Fecundity and spawning of the Atlantic horseshoe crab, Limulus polyphemus, in Pleasant Bay, Cape Cod, Massachusetts, USA
Abstract
This study provided the first comprehensive analysis of Atlantic horseshoe crab (Limulus polyphemus) fecundity. Limulus appear to be a determinate spawner, maturing all eggs for the breeding season before spawning begins. On average, larger females held a larger number of eggs (63,500) than smaller females (14,500). By the end of the breeding season there was an average of 11,600 mature eggs per female left undeposited, regardless of female size. Larger females laid a higher percentage of the eggs they contained. Thus they not only contain more eggs, but are more effective at laying them as well. Size of spawning females ranged from about 185–300 mm prosomal width, with by far the highest concentration in the mid-size ranges. Although on an individual basis large females carry and lay the greatest number of eggs, mid-size crabs as a group contributed more to the horseshoe crab population in Pleasant Bay because they were more plentiful (net fecundity was highest for mid-size crabs). These results have implications for the management of this important species, which is harvested for bait, scientific, and biomedical uses. Incorporation of these results into models and other management tools can help predict growth rates, effects of size-selective harvest, reproductive value, and stable stage distribution of populations.
Introduction
The Atlantic horseshoe crab, Limulus polyphemus, native to the east coast of North America from the Gulf of Maine to the Yucatan, is important ecologically and economically. Limulus eggs are a major food source for migrating shorebirds along the Atlantic coast (Castro & Myers 1993; Clark et al. 1993; Botton et al. 1994; Tsipoura & Burger 1999). Horseshoe crab adults are commercially harvested for the blood-clotting compound Limulus Amoebocyte Lysate (LAL), which is widely used to detect endotoxins on surgical instruments and implants (Novitsky 1984; Atlantic States Marine Fisheries Commission 1998; Rutecki et al. 2004). The eel and whelk fisheries harvest Limulus, especially the large, egg-laden females (Manion & Unsworth 2000; Ferrari & Targett 2003), for use as bait. Horseshoe crabs are also used as biomedical models to study vision, cell biology, neurobiology, drug development, and immunology (Rutecki et al. 2004).
Populations of horseshoe crabs are thought to be declining due to a combination of harvest pressure and habitat destruction (Rudloe 1982; Swan et al. 1996; Widener & Barlow 1999). The importance of this ancient species and concern that its numbers are dwindling has prompted demand for information on horseshoe crab populations and life-history variables that can be useful in management and conservation efforts (Berkson & Shuster 1999; Eagle 2001). While much research has been done on Limulus, lack of reliable information on their fecundity has constrained the ability of scientists and resource managers to develop models and other management tools. For example, data on ‘net fecundity’ (defined here as the total annual contribution of eggs by a given size class of females) could be used to determine which crabs contribute most to a population's reproductive success. In areas such as Delaware Bay, where Limulus eggs help nourish shorebirds on their annual migration, fecundity data can be used in estimating how many shorebirds can be sustained from this food source. Basic information would also aid development and refinement of models, currently in demand to predict population growth rates, effects of size-selective harvest, reproductive value, and stable stage distribution of populations.
Previous research in Delaware Bay suggested that, on average, each adult female horseshoe crab produces 88,000 mature and immature eggs (Shuster & Botton 1985). There are several issues still to be addressed regarding the interpretation of this number. First, in related invertebrates such as spiders, insects, crustaceans, and the Indian horseshoe crab (Carcinoscorpius rotundicauda) larger females carry greater numbers of eggs than smaller individuals (Chatterji & Parulekar 1992; Stella et al. 1996; Sukumaran & Neelakantan 1997; and many others). It is not known whether a similar relationship between female size and number of eggs occurs in Atlantic horseshoe crabs. If so, the mean number of eggs measured among Delaware Bay horseshoe crabs, which are among the largest in their range (Shuster 1955, 1982), may not be representative of all populations. To understand the relative contribution of reproductive effort by assemblages of female horseshoe crabs of different sizes, it is necessary to more clearly define the relationship of egg production to female size in this species.
Second, there is also little known about the pattern of egg maturation in horseshoe crabs and how this may relate to the number of eggs laid by an assemblage of females each year. Some fish and insects continuously develop and replenish eggs laid throughout the course of the breeding season while others mature all of their eggs prior to each season, with remaining immature eggs not maturing until the following year or later (‘determinate’ spawners) (Wallace & Selman 1981; Hunter et al. 1985, 1992; Watanabe & Adachi 1987; Murua et al. 1996; Jervis et al. 2001). Determinate spawners generally contain only mature and immature eggs during the breeding season – there are no intermediate developmental stages present. Also, some of these animals retain and/or resorb mature eggs at the end of the breeding season (Bell & Bohm 1975; Rivero-Lynch & Godfray 1997; Rosenheim et al. 2000). An understanding of the strategy used by horseshoe crabs would help determine which eggs should be counted when measuring fecundity and refining estimates of reproductive potential.
Breeding patterns of horseshoe crabs, including length of spawning season, size-frequency distribution of spawning females, clutch size, and patterns and timing of egg release, also affect net fecundity. Much is already known about horseshoe crab breeding from previous studies (e.g.Rudloe 1980; Cohen & Brockmann 1983; Barlow et al. 1986; Brockmann 1990, 1996, 2003; Brockmann & Penn 1992; Penn & Brockmann 1994, and many others). For example, pairs of crabs in amplexus (male clasping posterior of female's carapace) typically come ashore with the high tides onto protected beaches in spring to breed (Sekiguchi 1988). Females deposit eggs in multiple small clutches in nests 10–20 cm deep in the sand. As the eggs are laid, they are fertilized externally by the male in amplexus, and often by aggregations of satellite males as well (Rudloe 1980; Sekiguchi 1988; Brockmann & Penn 1992; Brockmann 1996). Females return to the beach to spawn more eggs over several days (Rudloe 1980; Cohen & Brockmann 1983). Though these behaviors have been studied elsewhere, these generalized spawning habits vary by location, making it essential to gather information about breeding habits in our study location to provide a behavioral context to corroborate fecundity estimates. We therefore examined these patterns as an ancillary, confirmatory test of our fecundity results.
We addressed the lack of information on horseshoe crab fecundity by examining a representative population that has received study in Pleasant Bay, Cape Cod, Massachusetts. This Bay sustains a large, actively breeding population of horseshoe crabs with known spawning areas (Shuster 1982; James-Pirri et al. 2002, 2005; Carmichael et al. 2003). In this study, we quantified size-specific potential fecundity (number of eggs carried by females of different sizes during the breeding season), and realized fecundity (number of eggs actually laid by different sized females). We delineated the breeding season. To corroborate realized fecundity estimates with spawning patterns, we determined number of eggs laid per spawning episode (i.e. visit to the beach) by individual females, and patterns of returns to the beach. Size-frequency distribution of spawning Limulus females in Pleasant Bay was determined. We then used this information together with data on abundance and fecundity to calculate net fecundity (Fx).
Methods
Breeding season
To define the length of the breeding season, we monitored a known spawning area (Fig. 1) on the eastern shore of Pleasant Bay on Cape Cod, Massachusetts (James-Pirri et al. 2002, 2005; Carmichael et al. 2003) during daytime high tides from early March to early August, a time period that spans the spawning season described in Pleasant Bay and other sites (Rudloe & Herrnkind 1976; Barlow et al. 1986; Carmichael et al. 2003; James-Pirri et al. 2005). We also visited the site on one night-time high tide during this period, but safety concerns prevented further nocturnal visits. We visited the site, which spanned a 0.5 km section of beach, every 2 weeks until spawning began, and then on six occasions throughout the season while spawning activity was high. On one of these occasions, we were there for 6 days in a row. To study egg development in the off-season, we returned to the area in August and collected females from the shallows using snorkels. In November and January we obtained crabs that had been recently collected offshore by the Marine Biological Laboratory in Woods Hole.
Study area in Pleasant Bay, Cape Cod, Massachusetts.
On six of the visits during the breeding season, we collected crabs in each of three size categories: small (≤210 mm prosomal width (PW) measured across the widest part of the prosoma, or anterior part of the carapace), medium (211–240 mm) and large (>240 mm). Approximately five crabs from each size category were collected each time (15 crabs per visit), for a total of approximately 90 crabs. We killed females in an anesthetic clove oil bath (Keene et al. 1998; Peake 1998), removed the carapace from the prosoma and took out the entire volume of eggs. Other tissue was separated from the eggs by stirring the mixture in seawater; the heavier eggs settled to the bottom, allowing other tissue to be decanted off. In the off-season, we collected 6–10 crabs in each of the 3 months. Females were killed and dissected as above, but eggs were examined only for size, not number.
Egg maturation
To estimate net fecundity, we first assessed whether eggs mature before or continuously during the spawning season, and then used this information to determine whether to count immature eggs in the size-specific fecundity estimates (mxi) (mean contribution of eggs by a single female in a given size class). We measured egg diameter to the nearest 0.1 mm under a dissecting microscope in samples removed from the total volume of eggs collected from 6–10 crabs from all size categories on each of four occasions, during the spawning season (May), and after the season in August, November, and January. Samples of one hundred eggs from each crab were measured. Gardiner (1927) and Dumont & Anderson (1967) described mature horseshoe crab eggs (approximately 1.7 mm diameter) and immature eggs (<0.5 mm). Using these values of egg sizes throughout the spawning season and beyond, we were able to establish in the following way whether horseshoe crabs are determinate or indeterminate spawners and therefore whether we should count immature eggs as well as mature ones. If determinate, crabs would contain a range of developing egg sizes over the winter, but by the onset of spawning only mature and immature eggs would appear, indicating the crabs matured all of their eggs for the current season before breeding began; remaining immature eggs would therefore not be laid until a subsequent year and should not be counted in the current year's fecundity estimates. If indeterminate, females would contain a range of egg sizes during the season, indicating that eggs were continually maturing as the breeding season progressed; immature eggs should be counted in this case.
Size-specific fecundity
To relate female size to fecundity, we determined the total number of eggs in each female and related that to her prosomal width. Egg number was determined as follows: the entire volume of eggs from each female was dried in an oven (66 °C) for approximately 1 week and weighed (Turra & Leite 2001). To convert egg weight to egg number, five aliquots of 200 eggs from different crabs were dried and weighed to derive a conversion factor. Size-specific fecundity (mxi) was calculated by subtracting the number of eggs retained at the end of the breeding season by females of various sizes from the ‘potential’ fecundity (number of eggs in a female before spawning begins), to obtain ‘realized’ fecundity, defined here as the number of eggs actually laid (Wallace & Selman 1981; Hunter et al. 1985).
Though population ecologists generally use number of female eggs (eggs destined to become females) in their calculations, we did not do that here. Since horseshoe crab eggs have ecological uses beyond reproduction where sex is irrelevant, such as food for shorebirds, it was more useful to consider the total number of eggs. Female eggs can be assumed to be 50% of the total eggs (R.H. Carmichael, unpublished data), so number of female eggs can simply be obtained by dividing total number of eggs by 2, should the reader have need for this information.
Breeding behavior
We needed to define certain features of local breeding behavior to interpret our fecundity data. The needed behavioral information included number of eggs deposited per spawning, number of spawning episodes likely to take place per breeding season, and the proportion of eggs laid during the entire season. The number of eggs carried by a female at the start of breeding should correlate with the number of eggs she deposits during a spawning event and the number of times she spawns; we confirmed this correlation from three lines of evidence. First, we directly measured the number of eggs deposited in a spawning episode on two daytime high tides by marking nests where crabs were spawning. We estimated size of spawning females by holding a ruler over them without interrupting the spawning process (Cohen & Brockmann 1983). After the tide receded, we gently excavated the eggs and collected the discrete clutches (Cohen & Brockmann 1983; Shuster & Botton 1985; Brockmann 1990).
Second, to determine whether females returned to spawn more than once, either during a tidal cycle or later, we tagged 315 females over 4 days (12–15 June), including one nighttime visit during the marking period (2 am 15 June). Female crabs were marked with durable and long-lasting numbered thumbtacks inserted into the prosoma postero-lateral to the right compound eye (Sokoloff 1978; Cohen & Brockmann 1983). Sokoloff (1978) determined that tacks could remain in the carapace for at least 2 years. We searched for tagged crabs on all daytime high tides on return visits during the tagging period and the following 2 days (16–17 June), and again 2 weeks (28–29 June) and 4 weeks (14 July) later.
Third, to roughly estimate the proportion of eggs released during spawning episodes (one visit to the beach), we measured number of eggs carried by females arriving on and leaving the breeding beach on two daytime high tides. Thirty female crabs in amplexus (15 arriving, 15 leaving) were collected on each of these dates. The number of eggs found in the arriving group was compared to that in the departing group.
Net fecundity
To calculate net fecundity we measured size distribution of the female breeding population. We measured 222 females found in the spawning area over the course of the breeding season. Adult females were recognized by the absence of monodactylus pedipalps (‘boxer claws’ found on mature males) and the structure of genital pores (Shuster 1982; Sekiguchi 1988). The measurements were grouped into bins of 20 mm for the calculations.
We used data from R.H. Carmichael on size-frequency distribution of the whole Pleasant Bay female horseshoe crab population together with our data for size frequency of the breeding population to extrapolate an estimate of the total breeding population by size (Nxi) in Pleasant Bay. Net fecundity (Fxi) (the total number of female eggs produced by all females of a given size category in a season) was calculated by multiplying (Nxi) by size-specific fecundity (mxi). We assumed a stable population structure, an assumption supported by Carmichael et al. (2003). Standard errors were provided except where they could not be calculated because at least one variable had an N of 1. SE was propagated according to Meyer (1975).
Results and discussion
Breeding season
Number of female crabs in the spawning area of our study site went from one on 30 April to an estimated 60–100 from mid-May to mid-June. By 28 June, only 10 females were observed. This number remained consistent until observations ceased in mid-August. This timing of spawning agrees with that of Barlow et al. (1986) for Limulus spawning in Mashnee Dike, Cape Cod, and of James-Pirri et al. (2005) for Pleasant Bay. Although other studies have found spawning activity to be concentrated around the full and new moon high tides (Rudloe 1980; Cohen & Brockmann 1983; Barlow et al. 1986), often at night, we observed a similar level spawning activity on all the daytime high tides we visited during the period from mid-May to mid-June, regardless of lunar phase. In their study of horseshoe crab spawning on Cape Cod, James-Pirri et al. (2005) had similar findings in Pleasant Bay; they concluded that spawning densities in this area were not significantly affected by day of survey. Due to safety issues (flooded, ditched marsh), we made only one night time high tide visit to the marsh. We observed very few spawning pairs on that tide (total of 8), although visibility and water clarity were excellent. James-Pirri et al. (2005) also found that Pleasant Bay daytime spawning densities were higher than night time surveys. They noted that our study site was submerged under 0.5–1 m of water at night time high tides, which we also observed, and that crabs may therefore not spawn at this location at night. Based on James-Pirri et al.'s (2005) conclusion and our own observations we therefore limited future visits to daytime high tides and assumed that spawning activities observed on those dates were representative of those throughout the season.
Egg maturation
During the spawning season (eggs examined in May), all eggs were either immature or mature (Fig. 2, top). No developing, mid-sized eggs were present. This was apparent in all the crabs dissected during the spawning season, though we did not measure eggs from every crab; eggs were a very consistent size with a firm texture, regardless of female size. In contrast, eggs after the spawning season, in August (Fig. 2, middle), showed a wide range of sizes, with more eggs in the smaller sizes and very few mature-sized eggs. As the winter progressed (data from November and January were pooled as they did not differ significantly) (Fig. 2, bottom), some crabs contained mature eggs (although these did not yet exhibit the firm texture of mature eggs during the spawning season), with smaller, developing eggs still present. These results suggest that horseshoe crabs are determinate spawners; that is, all eggs to be laid in a given breeding season are mature at the onset of spawning, having matured gradually over the non-breeding season. We therefore did not include immature eggs in our seasonal fecundity estimates.
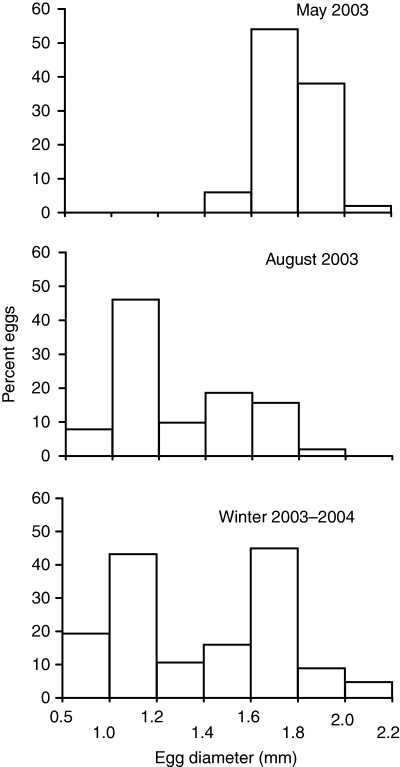
Egg diameter in the Atlantic horseshoe crab, Limulus polyphemus, in Pleasant Bay, Massachusetts during the spawning season (May 2003), and after the main spawning season in August 2003 and winter 2003–2004. Winter data comprise pooled data from November 2003 and January 2004, which did not differ significantly. All crabs contained immature eggs (<0.5 mm); only non-immature (i.e. developing or mature) egg sizes are graphed.
Dry weight of aliquots of mature eggs was uniform (mean ± SE = 0.337 g ± 0.002 or 1.69 × 10−3 g per egg), as was mature egg diameter (1.76 mm ± 0.015) among females during the spawning season, enabling us to obtain a reliable conversion factor for egg weight to egg number (588.2 ± 5.0 eggs·g−1).
Size-specific fecundity
In spite of the scatter in the individual points, on average the number of mature eggs (≥1.76 mm) per female increased with size of the female during the period of high spawning (14 May–3 June) (F = 18.693, P < 0.0001) (Fig. 3). [Data were log transformed to meet the assumptions of regression analysis (Somers 1991; Valiela 2001). This transformation did not change the significance of results; data are presented arithmetically for clarity.] The regression lines for each of the surveys done between 14 May and 3 June were significant, but did not differ among the different dates (ancova, P = n.s. for all pair-wise comparisons). In contrast, the survey taken at the end of peak spawning (16–17 June) shows far fewer eggs per female, and no relationship between female size and egg number (F = 0.088, P = n.s.); this may suggest that the females had spent their supply of eggs, and that a residue of eggs (mean ± SE = 11,700 ± 2200 eggs) were not deposited. If these eggs are in fact retained, they likely do not contribute to the population of the current breeding year. In case these eggs were in fact laid during the last few days of the season, we also provide conservative fecundity estimates assuming all eggs carried by females were actually laid, that is, assuming realized fecundity equaled potential fecundity. Though several studies (Packard 1870, 1885; Kingsley 1892, 1893; Munson 1898; Dumont & Anderson 1967) have looked at development and embryology of Limulus eggs, we are not aware of any that correlate vitellogenesis with time of year. Such histological investigations could clarify whether unlaid eggs are in fact resorbed at the end of the spawning season.
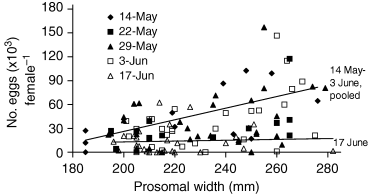
Egg number versus female size in the Atlantic horseshoe crab, Limulus polyphemus, in Pleasant Bay, Massachusetts on different collection dates during the 2003 spawning season (14 May–17 June). Each point represents data from one crab. Difference between regression lines for surveys during the season (14 May–3 June) were significant, but did not differ among the different dates (ancova, P = n.s. for all pair-wise comparisons). When data from 14 May–3 June were pooled, regression was significant (F = 23.5, P < 0.0001. y =0.5684x − 98.252). There was no correlation between female size and fecundity at the end of the season (16–17 June).
While values for the size-specific realized fecundity (Fig. 4, bottom) decreased relative to potential fecundity estimates (Fig. 4, top), the positive female size: egg number relationship was preserved. Unless otherwise noted, subsequent calculations were performed using realized fecundity, with the assumption that eggs were retained.
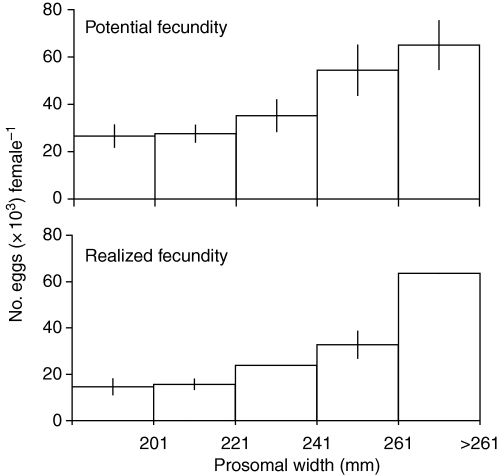
Potential fecundity (top) and realized fecundity (bottom), in the Atlantic horseshoe crab, Limulus polyphemus, in Pleasant Bay, Massachusetts. Realized fecundity = potential fecundity – number of retained eggs, estimated from data in Fig. 3. Standard errors are given where sample sizes were large enough to calculate them.
While small sample sizes in some of the late season size classes resulted in large SEs, there appeared to be a positive relationship between female size and percent of total eggs laid; i.e. larger crabs laid a higher percentage (and retained fewer) of the eggs they contained than smaller females (Fig. 5). Large females, then, appear to hold at least two advantages over small ones with regard to their breeding efficiency: they contain more eggs, and they are more effective at laying those eggs. These advantages may be due to behavioral or physiological differences, as yet unidentified. Difference in retention rates, for example, may be due to smaller crabs’ inexperience in finding suitable nesting sites or in the actual release of eggs.
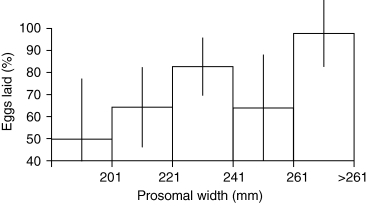
Percent of total eggs laid (realized fecundity divided by potential fecundity × 100) versus size in Limulus polyphemus, in Pleasant Bay, Massachusetts. When 241–261 mm size category is removed from analysis due to small sample size, P = 0.0018.
The fact that larger females carry and lay more eggs than smaller ones may make them more attractive as mates. Brockmann (1996) found that larger females attract more satellite males than smaller females. If eggs produce a chemical signal which is detected by males, as suggested by Ferrari & Targett (2003) and Hassler & Brockmann (2001), it is feasible that more eggs produce more signal, thereby making larger females more desirable.
Breeding behavior
The number of eggs found in individual clutches in nests excavated on two dates during peak spawning was quite variable; modal frequency was 640–1280 eggs per clutch (Fig. 6). This clutch size falls within the range reported in other studies (Table 1). Using the mean of this range, 960 eggs per clutch, and dividing it by the average number of eggs laid by females of a given size, we found that clutch size was small in relation to female egg load, representing between 2% and 7% of the total eggs laid by spawning females (Table 2). The number of eggs in a clutch showed no relationship to female size (F = 0.20, P = n.s.), consistent with findings by Cohen & Brockmann (1983) and Brockmann (1996).
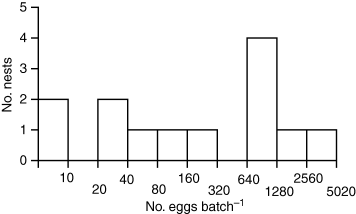
Frequency histogram of number of eggs per batch in clutches excavated from marked nests after crabs finished spawning. Data are plotted on a log2 scale.
| no. eggs per batch | study location | source |
|---|---|---|
| 900 | Atlantic Coast America (unclear where) | Sekiguchi & Nakamura (1979) |
| 960 | Pleasant Bay, MA | Current study |
| 1025 | Cold Spring Harbor, NY | Sokoloff (1978) |
| 1199–9610 | Florida | Cohen & Brockmann (1983) |
| 2236 | Florida | Brockmann (1990) |
| 3650 | Delaware Bay | Shuster & Botton (1985) |
| prosomal width | mean no. total eggs laid per female per season [mx (× 103)] | % total eggs in one clutch [(960/mx) ×100] |
|---|---|---|
| 201 | 14.5 | 7 |
| 221 | 15.7 | 6 |
| 241 | 23.9 | 4 |
| 261 | 32.7 | 3 |
| >261 | 63.5 | 2 |
Returns from our mark and recapture study indicated females returned to the study area most frequently during the same week, but also occasionally 2 and 4 weeks later (Table 3). Out of 315 females tagged, 35 (11.1%) were found again in the study area, and another 23 within a kilometer of the site, within 5 days of marking. Two weeks later, we saw three marked crabs; a month after marking, one marked crab was found (Table 3). Only six crabs were seen three times (PW data were only available for two of the six), and none more often, suggesting that many females may complete their spawning in two visits to the beach. This pattern of returns is supported in the literature (Rudloe 1980; Cohen & Brockmann 1983), though our number of returns may have been higher if we had marked crabs earlier in the season.
| elapsed time | no. recovered | % of marked | % of returns |
|---|---|---|---|
| 1 week (June 13–17) | 35 | 11.10 | 90 |
| 2 weeks (June 28) | 3 | 0.95 | 7.7 |
| 4 weeks (July 14) | 1 | 0.32 | 2.6 |
| total | 39 | 12.4 | 100 |
Clutch size data, in combination with returns during the first week of our mark and recapture study, confirm that Pleasant Bay horseshoe crabs, like populations studied elsewhere (Shuster & Botton 1985; Brockmann 1990; Penn & Brockmann 1994; Carmichael et al. 2003), sometimes come ashore more than once, primarily within 1 week, laying part of their total load of eggs on each trip. Size of individual clutches of eggs compared to total egg number carried by females confirms that crabs are laying eggs in multiple small clutches, rather than all at once.
If clutches contain 2–7% of a female's total eggs, as we report, we would predict her laying between 14 and 50 clutches. Larger females would need to lay more clutches or nests, or return more often to the beach to exhaust their egg supply, since they do not appear to lay bigger clutches. In fact, larger females did appear to return more often than smaller crabs (Fig. 7). The percentage of returns (normalized to percent of the population) was higher in larger crabs, and large crabs were the only ones we saw return for a third visit (Fig. 7). Brockmann (1990) reports that females laid 1–15 batches of eggs (mean 3.4 ± 2.3, N = 133) per nest (series of clutches in one place), and that more than one nest per female on one tide was not unusual. Given these numbers, a female would certainly have the potential to lay all of her eggs over two or three tidal cycles. For example, a large female could lay eight clutches of 1200 eggs in each of three different nests, return a second time and do the same, and lay a total of 58,000 eggs, which fits within the mean fecundity for large females (Fig. 5).
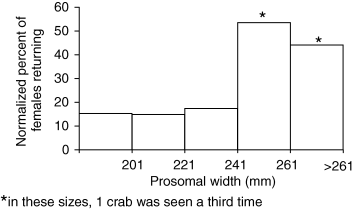
Percent of females (normalized by percent of population) by size recaptured in our mark and recapture study. Asterisk (*) denotes crabs found in spawning area a third time (one crab in each marked size class). Return data from the study site were consolidated with returns within a kilometer of the site, to increase sample size.
There was no detectable difference in the number of eggs between incoming and outgoing crabs (Fig. 8, ancova, P = n.s.). If crabs return to the beach only two or three times at most to spawn, as suggested by our mark and recapture study as well as previous studies, eggs laid at each spawning should presumably comprise 30–50%, an amount which should be detectable, of a female's potential fecundity. The fact that we did not detect such a difference in egg load between arriving and departing crabs implies another likely pattern: that crabs arrived to spawn in waves, as suggested by Shuster & Botton (1985). Estimates of egg deposition obtained by counts in females entering and leaving spawning areas are likely to be variable (Fig. 8). The crabs could have been newly arrived still containing their full load of eggs, or have spawned previously. In any case, the central point of Fig. 8 is that deposition of eggs during any one particular spawning involved much smaller numbers of eggs than were carried by the females during most of the spawning season; therefore, no difference was detectable between these two groups. It is not until the very end of the season (16–17 June) when no new crabs were arriving, and most of the females had presumably spawned, that we saw a consistent depletion of number of eggs in all female sizes.
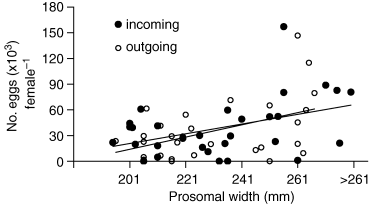
Egg number versus female size in the Atlantic horseshoe crab, Limulus polyphemus, in Pleasant Bay, Massachusetts for crabs coming in on the rising tide, and those leaving the beach on the outgoing tide. Difference between groups was n.s.
Net fecundity
There was a relatively wide range of female sizes among the 222 crabs we measured (Fig. 9). Females between 200 and 265 mm were the most common, with very few smaller or larger individuals. The lower number of crabs in the smaller size categories is likely due to two factors. First, it indicates that most females do not enter the spawning population until around 200 mm PW, so crabs smaller than this are scarce in the breeding area. In addition, because we measured size and not age, and growth slows with age (Sekiguchi 1988; Carmichael et al. 2003), the number of crabs accumulates in the mid-size ranges, until death or reproductive senescence likely removes them from the breeding population in the largest sizes (Carmichael et al. 2003). Other studies have found similar size ranges of adult females in Pleasant Bay (Table 4). It is unlikely that the drop-off in number of large females is due to harvest, even though the biomedical and bait harvests in Pleasant Bay both selected for large females (Rutecki et al. 2004). In a study of the impact of harvest on the Pleasant Bay horseshoe crab population, Rutecki et al. (2004) note that the biomedical harvest is associated with low (10–15%) mortality (also Rudloe 1983), and the bait and scientific harvests are very small. Approximately 120 of the largest females (>261 mm PW) were harvested by the biomedical industry, possibly resulting in 12–15 deaths. The total bait catch for the Bay was 187 crabs in 2001 (Rutecki et al. 2004); data were not broken down by size class. Scientific harvest, which targets smaller adults, was insignificant in the large size range. Even assuming a very conservative estimate that 50% of the bait catch was large females (94), the total harvest for females of that size would be about 107, which represents just over 2% of the 4100 females of that size in the Bay. This degree of harvest is unlikely to have an impact on the population structure of horseshoe crabs.
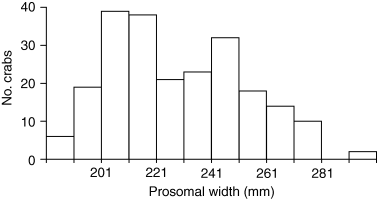
Size distribution of female Atlantic horseshoe crabs, Limulus polyphemus, in amplexus and presumed to be actively spawning, collected at our study site throughout the 2003 spawning season. N =222.
| range of PW (mm) | source |
|---|---|
| 186–272 | Shuster (1982) |
| 170–295 | James-Pirri et al. (2002) |
| 182–300 | Current study |
We calculated a total net fecundity (Fx) of 2.84 × 109 eggs produced in the Bay in one season (Table 5). The difference in contribution of realized fecundity (mxi) (data from Fig. 4, bottom) of different size classes multiplied by number of females of given sizes in the Bay (Nxi) to obtain net fecundity (Fxi = mxNxi) supported that crabs of intermediate size contributed by far the largest number of eggs to the population (Fig. 10). Large females were much more fecund that mid-size ones on a per crab basis, but there were fewer large females in the Bay than mid-size ones, so their relative importance in terms of net fecundity was dwarfed by the size-specific contribution to net fecundity by mid-size crabs.
| prosomal width (mm) | mean no. eggs (mx) (×103) | no. females with mature eggs in Pleasant Bay (Nx) (×103) | net fecundity (mxNx) = (Fx) (×108) |
|---|---|---|---|
| Eggs retained | |||
| 201 | 14.5 ± 6.88 | 22.9 ± 6.1 | 3.32 ± 0.0018 |
| 221 | 15.7 ± 4.65 | 56.5 ± 15.2 | 8.87 ± 0.0026 |
| 241 | 23.9 | 30.0 ± 8.1 | 7.2 |
| 261 | 32.7 ± 11.7 | 19.6 ± 5.3 | 6.41 ± .0029 |
| >261 | 63.5 | 4.1 ± 1.1 | 2.6 |
| Total | 133 | 28.37 ± 0.99 | |
| All eggs spawned | |||
| 201 | 26.6 ± 4.39 | 22.9 ± 6.1 | 6.1 ± 0.0019 |
| 221 | 27.7 ± 3.6 | 56.5 ± 15.2 | 15.6 ± 0.0047 |
| 241 | 35.2 ± 6.8 | 30.0 ± 8.1 | 10.6 ± 0.0019 |
| 261 | 54.4 ± 10.8 | 19.6 ± 5.3 | 10.6 ± 0.0047 |
| >261 | 65 ± 10.3 | 4.1 ± 1.1 | 2.6 ± 0.0035 |
| Total | 133 | 45.4 ± 0.98 | |
- Data from females containing no mature eggs were excluded. Eggs retained: (net fecundity) = (potential fecundity) − (retained eggs). All eggs spawned: (net fecundity) = (potential fecundity). No. females in Pleasant Bay was determined from Carmichael et al. (2003) by fitting data to our size classes and extrapolating to Bay population. Total net fecundity: estimated no. eggs produced in Pleasant Bay in one spawning season by all spawning females.
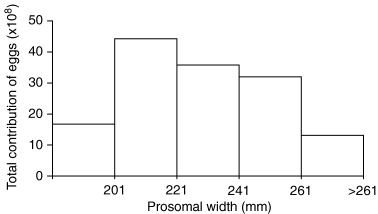
Net fecundity (Fxi) by size, or total contribution of eggs by each size class of female horseshoe crabs.
Our estimate of the total number of eggs produced (Fx) is almost an order of magnitude smaller than the number of eggs estimated by Carmichael et al. (2003) (1.3 × 1010) based on the Shuster & Botton (1985) estimate of 88,000 eggs per female. Our findings are consistent with egg densities found in our study area by James-Pirri et al. (2005). While they speculate that the difference between Carmichael et al.'s (2003) modeled findings and their empirical ones may be due to spawning disruption, it is more likely due to the former study's overestimation of egg numbers based on previous, less comprehensive estimates of number of eggs produced per female.
No estimates for shorebird populations are available for Pleasant Bay, and it is not known how much these animals rely on Limulus eggs for sustenance in this area, so it is not known whether available eggs limit the number of shorebirds or how significant an impact shorebird predation may have on Limulus egg survival. Our estimates of total egg production compared to those of Carmichael et al. (2003) greatly reduces the predicted number of shorebirds that the eggs of the current horseshoe crab population could sustain, to 2.8 × 103 birds·day−1 based on 8300 eggs·day−1 per eaten bird (Botton et al. 1994). Further studies of shorebird populations and their reliance on horseshoe crab eggs are needed to determine whether a relationship exists between bird populations and available eggs in Pleasant Bay.
In addition to influencing net fecundity, the size-frequency distribution of female horseshoe crabs may also contribute to an apparent lack of size-selectivity among amplexed mates. Unlike satellite males, amplexed males show only a very weak or no preference for larger females (Cohen & Brockmann 1983; Loveland & Botton 1992; Suggs et al. 2002). Suggs et al. (2002) found that overall, small and medium-sized males found mating partners more often than large males, and when large males did pair, it was with large females. Large males may simply have fewer large females to choose from, given the predominance of medium-sized females.
Our findings with regard to Limulus fecundity have implications for management of the Pleasant Bay horseshoe crab population and illustrate the importance of assessing net fecundity in terms of population structure rather than individual fecundity alone. If, for example, harvest pressure were focused on mid-sized females because they were individually less productive than larger crabs, consequences for the total egg production by the population could be significant. If enough mid-sized females were removed from the population, recruitment and population size could be negatively impacted. This in turn could negatively impact shorebird populations. The development of models using these data could quantify the effects of such manipulations of harvest pressure on the population.
Conclusion
We found a previously unreported correlation between female size and number of eggs in Limulus. Similar studies on fecundity in Delaware Bay and Florida, where crabs are generally larger and smaller, respectively, than in Pleasant Bay, could determine whether the size to fecundity relationship continues into size ranges typical for those areas. Horseshoe crabs appear to be determinate spawners, so we did not include immature eggs in our estimates. On average, crabs seem to retain eggs at the end of the season; it is therefore important to obtain data on realized fecundity, rather than just potential fecundity. Though large crabs laid the most eggs, their numbers in Pleasant Bay were so low that their total contribution of eggs to the population was far less than that of mid-size crabs. In addition to these novel data, results from our study site also confirmed those of other researchers that crabs returned repeatedly, primarily <3 times within the same week, to lay multiple small clutches of eggs. They appeared to arrive at the breeding beach in waves, perhaps due to distance they overwintered from shore, health, and other factors. Our estimates of fecundity can be incorporated into models to help manage this important species.
Acknowledgements
This project was partially funded by MIT Sea Grant 8247-5. Thanks to Ruth Carmichael for sharing her data and providing valuable assistance in preparation of this manuscript. Chuck O'Connell, Joe Francese, Joy Ramstack, and Allyson Papa provided help in the field and lab.




