biomass | Variability | 3 | 52 | 16.699 | <0.001 |
| Sequence (variability) | 4 | 52 | 0.372 | 0.828 | |
| Total abundance | Variability | 3 | 52 | 11.193 | <0.001 |
| Sequence (variability) | 4 | 52 | 2.068 | 0.098 | |
| Evenness | Variability | 3 | 52 | 1.693 | 0.180 |
| Sequence (variability) | 4 | 52 | 0.260 | 0.902 | |
| H′ | Variability | 3 | 52 | 2.645 | 0.059 |
| Sequence (variability) | 4 | 52 | 0.213 | 0.930 | |
| (b) | |||||
| Biomass | Variability | 3 | 52 | 30.275 | <0.001 |
| Sequence (variability) | 4 | 52 | 0.163 | 0.956 | |
| Total abundance | Variability | 3 | 52 | 26.223 | <0.001 |
| Sequence (variability) | 4 | 52 | 0.688 | 0.604 | |
| Evenness | Variability | 3 | 52 | 2.847 | 0.046 |
| Sequence (variability) | 4 | 52 | 0.788 | 0.539 | |
| H′ | Variability | 3 | 52 | 2.848 | 0.046 |
| Sequence (variability) | 4 | 52 | 0.788 | 0.538 | |
- For full details see the Material and Methods section. Results are presented for (a) Hartlepool marina, (b) Sunderland marina.
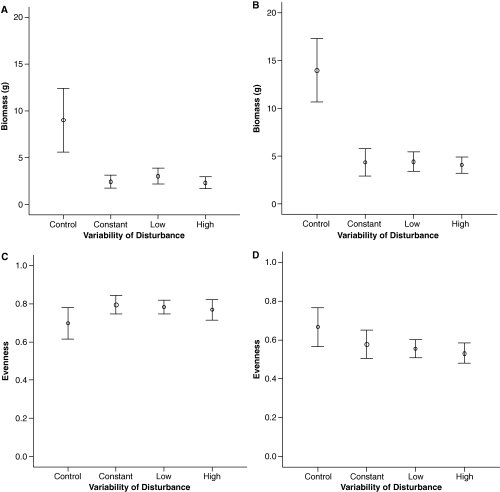
Mean ± SE biomass in (A) Hartlepool marina and (B) Sunderland marina, and mean ± SE evenness in (C) Hartlepool marina and (D) Sunderland marina under different levels of variability of disturbance.
Discussion
It has been previously suggested that changing the variance around the mean effect size of the predictor variable (i.e., the disturbance regime) can have important consequences for the response of a community assemblage (Benedetti-Cecchi 2003; Bertocci et al. 2005) however this has rarely been experimentally tested (but see Butler 1989; Navarrete 1996; Benedetti-Cecchi 2000). The aim of this study was to investigate whether the temporal variability in a disturbance regime has an effect on the species diversity and composition of marine fouling assemblages. The results presented in this paper show no support for this theory at either of the sites investigated.
It has also been highlighted by (McCabe & Gotelli 2000; Bertocci et al. 2005) that within regimes of equal disturbance variability, disturbances may occur in different sized clusters within any one regime. This may have severe implications for the ability of populations to re-colonise disturbed areas, depending on whether the specific timing of clusters coincide with reproduction or recruitment into a community (McCabe & Gotelli 2000; Bertocci et al. 2005). This aspect was investigated by manipulating the specific timing of disturbance events within the two levels of temporal variability and it was found once again that this had no effect on assemblages at either site.
At both study sites, the disturbances applied reduced the biomass and the total abundance of the fouling assemblage. This was expected as the nature of the disturbance was to create a loss in biomass and it was shown very clearly that there was an effect of the treatment. This effect remained evident because the disturbances were too frequent to allow the complete re-establishment of the fouling community, preventing a subsequent gain in biomass.
Elton (1958) suggested that a decrease in the diversity of a system restricts its functioning and lowers its ecological stability. To this day, his notion has inspired a great number of studies which both supported (McNaughton 1977; King & Pimm 1983; Tilman et al. 1996) or challenged (De Angelis 1975; Pimm 1984) the diversity-stability hypothesis. We conclude that the fouling assemblages in Hartlepool, due to the presence of 14 species with different ecological traits, were more stable towards disturbance than the assemblages in Sunderland, since community diversity at Hartlepool marina was maintained in response to disturbances. Former studies revealed that higher plant diversity led to a higher stability of grassland ecosystems towards disturbance (McNaughton 1977; King & Pimm 1983), while, more generally, it has been argued that community stability increases with increasing diversity (Pimm 1984; Odenbaugh 2001). Here the dominant competitor, Ascidiella aspersa, was efficiently removed by the disturbance events and could not re-colonise the freed space, while competitively inferior species, e.g.,Ectocarpus siliculosus and Botrylloides leachi, which were already present in the communities, exploited this resource quickly by lateral in-growth. The incapability of the ascidian for vegetative growth and its slow growth rates prevented it from regaining its competitive dominance.
The species-poor assemblages of Sunderland marina appeared to be less stable, as here diversity was decreased by disturbance. This decrease was due to the negative effects of disturbance on the competitively inferior species Balanus crenatus and Pomatoceros triqueter while the brown algae E. siliculosus was favoured. It monopolised the area after a disturbance event because it quickly invaded the free space which was created, which in turn reduced the evenness of the assemblage. The lack of functional diversity, i.e., more organisms capable of lateral growth, in this system made it less stable compared with the fouling assemblages in Hartlepool marina. This observation supports the diversity-stability hypothesis (Elton 1958).
With a dynamically changing environment and shifting global climate it is predicted that there will be large impacts on ecosystems, owing to the changes caused by increased sea surface temperatures, sea level rise and changed patterns of precipitation (Michener et al. 1997). One of the most important of these predictions is that the intensity, frequency, distribution and seasonal duration of large disturbances, such as hurricanes, tropical storms and periods of extreme heat, will become more variable and severe, with a clustering of events in short periods separated by large intervals (Smith & Buddermeier 1992; Michener et al. 1997). If this is the case then predicting the effects of a variable disturbance regime could be a very important tool to protect and conserve biodiversity.
In conclusion, it seems that although the inherent variation and sequence of disturbance events do not affect fouling assemblages of an early successional stage in the temperate subtidal systems investigated herein, disturbance events in general do, and they are an important force in structuring community assemblages. However, although there was no effect on the systems investigated this may not, nor should be taken as, the case for all types of assemblages. It is possible that this could be due to the confounding effects of recovery from recent disturbances to the point of sampling, for example, by having regular sampling throughout the study and taking the average response of assemblages we could gain a more thorough interpretation of the experimental treatments, better enabling the effects of variance and sequence to be separated from the recent history of disturbances. It seems that in marine hard bottom assemblages, diversity is increased under the influence of disturbances, adding support to the non-equilibrium concept of biodiversity. However, although variation within communities is the cornerstone of this paradigm (Landres et al. 1999) it seems, in this case, that the variability in the driving force, i.e., the disturbance regime, is unimportant. In terms of diversity the most crucial aspect was the actual disturbance itself.
Acknowledgements
The project was funded by The Stiftung Mercator Foundation. We are grateful to A. Henderson, Marina Director at Hartlepool marina and the staff at Sunderland marina for use of the sites. We would like to thank R. Sanderson and D. Hering as well as two anonymous referees for their invaluable comments on a previous version of the manuscript. J.C Thomason is supported by The Royal Society.




