Consumption of bacteria by larvae of a deep-sea polychaete
Abstract
We investigated whether trochophore larvae of the polychaete Hesiocaeca methanicola, which lives on exposed ice-like methane hydrates between 500 and 600 m, could consume near-bottom picoplankton. In laboratory trials larvae significantly reduced the growth rates of all types of picoplankton, including heterotrophic bacteria, Prochlorococcus sp., Synechococcus-type cyanobacteria and phototrophic eucaryotes <3 μm. Our findings suggest that these types of plankton may be important food sources for deep-sea planktotrophic larvae.
Problem
The vast majority of deep-sea animals with known life cycles either brood their young or reproduce by means of lecithotrophic (non-feeding) larvae (reviewed by Young 2003). Despite early predictions to the contrary (e.g.Thorson 1950), it has become apparent in recent years that planktotrophic larval development is a viable option for many species on the continental slope (e.g.Tyler et al. 1982; Young & Cameron 1989; Young & George 2000) and at least some abyssal species (Turner 1973; Bouchet 1976; Tyler & Gage 1984; Van Dover et al. 1985). A few planktotrophic species are known to migrate from abyssal depths to the euphotic zone, where they presumably feed upon phytoplankton (Rex & Waren 1982) or zooplankton (Epifanio et al. 1999), but migration models and experiments on thermal tolerances of echinoderm larvae indicate that migration is probably not possible for many other species (Young et al. 1996). Because living diatoms, dinoflagellates and other phytoplankton are typically not available below the permanent thermocline (Wishner 1980; Karl & Knauer 1984; Graf 1989), the question remains, ‘What do deep-sea planktotrophic larvae eat?’
Cyanobacteria and other picoplankton are not heavy enough to sink into the deep sea (Michaels & Silver 1988), but they may be transported into deep water by downwelling currents or in fecal pellets of mid-water plankton (Silver et al. 1986; Graf 1989; Pfannkuche & Lochte 1993). Heterotrophic bacteria, on the other hand, comprise a large proportion of the living deep-sea plankton, with typical densities of around 106 cells·ml−1 (Wishner 1980; Karl & Knauer 1984; Graf 1989; Pile & Young 1999). We have shown elsewhere that both heterotrophic bacteria and phototrophic picoplankton are important foods of benthic filter-feeders at a depth of 750 m on the Louisiana Slope (Pile & Young 1999). Several species of echinoderms and polychaetes from this depth and region are known to produce planktotrophic larvae. One such animal is the recently discovered ‘ice worm’Hesiocaeca methanicola (Desbruyères & Toulmond 1998), which lives on ice-like methane hydrates that breach the muddy sea floor between 500 and 600 m. Adults apparently graze on free-living chemosynthetic bacteria (Fisher et al. 2000). They are gonochoristic, free-spawning, and produce small (80 μm) eggs that are fertilized externally by ect-aquasperm (Eckelbarger et al. 2001). Embryos develop into rapidly swimming trochophore larvae in approximately 74 h. In the present study, we demonstrate that trochophore larva of H. methanicola are capable of consuming the same kinds of picoplankton that are eaten by cold-seep mussels (Pile & Young 1999). This is the first published demonstration of bacterivory in a deep-sea larva.
Material and Methods
Larvae of the polychaete H. methanicola reared in August of 1997 for developmental studies (Eckelbarger et al. 2001) were used for feeding trials. Embryos hatched as swimming trochophores approximately 74 h after fertilization and were maintained in culture for another 16 days (Eckelbarger et al. 2001). The larvae used for feeding experiments were 10–12 days old. By the time larvae hatched as feeding larvae, we had no access to fresh seawater from bathyal depths, so experiments were run using coastal seawater collected off Ft. Pierce, Florida USA and diluted 1:10 with 0.2-μm filtered seawater. After dilution, the concentrations of picoplankton were the same order of magnitude as those found in the Gulf of Mexico at 750 m (Pile & Young 1999), the approximate depth at which adult ice worms occur (Fisher et al. 2000). Trials were conducted using 20-ml glass scintillation vials to which 10 ml of seawater and 10 larvae were added (n = 3). Control vials (n = 3) had no larvae added. We incubated the vials for 5 h in a darkened incubator at 8 °C. One-milliliter water samples were collected from each vial at the beginning and end of the incubations and preserved for flow cytometry using standard protocols (Pile et al. 1996).
Picoplankton populations were quantified using an EPICS Elite flow cytometer (Coulter Electronics Corporation, Hialeah, FL, USA) at Harbor Branch Oceanographic Institution following the techniques of Marie et al. (1997). Orange fluorescence (from phycoerythrin), red fluorescence (from chlorophyll), and green fluorescence (from DNA stained with SYBR Green) were collected through band-pass interference filters at 530, 585, and 650 nm, respectively. Five measured parameters [forward- and right-angle light scatter (FALS and RALS), and orange, red, and green fluorescence] were recorded on 3-decade logarithmic scales, sorted in list mode, and analyzed with custom-designed software (Vaulot 1989). Picoplankton populations were identified to four general cell types: heterotrophic bacteria, Prochlorococcus sp., Synechococcus-type cyanobacteria, and autotrophic picoeucaryotes <3 μm. Cell types were visually confirmed, and mean cell diameter measured (n = 50 for each type) using epifluorescence microscopy.
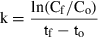
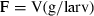
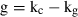
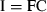
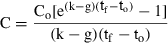
The significance of retention of each type of picoplankton was determined by comparing kc and kg using paired t-tests. Differences in the clearance rates between heterotrophic bacteria, Prochlorococcus sp., Synechococcus-type cyanobacteria, and picoeucaryotes <3 μm were determined using one-way analysis of variance after the assumption of homogeneity of variances was met with Levene's test (Zar 1984).
Results and Discussion
Trochophore larvae of H. methanicola significantly reduced the growth rates of all types of picoplankton (Fig. 1), indicating that larvae are capable of feeding on heterotrophic bacteria, Prochlorococcus sp., Synechococcus-type cyanobacteria, and phototrophic eucaryotes <3 μm. Mean clearance rates were 104 (±37 SD) μl·h−1·individual−1 for heterotrophic bacteria and 101 (±45 SD), 63 (±48 SD), and 105 (±48 SD) μl·h−1 for Prochlorococcus sp., Synechococcus-type cyanobacteria and autotrophic eucaryotes <3 μm respectively. Clearance rates were not significantly different from each other (ANOVA, F3,8 = 2.152, P = 0.172) indicating that H. methanicola larvae capture picoplankton unselectively. Ingestion rates for heterotrophic bacteria were 16 (±8 SD) cells·h−1·individual−1 and 35 (±14 SD), 47 (±16 SD), and 19 (±15 SD) cells·h−1·individual−1 for Prochlorococcus sp., Synechococcus-type cyanobacteria and autotrophic eucaryotes <3 μm respectively.
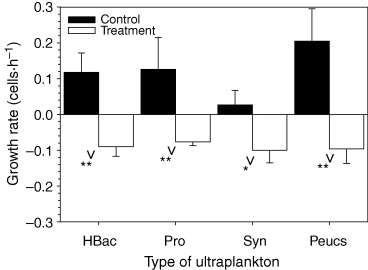
Net growth rate of heterotrophic bacteria (Hbac), Prochlorococcus sp. (Pro), Synechococcus-type cyanobacteria (Syn), and autotrophic eucaryotes <3 μm (Peucs) in the presence (Treatment, open bars) and absence (Control, solid bars) of trochophore larvae of the ‘ice worm’Hesiocaeca methanicola. Values are mean ± 1 SD. Significant differences between a treatment and control are indicated as *P < 0.05 or **P < 0.01.
Trochophore larvae of the ice worm graze unselectively on all types of picoplankton at picoplankton densities found near the adults. It may seem unusual for deep-sea larvae to have access to phototrophic picoplankton, but Prochlorococcus and other cyanobacteria are now known to occur in significant numbers well below the chlorophyll maximum (reviewed by Partensky et al. 1999) and are routinely found at the depths of the adult H. methanicola in the Gulf of Mexico (Pile & Young 1999; C. Everroad & M. Wood, personal communication). Additionally, the prokaryotic plankton of the meso- and bathypelagic is dominated by Archaea (Fuhrman et al. 1992; Karner et al. 2001; Herndl et al. 2005), and it seems likely that they are an overlooked important source of food for planktotrophic larvae. It is interesting to note that as these trochophore larvae grazed unselectively, there does not appear to be a limitation on the size of the particles captured or any selectivity to meet nutritional needs.
It is not unexpected to find that planktotrophic larvae of a deep-sea organism can graze on picoplankton, since shallow-water planktotrophic larvae in several phyla have this capability. Although few species have been studied, it appears from the available data that bacterial consumption is particularly prevalent in oligotrophic ecosystems such as the Antarctic (Rivkin et al. 1986) and coral reefs (Ayukai 1994). Echinoderm larvae in both of these systems are known to graze on hetero- and autotrophic prokaryotes (Rivkin et al. 1986; Ayukai 1994), although the energetic importance of picoplanktonic food sources has been called into question (Pearse et al. 1991). Our measured clearance rates are within the range of those measured for Antarctic echinoderms on heterotrophic bacteria associated with natural plankton communities (228 ± 49 μl·h−1·individual−1; Rivkin et al. 1986) and for tropical echinoderm larvae reared on two types of cultured cyanobacteria (58.7 ± 5.1 to 185 ± 80 μl·h−1·individual−1) (Ayukai 1994). Ingestion rates of cyanobacteria by H. methanicola larvae are nearly double the rate of 21 cells·h−1·individual−1 reported by Ayukai (1994).
Bivalve larvae from temperate embayments have been found with cyanobacteria in their guts (Raby et al. 1997) and such larvae have been shown to clear cyanobacteria (Gallager et al. 1994) and other types of picoplankton (Riisgård et al. 1980) in laboratory studies. However, the clearance rates reported for mussel veligers were much lower than our clearance rate for H. methanicola larvae at 11.4 ± 2.1 μl·h−1·individual−1 (Riisgård et al. 1980). Even crab (Carcinus maenas) zoeae, which are generally considered to be predators on larger planktonic organisms, can consume picoplankton-sized particles (Factor & Dexter 1993). In the only previous study of bacterivory in polychaete larvae (Gosselin & Qian 1997), it was shown that a diet of bacteria alone can sustain larvae of a shallow-water tropical polychaete, Hydroides elegans. All of the authors of the aforementioned studies speculate on the commonly overlooked importance of picoplankton to meet larval nutritional needs. Our results indicate that vertically migrating larvae are capable of taking all types of picoplankton, thereby obviating the need to enter the warmer waters of the euphotic zone to feed. Many deep-sea larvae cannot tolerate the temperatures found in the euphotic zone (Young & Cameron 1989; Young et al. 1996, 1998).
Thorson's (1950) widely cited hypothesis that food limitation constrains deep-sea animals to have direct or lecithotrophic development is now known to have many exceptions (reviewed by Pearse 1994; Young 1994), but the sources of nutrition for deep-sea planktotrophs remain mostly unresolved. Our finding that polychaete trochophores consume cyanobacteria and heterotrophic bacteria, the latter being among the most abundant potential food sources in the deep-sea water column, suggests that picoplankton may be a key to understanding how planktotrophy has been retained as a developmental mode by species invading the deep sea from shallow-water environments.
Acknowledgements
We thank the crews of the research vessel ‘Edwin Link’ and ‘JSL II’ submersible of Harbor Branch Oceanographic Institution and Sandra Brooke and Eva Ramirez Llodra for helping to rear the larvae. Ross Longley and the Division of Biomedical Marine Research at Harbor Branch Oceanographic Institution graciously provided access to the flow cytometer. This work was supported by grant 9539 from the NOAA National Undersea Research Program (NURP) at the University of North Carolina at Wilmington, NSF grant OCE-0118733, and a postdoctoral fellowship from Harbor Branch Institution to AJP. Contribution 1613 of Harbor Branch Oceanographic Institution.




