Legs of deception: disagreement between molecular markers and morphology of long-legged flies (Diptera, Dolichopodidae)
Beine der Täuschung: Widerspruch zwischen molekularen Markern und Morphologie von Langbeinfliegen (Diptera, Dolichopodidae)
Present address: Space Biology Group/BIOTESC, ETH Zurich /Technopark, Technoparkstr. 1, CH-8005 Zurich, Switzerland
Abstract
enConflicting hypotheses in phylogenetics and systematics, generated by different data sets (e.g. morphological versus molecular), are common in biology. The clarification of such instances may allow understanding general mechanisms involved in the speciation process in an evolutionary light. Here, we present and discuss the case of the Dolichopus plumipes species group in the long-legged flies, Dolichopodidae. A phylogenetic survey was performed based on both morphological and molecular data. The full data set comprises 31 morphological characters and 2252 molecular characters (mitochondrial – COI: 810; 12S: 343; 16S: 514; nuclear – ITS2: 585) of 49 different species, represented by 82 specimens. The molecular phylogenetic analysis revealed a clade (composed by the species D. plumipes, Dolichopus wahlbergi, Dolichopus polleti, Dolichopus simplex, and Dolichopus nigricornis) that disagrees with the traditional morphological view based on external characters. In particular, specimens of the species D. plumipes and D. simplex were indistinguishable with the molecular markers used here. Yet, we still consider D. plumipes and D. simplex as two distinct taxa and provide explanatory hypotheses on the evolutionary background. The conspicuous male secondary sexual characters (present in plumipes but not in simplex) are key factors in sexual selection and their presumably rapid reduction in D. simplex is thought to be of main importance for the explanation of the speciation process. The plumipes–simplex case may therefore be viewed as a paradigmatic illustration showing that a better integration of the molecular and morphological approaches is needed to understand and clarify the, in some cases, complex systematics and phylogeny of organisms.
Zusammenfassung
deWidersprüchliche Hypothesen in Phylogenie und Systematik durch die Verwendung unterschiedlicher Datensätze (z.B. morphologische und molekulare Daten) sind keine Seltenheit in der Biologie. Die schlüssige Klärung solcher Sachverhalte erlaubt vielfach ein Verständnis grundlegender evolutiver Mechanismen der Artbildung. Hier präsentieren und diskutieren wir einen solchen Fall anhand der Dolichopus plumipes Artengruppe der Langbeinfliegen, Dolichopodidae. Eine phylogenetische Analyse morphologischer und molekularer Daten wurde durchgeführt. Der Datensatz umfasst 31 morphologische und 2252 molekulare Merkmale (mitochondrial: COI: 810; 12S: 343; 16S: 514; nuklear: ITS2: 585) von 49 Arten, welche in 82 Individuen vertreten sind. Die Analyse der molekularen Daten ergab eine Gruppe (bestehend aus D. plumipes, D. wahlbergi, D. polleti, D. simplex, und D. nigricornis), welche sich nicht mit der traditionellen morphologischen Sicht deckt. Als Überraschung waren Individuen der Arten D. plumipes und D. simplex mit den verwendeten molekularen Markern ununterscheidbar. Wir betrachten D. plumipes und D. simplex dennoch weiterhin als unterschiedliche Taxa und stellen erklärende Hypothesen zum evolutiven Hintergrund dazu vor. Die auffälligen sekundären männlichen Geschlechtsmerkmale (vorhanden bei plumipes, fehlend jedoch bei simplex) sind Schlüsselfaktoren der sexuellen Selektion. Damit kommt der vermutlich schnellen Reduktion dieser Merkmale bei D. simplex groβe Bedeutung für die Erklärung des Artbildungsprozesses zu. Der plumipes-simplex-Fall soll auch als paradigmatische Illustration einer stärkeren Integration von molekularen und morphologischen Ansätzen dienen, was in einigen Fällen unverzichtbar ist für das Verständnis komplexer Systematik und Phylogenie von Organismen.
Introduction
Mechanisms of speciation are still far from being totally unravelled and appear to be so complex and diverse that no general assumption for all organisms seems to hold true. The same problem arises in defining species. Indeed, several species concepts have been proposed, starting with the modernization of speciation since Darwin and Mendel by Dobzhansky (1937), Mayr (1942) and Simpson (1945). The introduction of molecular methods and the revival of the species debate in the sense of the Modern Synthesis after Coyne and Orr (2004) even added a number of new species concepts (e.g. general Avise and Wollenberg 1997; review by Sites and Marshall 2003; de Queiroz 2007). In practice any species will always remain a well or poorly supported hypothesis, depending on the underlying data. And in this context, one might wonder if molecular data can really be used as a universal tool to separate closely related taxa. At present, this approach remains truly controversial. The DNA barcoding league strongly advocates genetic markers as the ultimate solution for the identification of all life forms (Hebert et al. 2003). DNA taxonomy sensuTautz et al. (2003) proposed molecular evidence as the crucial key to taxonomy, including phylogenetic research. At the other side stand the traditional taxonomists with a massive knowledge on morphology and natural history of their investigated taxa, but with scepticism towards ‘more modern’, molecular approaches. This scepticism seems to be confirmed by the fact that over time numerous examples have accumulated which prove the COI sequence insufficient and sometimes unreliable for easy differentiation in some insect taxa (general overview: Rubinoff et al. 2006; Diptera: Meier et al. 2006) or by introgression events (Diptera: Withworth et al. 2007). In our opinion and in agreement with Meyer and Paulay (2005) integrative taxonomy (see Dayrat 2005; Will et al. 2005), i.e. the combination of traditional morphological investigations with molecular data from several markers – both mtDNA and nuclear DNA – seems the most reliable method to gather sound arguments for the respective phylogenetic position of taxa investigated.
With over 7100 species worldwide, long-legged flies or Dolichopodidae represent one of the most diverse families in the order Diptera (Pape et al. 2009). More than 1700 species belong to the subfamily Dolichopodinae with Dolichopus and Hercostomus as the most species-rich genera in the family, encompassing about 580 and 470 species, respectively (Yang et al. 2006; see also Sinclair et al. 2008). Despite – or just because of – this staggering biodiversity, profuse male secondary sexual characters (MSSC) and elaborate courtship behaviour, hardly any attempt was made until recently to unravel the phylogeny of this family. Bernasconi et al. (2007b) confirmed the monophyly of the subfamily Dolichopodinae based on molecular data – as was previously suggested by Brooks (2005) on the basis of morphological data. In another study, Bernasconi et al. (2007a) provided support for the monophyly of the genus Dolichopus on the basis of both molecular (COI, Cyt-b) and morphological data. Whereas most of the clades within Dolichopus generated by molecular analyses correspond well with traditional morphological species groups, the Dolichopus plumipes species group sensuMeuffels and Grootaert (1989) did not. That species group originally comprised three species [D. plumipes (Scopoli, 1763), Dolichopus wahlbergi Zetterstedt, 1843 and Dolichopus polletiMeuffels and Grootaert 1989] that all share a highly conspicuous plumose mid tarsus, considered as a unique synapomorphy. Yet, in sharp contrast to this morphological evidence, the molecular analyses (Bernasconi et al. 2007a) apart from the additional species Dolichopus nigricornis Meigen, 1824, rather suggested a very close relationship between D. plumipes and Dolichopus simplex Meigen, 1824. This result is surprising since D. simplex entirely lacks a plumose mid tarsus and has therefore never been thought to be closely related with the D. plumipes species group.
Incongruence between morphological and molecular data sets can point out unexpected events or mechanisms of speciation in evolutionary biology. Furthermore, as Vogler and Monaghan (2007) propose, a better understanding of species delimitation is especially well achievable through incorporation of discrepant data.
With an extended Dolichopus data set (41 species), we hypothesize in this paper about possible evolutionary interpretations with respect of the disagreement between the phylogenetic relationships based on morphological and molecular data in the D. plumipes–simplex species pair within the D. plumipes species group. Apart from COI, we use the additional molecular mitochondrial markers 16S, 12S and the nuclear marker ITS2. The latter has recently proved to be useful for resolving phylogenetic relations in Diptera Brachycera (Song et al. 2008), in Homoptera Aphididae (Coeur d’acier et al. 2008), and is known to discriminate successfully even at population level (e.g. Hymenoptera Encyrtidae Alvarez and Hoy 2002).
Materials and Methods
Samples
Eighty-two samples (specimens) of 49 species of the subfamily Dolichopodinae were included in this study. The ingroup consists of 41 species of Dolichopus. Eight outgroup species from five genera of Dolichopodinae were used. Collection data on all samples are presented in Table S1. All samples were conserved in 100% ethanol at 4°C.
Morphological data and phylogenetic analyses
Table S2 presents the states of the 31 morphological characters used in this study, including 29 non-genital and 2 genital characters checked exclusively in male specimens (see also Appendix S1). All characters were equally weighted and treated as unordered.
Morphological data were analysed by both Maximum Parsimony (MP) and Bayesian (BAY) tree reconstruction methods. MP [using the heuristic search with stepwise addition option – tree bisection reconnection (TBR) – branch swapping and 100 additional replicates] was performed using PAUP*4.0b10 and the reliability of internal branches was assessed by bootstrapping with 1000 pseudo-replicates. MrBayes version 3.1.2 was used for the BAY analyses (characters considered as ‘unordered’ and ‘variable’). The Markov chain Monte Carlo search was run with four chains (one cold and three heated) for 1 500 000 generations, with trees being sampled every 100 generations. Two independent trials were performed with ‘temp’ parameter T = 0.2 and T = 0.07, respectively. To determine the ‘burn-in’, log-likelihood plots were examined for stationarity (where plotted values reach an asymptote). In both cases, stationarity was clearly reached after less than 100 000 generations (=1000 trees), but we discarded the first 3000 trees to ensure that stationarity was completely reached. Higher ‘burn-in’ did not alter the topology of the final 50% majority rule consensus tree(s). Bayesian posterior probabilities were therefore given by the percentage of runs that produced each branch and were calculated from the remaining trees generated from the two parallel runs. In the analysis performed with T = 0.07, the two independent runs executed in parallel converged very quickly, reaching average standard deviation values of the split frequencies of <0.05.
DNA extraction, amplification and sequencing
DNA was extracted from fly specimens using the Dneasy Tissue kit (Qiagen AG, Hombrechtikon, Switzerland) following the manufacturer’s instructions. Entire specimens were first mechanically triturated in a microtube using a TissueLyser (Mixer Mill MM 300; Qiagen AG). After digestion with Proteinase K (20 μg/ml), samples were applied to the columns for DNA absorption and washing. Finally, the DNA was eluted in 200 μl of the buffer from the kit and stored at −20°C.
PCRs
Standard PCR reactions were performed with 2 μl of the extracted DNA as template, 0.5 μM of each primer, 1 Unit Taq Polymerase (HotStarTaq Master Mix Kit; Qiagen AG) in a total volume of 50 μl (manufacturer’s buffer). For all genes (COI, 12S, 16S, and ITS2), the reaction mixtures were subjected to 15 min DNA denaturation at 94°C, 35 cycles of denaturation at 94°C for 1 min, annealing at 48–54°C for 1 min and elongation at 72°C for 2 min. The elongation was completed by a further 7 min step at 72°C. The PCR reactions were performed in a DNA Thermal Cycler (Perkin-Elmer Applied Biosystems, Rotkreuz, Switzerland). The amplification and sequencing primers (Microsynth GmbH, Balgach, Switzerland) are listed in Table S3 and unless mentioned otherwise, are the same or modified versions of those published in Simon et al. (1994), Lunt et al. (1996), Zhang and Hewitt (1997) and Masunaga (1999).
DNA sequencing
Templates for direct sequencing were prepared by a simple purification step of PCR products using the QIAquick PCR Purification Kit (QIAGEN AG) or the NucleoSpin Extract II Kit (Macherey-Nagel AG, Oensingen, Switzerland), following the manufacturer’s instructions in both cases. Alternatively, the purification of the PCR products was performed by adding to each PCR product 2 ml (1 U/ml) Shrimp Alkaline Phosphatase (Promega AG, Wallisellen, Switzerland) and 1 ml (20 U/ml) Exonuclease I [New England Biolabs (Bioconcept), Allschwil, Switzerland]. The ExoSAP protocol consisted of 45 min incubation at 37°C and 15 min deactivation at 80°C. Cycle sequencing reactions were performed in total volumes of 15 μl using an ABI Prism Big Dye Terminator Cycle Sequencing Kit (Perkin-Elmer Applied Biosystems), purified using DyeEx 2.0 Spin Kit (Qiagen AG) or NucleoSEQ Kit (Macherey-Nagel AG) on an ABI Prism 3100-Avant Genetic Analyser (Perkin-Elmer Applied Biosystems) or on an ABI 3730 DNA Analyser (Perkin-Elmer Applied Biosystems), again following the manufacturer’s instructions.
DNA sequence analyses
The gene sequences (COI, 12S, 16S and ITS2) were handled and stored with the Lasergene program Editseq (DNAstar Inc., Madison, WI, USA). Alignment of all gene sequences was performed using Megalign (DNAstar Inc.) and, when necessary, manually adjusted. ForCon (Raes and Van de Peer 1999), a software tool for the conversion of sequence alignments, was further applied. The partition-homogeneity test (Farris et al. 1994) implemented in PAUP*4.0b10 (Swofford 2002) was used to test whether the data sets could be combined. Phylogenetic reconstruction was carried out using BAY analysis, performed with MrBayes version 3.1.2 (Ronquist and Huelsenbeck 2003). Modeltest 3.5 (Posada and Crandall 1998) was used to help us to identify the evolutionary model(s) to apply to our data for the BAY analyses. Thus, for the BAY analyses, data were partitioned by gene kind (COI, 12S, 16S and ITS2) and the COI gene was further partitioned by codon (first-, second- and third-codon position). BAY analyses were allowed to use a mixed model (i.e. a model in which all genes have their unique GTR+I+G model) and the Markov chain Monte Carlo search was run with four chains (one cold and three heated) for 1 500 000–2 500 000 generations, with trees being sampled every 100 generations. The heating of the chains was adjusted to get the acceptance rates for the swaps between chains to 10–70% (‘temp’ parameter varied therefore from 0.01 to 0.2). Nine independent trials have been performed on two different computers until optimal values of the various parameters were reached (including the convergence of the two independent runs executed in parallel – average SD values of the split frequencies of <0.05). To determine the ‘burn-in’, log-likelihood plots were examined for stationarity (where plotted values reach an asymptote). Depending on the analysis conditions, stationarity was clearly reached after less than 100 000 (=1000 trees) or remarkably after more generations. In all the cases, BAY posterior probabilities were given by the percentage of runs that produced each branch and were calculated from the remaining trees generated from the two parallel runs. Preliminary analyses using the MP and the Neighbour Joining method were performed with MEGA (Molecular Evolutionary Genetics Analysis version 3.1; Kumar et al. 2004) and PAUP*4.0b10. All new sequences analysed here have been deposited in GenBank (see Table S1).
Results
Out of 31 morphological characters considered here, 30 were parsimony informative. The MP tree reconstruction method produced 22 equally parsimonious trees of length 127 (consistency index = 0.323; retention index = 0.653; rescaled consistency index = 0.211; homoplasy index = 0.677). A 50% majority rule consensus tree from the BAY analysis was derived from 24 002 trees (12 001 trees for each of the two parallel runs) and was based on the full morphological data matrix. The topology of the latter BAY tree was generally less resolved, but not conflicting with the strict consensus tree generated in the MP analysis. For this reason, in the MP strict consensus tree (Fig. 1), we added the node support derived from the posterior probabilities in the BAY analysis next to the bootstrap support derived from 1000 bootstrap pseudo-replicates.
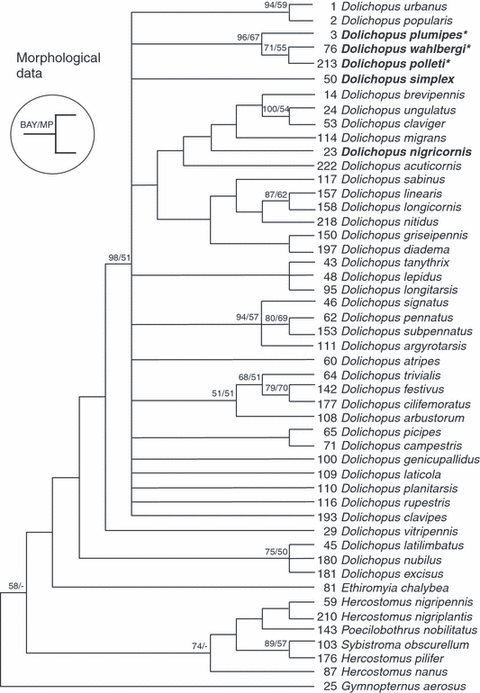
Maximum Parsimony strict consensus tree of 22 equally parsimonious trees of length 127 (consistency index = 0.323; retention index = 0.653; rescaled consistency index = 0.211; homoplasy index = 0.677) based on 30 parsimonious informative morphological characters in 49 dolichopodid species. Posterior probabilities over 50% (derived from a Bayesian analysis) and bootstrap support values from 1000 pseudo-replicates (MP analysis) are depicted above nodes. Members of the D. plumipes species group (sensu this paper) are indicated in bold. An asterisk (*) indicates the presence of a plumose mid metatarsus (character 17) as a synapomorphy. Note that the large clade including D. nigricornis is not characterized by any synapomorphy but is the result of a combination of characters
In the case of the molecular data, the partition-homogeneity test indicated that the four gene partitions were not significantly mutually incongruent (p = 1 for the gene partition; p = 0.74 for mtDNA versus ITS2) which justified the combination of the four molecular data sets, the further analysis is based upon. The results of the analyses are thus based on the total molecular evidence through concatenation of the four separate partitions. In preliminary analyses involving all samples available (see Table S1), specimens of nearly all species made monospecific clades, with the exception of the specimens of D. plumipes and D. simplex which formed together a mixed clade (see further). Therefore, only one specimen from each species has been included in the final phylogenetic analyses. The full data set comprises 2252 characters (16S: 514; COI: 810; 12S: 343, ITS2: 585) with 587 variable sites (16S: 101; COI: 275; 12S: 87, ITS2: 124). Phylogenetic relationships derived from 20 002 BAY trees (10 001 trees for each of the two parallel runs) based on combined 16S rDNA, COI, 12S rDNA and ITS2 sequences as established between 49 dolichopodine species are illustrated in Fig. 2.
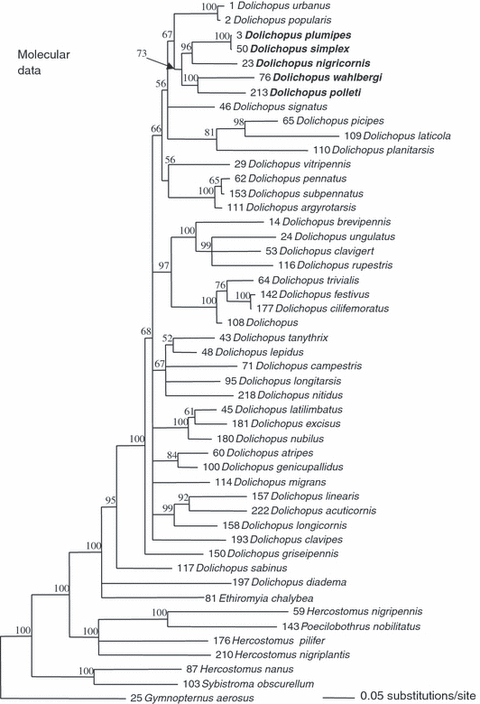
Phylogenetic relationships derived from 20 002 Bayesian trees based on combined COI, 12S rDNA, 16S rDNA and ITS2 sequences as established between 49 Dolichopodid species. The tree is a 50% majority rule consensus tree; values of posterior probabilities over 50% are indicated above branches (branches with posterior probabilities less than 50% are collapsed). The members of the D. plumipes species group (sensu this paper) are indicated in bold
Phylogenetic relationships between species not belonging to the D. plumipes species group are not in the focus of this paper and hence, are not further treated here. Most of them have already been discussed in detail by Bernasconi et al. (2007a,b) and, together with the structure and composition of the Ortochile genus group sensuBrooks (2005) (within the subfamily Dolichopodinae) will be further dealt with in a separate paper (Pollet M, Germann C, Tanner S, Bernasconi MV, unpublished data).
As expected on the basis of their morphology (the plumose mid metatarsus, in particular), D. plumipes, D. wahlbergi and D. polleti form a clade (Fig. 1) with moderate (MP analyses: 67%) to strong (BAY analyses: 96%) support. The position of D. simplex remains unresolved, while that of D. nigricornis lacked any statistical support (see Fig. 1). Dolichopus wahlbergi and D. polleti, however, grouped together with weak (MP analyses: 55%) or moderate (BAY analyses: 71%) support.
The molecular analysis, on the contrary, provides a different picture with a large clade (73% posterior probability support), including all five species (D. plumipes, D. simplex, D. wahlbergi, D. polleti and D. nigricornis) (Fig. 2). This clade encompasses three subclades, one consisting of D. plumipes, D. simplex and D. nigricornis (96% support), a second with D. plumipes and D. simplex (100% support) and a third comprising D. wahlbergi and D. polleti with 100% support. In particular, specimens of the species D. plumipes and D. simplex are indistinguishable with the markers we used. No mitochondrial haplotype nor the ITS2 sequence is characteristic for either species that would allow a clear genetic distinction between specimens of D. plumipes and D. simplex.
Discussion
All phylogenetic analyses – morphological and molecular – suggested, in general and with variable support, the close relationship between D. plumipes, D. polleti and D. wahlbergi (1, 2). Surprisingly, in the molecular tree, D. simplex clustered together with D. plumipes, as well as D. nigricornis. Furthermore, D. plumipes and D. simplex cannot be separated on the basis of the sequence data at hand (COI, 12S rDNA, 16S rDNA, ITS2) which might lead to the conclusion that D. plumipes and D. simplex must be considered as conspecific. This assumption, in turn, is strongly contradicted by ten unequivocal and consistent morphological differences between males of both species (Tables 1 and S2, Fig. 3).
| D. plumipes | D. simplex | D. wahlbergi | D. polleti | |
|---|---|---|---|---|
| Male | ||||
| Head | ||||
| Face: colour | Shining lemon yellow | Shining whitish yellow* | Shining golden yellow | Shining golden yellow |
| Antenna, first flagellomere: colour | Pale yellow with apical 2/3 black | Entirely black* | Pale yellow with apical 2/3 black | Pale yellow with apical 2/3 slightly darkened |
| Antenna, scape and pedicel: colour | Entirely pale yellow | Pale yellow, with pedicel darkened on inner face* | Entirely pale yellow | Entirely pale yellow |
| Legs/wings | ||||
| Femora I–III and tibiae I, III: colour | Yellow | Yellow | Whitish yellow | Whitish yellow |
| Femur II: ventral tubercle | Very weak, at basal 1/3 | Very weak, at basal 2/5* | Weak, at about basal 1/3 | Weak, at about basal 2/7 |
| Femur II: ventral pile | From basal 1/3 till apex | Absent* | From basal 1/5 to basal 1/3 | From basal 1/5 to basal 1/3 |
| Tibia II: colour | White with distinct dark brown stripe | Whitish yellow* | Pale brown | Yellowish white |
| Tibia II: dorsal face | Bare | Entirely pubescent* | Bare | Bare |
| Tibia II: attenuation (dorsoventral flattening) | Very strong | Very weak* | Strong | Strong |
| Tibia III: pile (character 27, Appendix S1) | Rather narrow patch at basal 1/5, narrowing into stripe towards apex | Absent* | Rather broad patch at basal 1/6, narrowing into stripe towards apex | Rather broad patch at about basal 1/5, narrowing in stripe towards apes |
| Wing: costal stigma | Small | Absent* | Small | Small |
| Hypopygium | ||||
| Aedeagus: shape (lateral view) | Slender with gentle subapical bend | Slender with gentle subapical bend | Stout, short and straight | Robust, long and with strong subapical bend |
| Basoventral epandrial lobes | Strongly asymmetrical | Strongly asymmetrical | Slightly asymmetrical | Slightly asymmetrical |
| Apicoventral epandrial lobe: shape | Smaller, with dorsal acute process | Smaller, with dorsal acute process | Larger, rounded rectangular | Larger, rounded rectangular |
| Ventral lobe of surstylus: shape | With gentle basidorsal bend | With gentle basidorsal bend | With strong basidorsal bend | With strong basidorsal bend |
| Cercus: shape | Ovoid | Ovoid | Rounded rectangular | Subcircular |
| Female | ||||
| Head, clypeus: | Bare | Bare | Pubescent | Pubescent |
| Antenna, 1st flagellomere: colour | Pale yellow with apical 2/3 black | Entirely black* | Pale yellow with apical 2/3 black | Pale yellow with apical 2/3 slightly darkened |
| Tarsus II: colour | Black, with only extreme basis yellow | Mostly yellow with dark apex, sometimes entirely brownish yellow* | Whitish yellow on basal 1/2 | Entirely black |
- Unequivocal characters (10 in males, two in females) to separate D. simplex from D. plumipes are marked with an asterisk (*). The remarkable absence of conspicuous MSSCs in D. simplex is marked in bold.
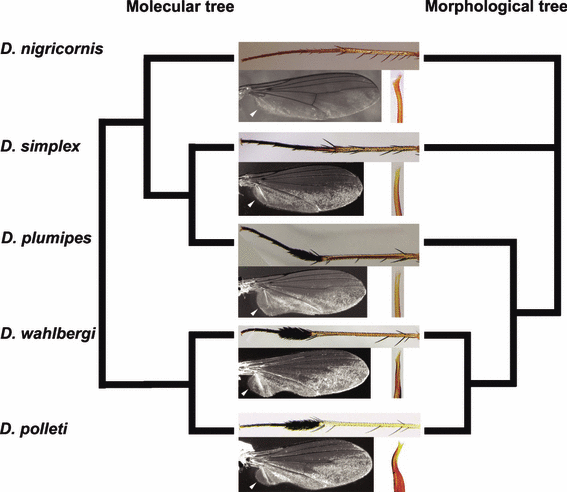
Simplified excerpt of the phylogenetic trees; modified mid tibia and tarsus, wing (MSSCs), and apex of aedeagus in the five D. plumipes species group members sensu this paper. Note that (i) the MSSCs are absent in D. simplex and D. nigricornis, (ii) the laterally compressed fifth tarsomere of the fore tarsus (MSSC) in D. nigricornis is not included, (iii) the aedeagus apex is very similar in the species pair plumipes–simplex and (iv) the results of the morphological and molecular analysis are clearly not congruent (see text)
Meuffels and Grootaert (1989) considered D. plumipes, D. wahlbergi and D. polleti close relatives featuring a number of morphological characters regarded as synapomorphous to this species group: in particular, an attenuated mid tibia and plumose mid metatarsus (Fig. 3). The plumose mid metatarsus is known to play an important role in courtship behaviour (Zimmer et al. 2003). During this process, male D. plumipes takes a position in front of the female after hovering over it. After spreading and vibrating the wings, the mid legs (with plumose tarsi) are lifted and alternatively moved while the abdomen with the genital apparatus is bent forward (Lunau 1996).
In contrast, D. simplex does not possess the plumose mid metatarsus and was previously never considered a member of the species group. Furthermore, a costal stigma, an undulating posterior wing margin, ventral pile of the mid femur and posteroventral pile of the hind tibia – four MSSCs possessed by the other three species – are absent in D. simplex as well (Tables 1 and S2). Nevertheless, its genetic profile being identical to that of D. plumipes seems to be reflected in more general morphological characters that it shares with the latter species. Although genital appendages are consistently different in both species (details not shown), they show obvious similarities and are clearly different from those of D. polleti and D. wahlbergi (Table 1). Finally, females of D. plumipes and D. simplex can only be separated on the basis of the colour of the antennal first flagellomere and of the mid metatarsus. Neither has D. nigricornis ever been considered a member of the Dolichopus plumipes species group, due to its considerably different morphology (laterally compressed fifth tarsomere of the fore tarsus, see trait 14 in Table S2) and general habitus (bigger size, elongated wings and more slender legs).
The molecular data suggest that both D. simplex and D. nigricornis clearly belong to the D. plumipes species group. Based on morphological data, the position of both species remains unresolved (see Results). While intentionally disregarding the conspicuous MSSCs, a close morphological relationship between at least D. plumipes and D. simplex seems probable. Whether we deal with a secondary loss of MSSCs in the case of D. simplex (and/or a shift in D. nigricornis) or with a less parsimonious parallel (and thus independent) development of nearly identical MSSCs in D. plumipes, D. wahlbergi and D. polleti cannot be answered coherently on the basis of our data. However, the evolutionary process itself has undoubtedly been affected by or is affecting the mating behaviour of D. simplex as all MSSCs present in the other species are used during courtship. Regardless of the above assumptions, the fact remains that a disagreement between the morphological and molecular analysis is observed in the D. plumipes–simplex species pair (see Fig. 3). In what follows, we hypothesize about a possible evolutionary interpretation.
In general, three different types of explanations can be considered to justify the alteration or loss of MSSCs (reviewed in Wiens 2001): environmental, social and random factors (genetic drift). We did not consider the third type as data on this aspect are not available, but we discuss the first two types (ecophenotypes as environmental; sexual selection as social), and add two alternative types, specifically applicable to the present case.
Demasculinization
Demasculinization, i.e. the reduction of male primary and secondary sexual characters in Dolichopus species induced by parasitic nematodes. The impact of these parasites on the development of male genitalia was only recently reported by Kahanpää (2008) and observed in D. fraterculus Zetterstedt, 1843, D. lepidus Staeger, 1842, D. longitarsis Stannius, 1831, D. picipes Meigen, 1824, D. popularis Wiedemann, 1817 and D. plumipes, mostly from Finland. Yet, the reported latter case of demasculinised D. plumipes specimen and the modifications mentioned by the author (wider, female-alike face) show that these are clearly different from D. simplex which falsifies this hypothesis. Furthermore, no nematodes were found in the D. simplex specimen examined in this study.
Wolbachia
Wolbachia is a gram-negative bacterium that infects arthropods, especially insects with rates from 17% to 76% (overview in Hoy 2003). Just recently Withworth et al. (2007) reported on a case of mitochondrial markers COI and COII in the dipteran genus Protocalliphora (Calliphoridae) being affected by Wolbachia. This considerably reduced the differentiation between the species as a result of introgressive hybridization associated with this Wolbachia infection. Typically this occurs between very closely related species where rare hybridization events admit the transfer of Wolbachia. So the presented identical genetic profiles of D. simplex and D. plumipes might be explained by the same phenomenon. However, all samples of the D. plumipes species group sensu this paper have been tested for Wolbachia via PCR, with negative outcome (manuscript in preparation). With these results, we can exclude Wolbachia infection as an explanation in the present case as well.
Different ecophenotypes
Different environmental factors as, e.g. predation risk, changed preconditions for an optimal signal transmission or limited availability of nutrients especially put pressure on high cost MSSCs that might ultimately lead to the alternation of morphological characters (Wiens 2001). Yet, in the present case, D. plumipes is one of the most widespread dolichopodid species in the Palaearctic (Pollet 2004b) and even occurs in Greenland (Pollet and Böcher 2005) and North America (Pollet et al. 2004). It inhabits all kinds of habitats with a preference for humid mesotrophic to eutrophic grasslands (Pollet 2001). D. simplex, on the contrary, shows a much more restricted distribution (Pollet 2000) and clearly prefers more oligotrophic habitats like peatmoors and humid heathlands. As a result, sympatry in these species is only very rarely observed. These ecological differences might act differently on the respective inhabiting populations and as such a precondition for two ecophenotypes of the same species might be given, a possible first step in an ecological speciation event. But as MSSCs are involved, an alternative explanation or even one in combination with the acting hypothesis is given hereunder.
Sexual selection: a case of rapid morphological evolution
Strong female choice is well known in Dolichopodidae (Lunau 1996; Zimmer et al. 2003) and a sound precondition for assuming sexual selection as a strong speciation force. Female choice has been reported in species with well-developed MSSCs as Poecilobothrus nobilitatus (Linnaeus, 1767) (Lunau 1992, 1996; Land 1993a,b) and Dolichopus brevipennis Meigen, 1824 as well as in species that even lack conspicuous MSSCs like Dolichopus acuticornis Wiedemann, 1817 (Zimmer 1999) and D. ungulatus (Linnaeus, 1758) (Lunau 1996). From a molecular point of view, it seems that we have reached the limits of the classical (or most widely used) genetic markers. In other words, we obtain insufficient resolution, but why? A case of rapid evolutionary process might be a possible explanation. MSSCs represent a pre-mating barrier and D. simplex might be in an early stage of the speciation process, which seems to be reflected by the fact that morphologically it strongly resembles D. plumipes, but only lacks the MSSCs of the latter. But is a rapid reduction of such an apparently complex structure realistic? In general, it is well known that sexually dimorphic traits evolve at a higher rate than most other morphological traits (Andersson 1994; True et al. 1997; Civetta and Singh 1998; Kopp and True 2002). The gain or loss of MSSCs is a fast process [general review: Wiens 2001; sex combs in Drosophilidae (Diptera): Kopp and True 2002; Mishra and Singh 2006; toothed femora in Sepsidae (Diptera): Puniamoorthy et al. 2008], and at least partly driven by the changes in female preferences (Ng and Kopp 2008).
The plumose mid metatarsus of members of the D. plumipes species group shows, indeed, an extraordinary similarity with the sex combs in Drosophila and most probably shares similar molecular regulation pathways. Minor changes within these regulatory pathways already lead to differences in morphology (Stern 1998). Furthermore, even in remotely related Oncopeltus (Heteroptera) (Angelini and Kaufman 2005), the mostly sex-specific expression of MSSCs on tarsi proved to depend on the same HOX genes (Scr, Sex combs reduced) as in Drosophila. This only illustrates the general and widespread nature of these pathways in insects. Not only a fast change in apparently complex MSSCs – even at microevolutionary timescales – is probable (sex combs: Barmina and Kopp 2007; wing spot: Prud’homme et al. 2006) but also the repeated loss and gain of complex structures is well supported (Kopp and True 2002; Kulathinal and Singh 2004; Barmina and Kopp 2007). So the possibility that very closely related (and, in the present case, genetically indistinct) species present morphologically very different traits (MSSCs) is, indeed, absolutely realistic.
Based on our data, we cannot confirm or reject with certainty two of the four hypotheses given above. Furthermore, also a combination of both of them is possible, but an estimation of how much each of them might contribute to the evolutionary process remains difficult. Nevertheless, as distinct MSSCs are involved, the sexual selection component likely outweighs the other hypotheses and might contribute the most to an explanation with a sound evolutionary background.
Conclusion
In this paper, we presented a case in which both a purely molecular approach as well as a merely morphological treatment would have failed to unravel the phylogenetic relationships between closely related dolichopodid species. On the one hand, absent MSSCs in D. simplex are likely to deceive the morphological worker focused on apparently complex morphological structures, interpreted as synapomorphies, that might be regulated by only a few pleiotropic (HOX) genes. The molecular worker, on the other hand, might be inclined to consider D. plumipes and D. simplex as conspecific without taking into account potential rapid speciation events, provoked by sexual selection pressure, that largely precedes sequence divergence in the most often used markers. Combining both disciplines enabled us to hypothesize on a remarkable evolutionary process that might be more common than currently expected, but perhaps went largely unnoticed up until now. The next step would be a further, detailed morphological study and the use of more sensitive molecular markers, e.g. microsatellites, broadly used in population genetics. This, together with an extensive specimen sampling of both species from various localities, will provide further insights into the relationship between these two species. Also ethological research, including the study of the courtship strategy in D. simplex in particular, might provide us with the ultimate trigger that led to the current deception and the key to its evolutionary origin.
Acknowledgements
This paper is dedicated to the memory of our friend and colleague Paul I. Ward (director of the Zoological Museum), who passed away prematurely on April 19, 2008. We thank Andrea Gubler (Zoological Museum, University of Zurich, Switzerland) for checking our samples for Wolbachia infection. We are grateful to Kazuhiro Masunaga (Oroshimo Kusatsu Shiga, Japan) for kindly providing us with the primer sequences (ITS2 region) previously published in his paper (Masunaga 1999) written in Japanese. We thank Marcel Güntert (director of the Natural History Museum, Bern) for the use of infrastructure by the first author. Sincere thanks are due to Martin Schäfer (Zoological Museum, University of Zurich, Switzerland) for helpful discussions, and to two anonymous referees who provided useful comments. This work was supported by a grant of the Swiss National Science Foundation (Grant 3100A0-115981) to M. V. Bernasconi. This paper is part of the PhD research project of the first author and is a contribution of the Zoological Museum, University of Zurich, Switzerland.




