Do Harpacticoids (Copepoda) Use Water-Borne Cues to Aid in Locating Food Parcels?
Abstract
Abstract. Harpacticoid copepod food is patchily distributed and individuals must episodically move to new food patches. Key questions in harpacticoid feeding ecology include how individuals detect food patches and how rapidly they move toward patches. Based on literature reports, we hypothesized that harpacticoids locate food parcels by sensing a water-borne cue. We tested this idea using individuals of Leptastacus coulli Huys, 1992 and Praeleptomesochra similis Lang, 1965 from a sandy beach in north Florida (29.948° N, 84.341° W). Individuals from both species selected seawater that had been exposed to food significantly more frequently than they selected control seawater. The rate of movement of these species was millimeters per second, which agreed with previous reports.
Problem
Harpacticoid copepods feed on many foods, including microalgae (Carman & Thistle, 1985), bacteria (Rieper, 1978, 1982), and flesh (Lopez, 1982; Guidi, 1984; Seifried & Dürbaum, 2000). Such resources are distributed patchily in the natural environment. For example, a patch of decaying flesh occurs where a benthic organism has died (Lopez, 1982; Olafsson, 1992), and patches of microbes occur in depressions on sand grains (Meadows & Anderson, 1968; Miller, 1989). As a consequence, a fundamental feature of harpacticoid ecology is each individual's constant need to find concentrations of food. Work on other crustacean taxa suggests that a food parcel can be located if the animal can sense water-borne chemicals leaking from the parcel (Zimmer-Faust, 1987; Webster & Weissburg, 2001). Although the study of the mechanisms of food location in harpacticoids is in its infancy, some species are known to be able to detect a food parcel at a distance of many body lengths (Seifried & Dürbaum, 2000). In concert, these facts suggest that harpacticoids also use water-borne cue(s) to detect food parcels at a distance. We tested this inference for two harpacticoid species.
To reach a food parcel, an individual must both detect it and move toward it, so an additional parameter in harpacticoid feeding ecology is the rate at which an animal moves toward a food parcel. Very little information exists on harpacticoid movement rates, either in general or during movement toward a food parcel; here we report estimates of both.
Material and Methods
We studied two harpacticoid species from the intertidal zone of the public beach of Bald Point State Park at the mouth of Ochlockonee Bay, Florida (29.948° N, 84.341° W). Species selection was a practical matter; other species were too rare to study. The sediment was a well-sorted, coarse sand (median particle diameter = 0.54 mm). Water content was 18.2%, and organic carbon content was 0.4%. At the time of our collections, the seawater temperature was 22.2 °C, and the salinity was 27.3.
We chose a test material that occurred in the habitat and consisted of more than one harpacticoid food to increase the probability of detecting a response. Pieces of fish flesh occur naturally in the seabed, because carcasses and pieces of fish flesh fall from the water column after some predation events. Fish flesh itself should attract flesh-eating harpacticoids (Lopez, 1982; Guidi, 1984; Seifried & Dürbaum, 2000). Bacteria co-occur with decaying flesh because decay begins instantly (Guidi, 1984), making fish flesh also attractive to harpacticoids that consume bacteria (Rieper, 1978, 1982; Carman & Thistle, 1985).
Striped mullet (Mugil cephalus Linnaeus, 1758, Actinopterygii, Mugiliformes), a local fish, was selected for use in the decaying-fish-flesh treatment. We filleted 10 specimens that had been kept on ice for 3 days. We cut a total of 15 regular pieces from each fillet and weighed and measured each one to ensure similarity of surface area and size. Pieces weighed 4.9–5.1 g and measured either 30 × 20 × 10 mm or 25 × 25 × 10 mm. Each piece was individually wrapped in aluminum foil. One piece from each fillet was placed in each of 15 jars and frozen at −80 °C. Before a set of trials, a jar was taken out of the freezer and 10 fish pieces were unwrapped and placed in individual beakers. Each beaker was filled with seawater to the 100-ml mark and left to thaw for ca. 12 h before the first trial. We used artificial seawater of salinity 32 throughout the experiment. The order in which the pieces from the different individual fish were used was determined at random, and no two trial days had the same order. Control beakers contained seawater only.
To test for a water-borne cue to decaying fish flesh, we conducted a y-maze preference experiment. Our y-maze (Fig. 1) consisted of glass tubing with a 0.4-cm lumen. A peristaltic pump was used to move seawater from reservoirs to the maze. Seawater entered via tubes A and A′ simultaneously, passed through tubes B and C, and exited by tube D. A vent in tube C allowed air bubbles to escape and experimental animals to be introduced. One individual was placed in the maze for each trial. Two marks on tube B (M1 and M2), separated by 1.2 cm, allowed us to measure the rate of progress of the individual against the flow through the tube.
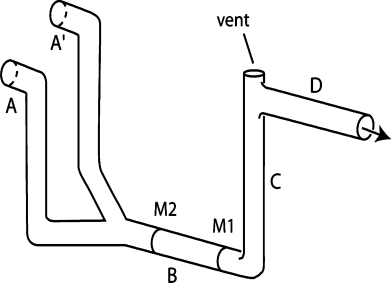
Sketch of the maze showing the tubes by which the two types of seawater entered (A, A′), the test section (B) with the two marks for the measurements of swimming speed against the flow (M1, M2), the connecting tube C, and the tube by which the seawater left the maze (D). Test individuals were introduced into the maze through the vent in the top of tube C. The arrow indicates the direction of flow. M1 was 1.2 cm from M2.
Sediment from our site was kept in the laboratory at 22 °C under dim light. To obtain test individuals, we transferred an aliquot of sediment to a sorting dish with a Pasteur pipette and, under a dissecting microscope, moved harpacticoid individuals into a second dish with a micropipette.
At the beginning of each trial, the lower portion of the maze was submerged in a water bath so the investigator could see the test individual with a dissecting microscope. Harpacticoids can be sensitive to light (Palmer, 1984), so the microscope was fitted with a ring light intended to minimize directional bias. We used a micropipette to move one haphazardly chosen, unidentified individual into tube C. Once the animal could be seen in tube B, the pump was turned on (volume pumped = 0.0442 cm3·s−1 from each reservoir). The flow through the maze was determined to be laminar (Re ≈ 27) on the basis of Reynolds number (= Re) calculations (Vogel, 1981) and observations of dyed fluid. To determine swimming speed against the flow, we used a stopwatch to measure the time the individual took to swim the distance from M1 to M2. If the individual swam into tube A or A′, its choice was recorded. The animal subsequently was identified to species and sex, and its length measured with the aid of a camera lucida.
We flushed all tubing between trials, first with distilled water and then with seawater, to remove the fluid from the previous trial. We also alternated which tube (A or A′) received the seawater that had been exposed to decaying fish flesh (= treated seawater) and which the control seawater. At the end of each day, a 0.5-M sulfuric acid solution was flushed through the system for 5 min before being replaced by distilled water, which was left inside overnight.
Individuals could only be identified under the compound microscope, so each was identified only after its trial. We decided a priori to include only adults in our study because of the risk of mistakes in the identification of juveniles. Most of the adults we tested were found to be individuals of Leptastacus coulliHuys, 1992 or Praeleptomesochra similisLang, 1965. Results of the few trials done with individuals of other species are not given because too few replicates were available for meaningful statistical tests.
To analyze preferences, we used binomial probabilities when the number of individuals in a comparison was less than 14 and a chi-square test when abundances were greater. For the analysis of swimming speed, we used a t-test (equal variances not assumed). All tests were two-tailed, and in this exploratory study we did not correct for multiple testing.
Results
Leptastacus coulli (adult female body length including furca ∼1 mm) and Praeleptomesochra similis (adult female body length including furca ∼0.5 mm) are species of Noodt's (1971) C3 Lebensformtypen, sediment-dwelling forms that are relatively slender and vermiform (Fig. 2). In 62 of 86 trials, individuals of L. coulli chose to swim up the tube that carried treated seawater, a significant difference from random choice (P < 0.005) (Table 1). In 11 of 13 trials, individuals of P. similis chose to swim up the tube that carried treated seawater, also a significant difference from random choice (P < 0.024) (Table 1). We also examined males, non-gravid females, and gravid females separately for each species (Table 1). In all cases, more individuals selected the treated than the control seawater.
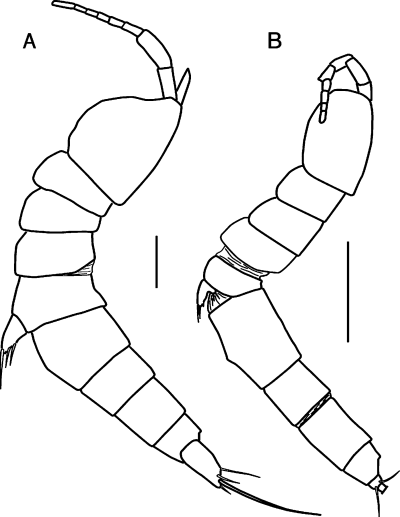
Habitus sketches of adult females of (A) Leptastacus coulli and (B) Praeleptomesochra similis in lateral view, showing their narrow, elongate bodies. Most appendages have been omitted. Scale lines = 0.01 cm.
| treated | control | percentage | |
|---|---|---|---|
| Leptastacus coulli | |||
| total | 62 | 24 | 72.1 |
| males | 34 | 9 | 79.1 |
| non-gravid females | 24 | 13 | 64.9 |
| gravid females | 4 | 2 | 66.6 |
| Praeleptomesochra similis | |||
| total | 11 | 2 | 84.6 |
| males | 4 | 1 | 80.0 |
| non-gravid females | 5 | 0 | 100.0 |
| gravid females | 2 | 1 | 66.6 |
- Both species and all subgroups chose the treated seawater more often than the control seawater.
Table 2 summarizes our results for swimming speed against the flow. For L. coulli, we had sufficient replication to report separate results for males and females (gravid and non-gravid combined). The results for trials in which the test individual chose the treated seawater are given separately because such individuals might have increased their speed in response to treated seawater. We found no significant differences in speed between individuals that selected treated seawater and those that did not.
| speed | n | ||||
|---|---|---|---|---|---|
| [mm·s−1] | [body lengths·s−1] | ||||
| average | range | average | range | ||
| Leptastacus coulli males | |||||
| treatment | 0.53 | 0.06–1.30 | 1.40 | 0.15–3.59 | 34 |
| control | 0.84 | 0.21–2.29 | 2.32 | 0.51–6.55 | 9 |
| Leptastacus coulli females | |||||
| treatment | 0.62 | 0.08–1.38 | 1.44 | 0.21–3.20 | 28 |
| control | 0.42 | 0.10–0.92 | 1.07 | 0.26–2.90 | 15 |
| Praeleptomesochra similis males and females | |||||
| treatment | 0.64 | 0.09–1.42 | 1.66 | 0.19–3.94 | 11 |
| control | 0.50 | 0.25–0.74 | 1.38 | 0.50–2.26 | 2 |
- For still-water swimming speeds, see text. n = number of individuals tested.
Discussion
1. Cues
Laboratory choice experiments sometimes reveal only potentialities because the experimental circumstances differ too greatly from conditions in the animal's habitat. Leptastacus coulli is an interstitial species (Huys, 1992). Praeleptomesochra similis has an elongate body and reduced swimming legs, and the specimens we studied were collected from a sand beach, implying that it too is an interstitial species. In the maze, we mimicked the flow in the interstitial spaces of beaches and shelf sands. In such sediments, pressure differences caused by breaking waves (Riedl & Machan, 1972) or surface gravity waves (Webb & Theodor, 1968, 1972) pump water through the interstitial spaces. These interstitial flows are laminar (Re on the order of 1). To match this condition, we created laminar flow in the maze. Thus, our experiment presented interstitial species with an interstitial flow regime. Although all possibility of experimental artifact can never be eliminated (Hurlbert, 1984), the counterbalancing in our experimental design should have kept any bias in the delivery of the two types of seawater to the test animals from influencing our results.
Our species were able to use water-borne chemical cues under these circumstances to move toward decaying fish flesh. This result suggests that this strategy of locating food could be used in natural interstitial spaces. Further, it would not be surprising if interstitial animals could follow water-borne cues to other food sources or to mates.
In our experiments, some individuals did not swim toward either the control or the treatment. The cause of this behavior is not clear. They may have been in poor health when selected and/or been traumatized by experimental procedures. Alternatively, they may have been those that had large energy reserves and thus had little need to move toward a concentration of food. Carman et al. (1991) and Thistle et al. (1995) have shown that individual harpacticoids vary substantially in the extent of their stored energy reserves.
2. Swimming speed
To arrive at a food patch, an individual must both detect its presence and travel to it. Our species swam rather than crawled through the maze. To estimate their swimming speed, we began by calculating the speed of the opposing flow. The flow in the maze was laminar, so its speed decreased parabolically from the center of the tube to the walls. The maximum speed that an individual would have had to swim against was 14 mm·s−1 (Hagen-Poiseuille equation; Vogel, 1981). To estimate the minimum speed that an individual would have had to swim against, we calculated the speed 0.025 mm from the wall (our species were ∼0.05 mm wide) and found it to be 0.35 mm·s−1. Therefore, minimum and maximum still-water swimming speeds can be estimated by addition of 0.35 or 14 mm·s−1 to the values in Table 2. Seifried & Dürbaum (2000) reported still-water swimming speeds of 2.4–10.2 mm·s−1, values comparable to ours (see also Palmer et al., 1992). Although more measurements are needed, harpacticoid still-water swimming speeds seem to be millimeters per second.
Conclusions
We have shown that two species of interstitial harpacticoids chose seawater that had been exposed to decaying fish flesh significantly more than control seawater. We infer that they responded to a water-borne cue from a source of food. Because of the orderly flow in the pore spaces, we suspect that such cues are useful in the interstitial environment and that other interstitial species use them for locating such things as food and mates.
Acknowledgements
We received technical assistance and advice from L. Sedlacek and M. Teasdale. L. Dewhirst helped in the field. The manuscript has been improved by the comments of B. C. Hippolyte, L. Sedlacek, M. Teasdale, and A. B. Thistle. We wish to express our thanks for this kind help. Contribution number 1103 of the Florida State University Marine Laboratory.




