Phylogeny of Thalassinidea (Crustacea, Decapoda) inferred from three rDNA sequences: implications for morphological evolution and superfamily classification
Phylogénie de Thalassinidea (Crustacea, Decapoda) déduite de trois rDNA séquences: Implications pour l’évolution morphologique et la classification des superfamilles
Abstract
enThe infraorder Thalassinidea is a group of cryptic marine burrowing decapods of which the higher taxonomy is often contentious. The present analysis attempts to reconstruct phylogenetic relationship among 12 of the 13 currently recognized families using partial nuclear 18S, 28S rDNA and mitochondrial 16S rDNA sequences. The infraorder is divided into two distinct clades, with the first clade consisting of Thalassinidae, Laomediidae, Axianassidae and Upogebiidae, and the second clade including Axiidae, Calocarididae, Eiconaxiidae, Callianassidae, Ctenochelidae, Micheleidae, Strahlaxiidae and Callianideidae. Within the first clade, the Upogebiidae is the basal family. The Axianassidae shows low affinity to other laomediid genera indicating that it is a valid family. The interfamilial relationships are less well resolved in the second clade. The Axiidae is paraphyletic with respect to Calocarididae and Eiconaxiidae. Thus, the status of these two latter families is not supported if the currently defined Axiidae is maintained. All three families appear to be basal in the thalassinidean clade. The Micheleidae is closely related to the Callianideidae and they form a sister group to the Strahlaxiidae. The monophyletic Callianassidae aligns with the Micheleidae + Callianideidae + Strahlaxiidae clade. The relationship among the Axiidae + Calocarididae + Eiconaxiidae clade, Callianassidae + Micheleidae + Callianideidae + Strahlaxiidae clade and the Ctenochelidae cannot be resolved which might be due to a rapid radiation of the three lineages. Our results do not support the generally used classification scheme of Thalassinidea and suggest that the infraorder might be divided into two superfamilies instead of three as suggested based on larval morphology, second pereiopod morphology in adults and gastric mill structure. The two superfamilies are Thalassinoidea (i.e. Thalassinidae, Laomediidae, Upogebiidae and Axianassidae) and Callianassoidea (i.e. Axioidea + Callianassoidea, as defined in Martin and Davis (2001) but excluding Laomediidae and Upogebiidae). It also appears that gill-cleaning adaptations are important in thalassinidean evolution while the presence of linea thalassinica is a result of parallel evolution.
Résumé
kaL’infraordre des Thalassinidea est un groupe de décapodes marins fouisseurs cryptiques dont la taxonomie au niveau supérieur est souvent controversée. Cette analyse tente de reconstruire les relations phylogénétiques entre 12 familles sur les 13 actuellement reconnues en utilisant les séquences partielles de rDNA nucléaire 18S, 28S et de rDNA mitochondrial 16S. L’infraordre est divisé en deux clades distincts, le premier comprenant les Thalassinidae, Laomediidae, Axianassidae et Upogebiidae, et le deuxième comprenant les Axiidae, Calocarididae, Eiconaxiidae, Callianassidae, Ctenochelidae, Micheleidae, Strahlaxiidae et Callianideidae. Dans le premier clade, les Upogebiidae est la famille basale. Les Axianassidae montre peu d’affinité avec les autres genres de laomedidés, ce qui indique que la famille est valide. Les relations interfamiliales sont moins bien résolues dans le second clade. La famille des Axiidae est paraphylétique par rapport aux Calocarididae et Eiconaxiidae. Ainsi le statut de ces deux dernières familles n’est pas supporté si la famille des Axiidae est maintenue dans sa définition actuelle. Toutes les trois familles apparaissent basales dans le clade thalassinidéen. La famille des Micheleidae est très proche des Callianideidae et elles forment un groupe frère des Strahlaxiidae. La famille monophylétique des Callianassidae s’aligne avec le clade Micheleidae + Callianideidae + Strahlaxiidae. La relation entre le clade Axiidae + Calocarididae + Eiconaxiidae, le clade des Callianassidae + Micheleidae + Callianideidae + Strahlaxiidae et la famille des Ctenochelidae ne peut être résolue, ce qui pourrait être dûà une radiation rapide des trois lignées Nos résultats ne supportent pas le schéma de classification généralement utilisé pour les Thalassinidea et suggèrent que l’infraordre pourrait être divisé en deux superfamilles au lieu de trois comme suggéré sur la base de la morphologie larvaire, de la morphologie du deuxième péréiopode de l’adulte et de la structure du moulin gastrique. Les deux superfamilles sont: les Thalassinoidea (c’est-à-dire Thalassinidae, Laomediidae, Upogebiidae et Axianassidae) et Callianassoidea (c’est-à-dire Axioidea + Callianassoidea, comme définis dans Martin et Davis 2001 mais excluant les Laomediidae et les Upogebiidae). Il apparaît aussi que les adaptations pour le nettoyage des branchies sont importantes dans l’évolution thalassinidéenne alors que la présence de la linea thalassinica est le résultat d’une évolution parallèle.
Introduction
The Thalassinidea (mud shrimps or ghost shrimps) is a group of cryptic, marine burrowing decapods that occur worldwide. They have attracted increasing attention in ecological research on the effects of their burrowing activities on benthic community structure and tidal-flat nutrient composition (Posey et al. 1991; Ziebis et al. 1996; Dworschak 2000). In spite of their ecological importance, the higher taxonomy of these animals is rather confusing (e.g. de Saint Laurent 1973; Kensley 1989; Sakai and de Saint Laurent 1989). The most recent classification scheme of Martin and Davis (2001) followed the revision of Poore (1994) who recognized 11 families that are classified into three superfamilies: Axioidea, Thalassinoidea and Callianassoidea.
The classification of the Thalassinidea is extremely controversial, and the monophyly of the infraorder and its superfamilies and families has always been in dispute. Borradaile (1903) first designated four families: Axiidae (Huxley, 1879), Laomediidae (Borradaile, 1903), Thalassinidae (Dana, 1852) and Callianassidae (Dana, 1852) (with two sub-families: Callianassinae and Upogebiinae). However, Gurney (1938) proposed a phylogenetic relationship among the four thalassinidean families based on larval morphology. In his scheme, the Thalassinidea was paraphyletic and divided into two lineages: a ‘Homarine group’ consisting of the Axiidae and Callianassidae, and an ‘Anomuran group’ containing the Upogebiidae and Laomediidae. Since then many authors (e.g. de Saint Laurent 1973, 1979a; Kensley 1989; Sakai and de Saint Laurent 1989; Manning and Felder 1991; Sakai 1992a, 1999; Sakai and Ohta 2005) have erected new families or sub-families as well as removed existing ones, leading to a very confusing higher classification of this group. de Saint Laurent (1979a,b) and de Saint Laurent and Le Loeuff (1979) proposed a classification scheme essentially similar to that of Gurney (1938) but based on adult morphology. On the other hand, Poore (1994) published the first extensive cladistic analysis of the infraorder based on morphology and proposed a new classification scheme and a phylogenetic tree for the 22 genera representing 11 families and three superfamilies studied. He considered the Axioidea to be the sister group of the Thalassinoidea and Callianassoidea, and established one more family, Strahlaxiidae. The family Ctenochelidae was found to be paraphyletic (but see Tudge et al. 2000; who argued for monophyly of the Ctenochelidae). Poore’s (1994) result also supported the monophyly of the infraorder. However, subsequent studies based on morphology (Tudge et al. 2000; Dixon et al. 2003; Ahyong and O’Meally 2004) and gastric mill morphology (Sakai 2005) challenged the relationships of the families of Poore (1994) and more families have been added (Tudge and Cunningham 2002; Sakai 2005). On the other hand, recent evidence from spermatozoa and spermatophore morphology (Tudge 1995, 1997) and molecular analyses (Morrison et al. 2002) has put the monophyly of the Thalassinidea into question.
Burrowing behaviour is a characteristic feature of thalassinideans. It is believed to be a derived character for the Thalassinidea and various morphological adaptations have evolved to facilitate the burrowing lifestyle, including modified appendages (Coelho and Rodrigues 2001) and the presence of the linea thalassinica (Poore 1994). These characters are important to thalassinidean evolution and are highly informative in systematics and phylogenetic reconstruction of the Thalassinidea. However, the appendages are usually involved in multiple functions and homoplasy in functionally important characters hamper a reliable definition in cladistic analysis. Thus, resolving the phylogeny of Thalassinidea could provide valuable information on the role of character evolution as a result of adaptations.
Tudge and Cunningham (2002) made the first attempt to resolve the phylogenetic relationships among thalassinidean families using DNA sequence data of nuclear 18S rDNA and mitochondrial 16S rDNA. Their result based on 14 species from five families revealed two major clades with strong support: Upogebiidae + Laomediidae + Thalassinidae and Strahlaxiidae + Callianassidae. The Laomediidae is shown to be paraphyletic and the support for the monophyly of the infraorder was low. In a recent study on reptant decapod phylogeny, Ahyong and O’Meally (2004) also recovered the same two clades using morphology and three molecular loci (18S, 28S and 16S rDNA). These two clades to some extent correspond to the grouping proposed by Gurney (1938), de Saint Laurent (1979a,b) and Sakai (2005). However, these two molecular phylogenetic analyses suffer from limited taxon sampling of thalassinideans. The present study provides the first molecular systematic analysis based on an extensive taxon sampling of this group, involving 12 of the 13 families currently recognized (see Martin and Davis 2001; Tudge and Cunningham 2002; Sakai and Ohta 2005). A phylogeny was constructed among thalassinidean families to evaluate the current taxonomy using DNA sequence data from nuclear 18S and 28S rDNA and mitochondrial 16S rDNA. The goals of the present study are: (1) to test for the separation of the two major clades in the Thalassinidea; (2) to test for monophyly of the thalassinidean superfamilies; and (3) to reconstruct an overall phylogeny of the families within the Thalassinidea.
Materials and Methods
Taxon sampling and outgroup choice
A total of 27 species from 23 genera of 12 out of the 13 currently recognized families in the Thalassinidea were analysed in this study. The only family not represented was Thomassiniidae (superfamily Callianassoidea). Of these, 12 species were wholly or partially sequenced in this study, while the other sequences analysed were obtained from GenBank database or from Dr C.C. Tudge (see Tudge and Cunningham 2002). Sequences from Cherax glaber and Homarus americanus in the infraorder Astacidea were used as outgroups since Astacidea was found to be closely related and possibly basal to the Thalassinidea (Dixon et al. 2003; Ahyong and O’Meally 2004; Porter et al. 2005). Only outgroups from one infraorder were included because the major objective of this study focuses on the taxonomy and phylogeny within the Thalassinidea, and not the monophyly of this infraorder. Enoplometopus occidentalis (generally considered belonging to Astacidea) was also included in the analysis as the taxonomic status of Enoplometopus has been controversial and some authors believe that it is affiliated with axiids (e.g. Holthuis 1974, 1983; Kensley and Child 1986). Species included in the study, sample locations, GenBank accession numbers and voucher numbers are listed in Table 1.
| Taxon | Voucher ID | Sample locations | Gene | ||
|---|---|---|---|---|---|
| 16S | 18S | 28S | |||
| Thalassinidea | |||||
| Superfamily Thalassinoidea | |||||
| Thalassinidea | |||||
| Thalassina squamifera (de Man, 1915) | – | Tudge and Cunningham (2002) | – | – | – |
| Thalassina anomala (Herbst, 1804) | ZRC1998.2263 | GenBank | AY583896 | AY583969 | EF585476 |
| Superfamily Callianassoidea | |||||
| Callianassidae | |||||
| Biffarius arenosus (Poore, 1975) | – | GenBank | DQ079705 | DQ079739 | – |
| Biffarius delicatulus Rodrigues and Manning, 1992 | – | Tudge and Cunningham (2002) | – | – | – |
| Callichirus major (Say, 1818) | – | GenBank | DQ079707 | DQ079741 | DQ079777 |
| Callianassa subterranea (Montagu, 1808) | – | GenBank | DQ079706 | DQ079740 | – |
| Lepidophthalmus louisianensis (Schmitt, 1935) | – | GenBank | DQ079717 | DQ079751 | DQ079792 |
| Neocallichirus rathbunae (Schmitt, 1935) | – | Tudge and Cunningham (2002) | – | – | – |
| Neotrypaea californiensis (Dana, 1854) | – | GenBank | AF436042 | AF436003 | – |
| Sergio mericeae Manning and Felder, 1995 | – | GenBank | DQ079733 | DQ079768 | DQ079811 |
| Callianideidae | |||||
| Callianidea typa H. Milne-Edwards, 1837 | MNHNTh 1507 | New Caledonia | EF585448 | EF585459 | EF585470 |
| Ctenochelidae | |||||
| Ctenocheles balssi Kishunouye, 1926 | 530-2-1787 | Japan | EF585444 | EF585455 | EF585466 |
| Laomediidae | |||||
| Jaxea nocturna Nardo, 1847 | GenBank | AF436046 | AF436006 | – | |
| Laomedia astacina De Haan, 1841 | NTOUA00366 | Taiwan | EF585450 | EF585461 | EF585472 |
| Laomedia healyi Yaldwyn and Wear, 1970 | – | Tudge and Cunningham (2002) | – | – | – |
| Axianassidae | |||||
| Axianassa australis Rodrigues and Shimizu, 1992 | – | Tudge and Cunningham (2002) | – | – | – |
| Upogebiidae | |||||
| Austinogebia narutensis (Sakai, 1986) | NTOUA00416 | Taiwan | EF585443 | EF585454 | – |
| Gebiacantha plantae (Sakai, 1982) | – | Tudge and Cunningham (2002) | – | – | – |
| Upogebia affinis (Say, 1818) | – | GenBank | AF436047 | AF436007 | – |
| Superfamily Axioidea | |||||
| Axiidae | |||||
| Calaxius manningi Kensley et al., 2000 | NTOUA0053 | Taiwan | EF585447 | EF585458 | EF585469 |
| Calocarides chani Kensley et al., 2000 | NTOUA00423 | Taiwan | EF585445 | EF585456 | EF585467 |
| Eiconaxiidae | |||||
| Eiconaxius indicus (de Man, 1907) | NTOUA00829 | Pratas Is., South China Sea | EF585449 | EF585460 | EF585471 |
| Calocarididae | |||||
| Calastacus crosnieri Kensley and Chan 1998 | NTOUA00212 | Taiwan | EF585446 | EF585457 | EF585468 |
| Paracalocaris sagamiensis Sakai, 1991 | NTOUA00142 | Taiwan | EF585453 | EF585464 | EF585475 |
| Micheleidae | |||||
| Meticonaxius soelae Sakai, 1992 | NTOUA00094 | Taiwan | EF585451 | EF585462 | EF585473 |
| Strahlaxiidae | |||||
| Neaxius acanthus (Milne-Edwards, 1878) | NTOUA00421 | Taiwan | EF585452 | EF585463 | EF585474 |
| Neaxius glyptocercus von Martens, 1868 | – | Tudge and Cunningham (2002) | – | – | – |
| Astacidea | |||||
| Parastacidae | |||||
| Cherax glaber Riek, 1967 | – | GenBank | AF135978 | DQ079745 | DQ079783 |
| Nephropidae | |||||
| Homarus americanus H. Milne-Edwards, 1837 | – | GenBank | AF370876 | AF235971 | DQ079788 |
| Enoplometopidae | |||||
| Enoplometopus occidentalis (Randall, 1840) | – | GenBank | AY583892 | AY583966 | – |
- Accession numbers for the sequences obtained in this study are in bold. The sequences from Tudge and Cunningham (2002) do not have GenBank accession numbers.
Laboratory analysis
Total genomic DNA was extracted from pleopods using the commercial QIAamp Tissue Kit (Qiagen, Hilden, Germany). A partial mitochondrial 16S rDNA segment was amplified using the universal primers 16Sar and 16Sbr (Simon et al. 1994) or 1472 (Crandall and Fitzpatrick 1996). A partial 18S rDNA segment was amplified using primer set A and B (Apakupakul et al. 1999) while a partial segment of 28S rDNA gene was amplified using Rd3.2a and Rd5b (Whiting 2002). The amplifications were conducted in a reaction mix containing 1 μl of template DNA, 1X polymerase chain reaction (PCR) reaction buffer, 2 mM MgCl2, 200 nM of each primer, 200 μM dNTP, 1.5 units of Taq polymerase (Amersham, Uppsala, Sweden) and ddH2O to a total volume of 50 μl. The PCR profile for 16S rDNA was as follows: 3 min at 94°C for initial denaturation, then 33 cycles of 30 s at 94°C, 30 s at 47°C, 40 s at 72°C with a final extension for 3 min at 72°C. The same profile was employed for 18S and 28S rDNA with an annealing temperature of 53°C and extension time of 2 min in each cycle. The PCR products were then purified using the QIAquick gel purification kit (Qiagen) according to the manufacturer’s instructions. Sequencing reactions were performed using the same sets of primers for 16S and 28S rDNA. For 18S rDNA, sequences were obtained in three overlapping fragments using the following primer pairs: A and L, C and Y, O and B (Apakupakul et al. 1999). All sequences were generated using an Applied Biosystems (ABI, Foster City, CA, USA) 3100 automated sequencer using the ABI Big-dye Ready-Reaction mix kit, following the standard cycle sequencing protocol.
Sequence alignment and phylogenetic analysis
Sequences were aligned using CLUSTAL W (Thompson et al. 1994) with default gap weighting parameters, adjusted by eye and regions that could not be aligned unambiguously were excluded from the analysis. Sequence data from the three genes were first analysed separately to determine any nodes with high conflicts among different datasets. For a combined analysis, phylogenetic congruence among the two datasets was tested under parsimony (MP) criterion using incongruent length difference (ILD) test (Farris et al. 1994) implemented in PAUP* v4.0 b10 as the partition homogeneity test (Swofford 2000). Only 16S and 18S sequences were available for most of the taxa as many of the sequences were obtained from GenBank or Dr C.C. Tudge. Thus, a dataset of these two genes for a larger number of taxa was analysed first to determine the overall grouping of different taxa. Then a dataset with three genes combined (16S, 18S and 28S) for a reduced number of taxa was analysed subsequently in order to obtain a higher resolution for interfamilial relationships.
Three methods of phylogenetic inference were applied to the datasets: MP and maximum likelihood (ML) using PAUP*, and Bayesian inference (BI) using MrBayes v.3.12 (Huelsenbeck and Ronquist 2001). MP analyses were performed using heuristic search and tree-bisection-reconnection (TBR) with 1000 random sequence addition replicates. Character states were unordered and equally weighted. Gaps were treated as missing data. Bootstrap (BP) support for the most parsimonious tree was evaluated using 1000 replicates with 100 random sequence addition replicates. Modeltest 3.7 (Posada and Crandall 1998) was used to select the best-fit model of nucleotide substitution for each dataset in ML and BI analyses. For ML analysis, the heuristic search was performed with 100 random sequence addition replicates and support for individual clades was obtained from 100 BP replicates with 10 random sequence addition replicates. The Bayesian analysis was run with four Markov Chain Monte Carlo (MCMC) chains for 2 000 000 generations starting from a random tree. The chain was sampled every 100 generations and the first 40% of trees were discarded as burn-in. A 50% majority rule consensus tree was constructed from the remaining trees to estimate posterior probabilities (PP). Three replicates of these Bayesian runs were conducted as described to ensure convergence was repeatable.
Results
The final aligned sequences consist of 317 bp of mitochondrial 16S, 1674 bp of nuclear 18S and 825 bp for 28S genes for a total of 1991 bp and 2816 bp in the two- and three-gene combined datasets, respectively. The alignment files are available from the corresponding author (K.H. Chu) upon request. The trees constructed from individual genes do not show great conflict in topology (nodes different among trees with support >50%). There is no significant incongruence between data from different genes in both the two- or three-gene combined datasets (p > 0.05) as revealed by ILD test and the BP supports are generally higher for the combined analysis, so that the phylogenetic trees from combined analyses are shown (1, 2). The best-fit model selected using Modeltest is General Time-Reversible (GTR) model in which the propotion of invariant sites (I) and the shape of gamma distribution (G) were estimated from the data for both of the combined datasets.
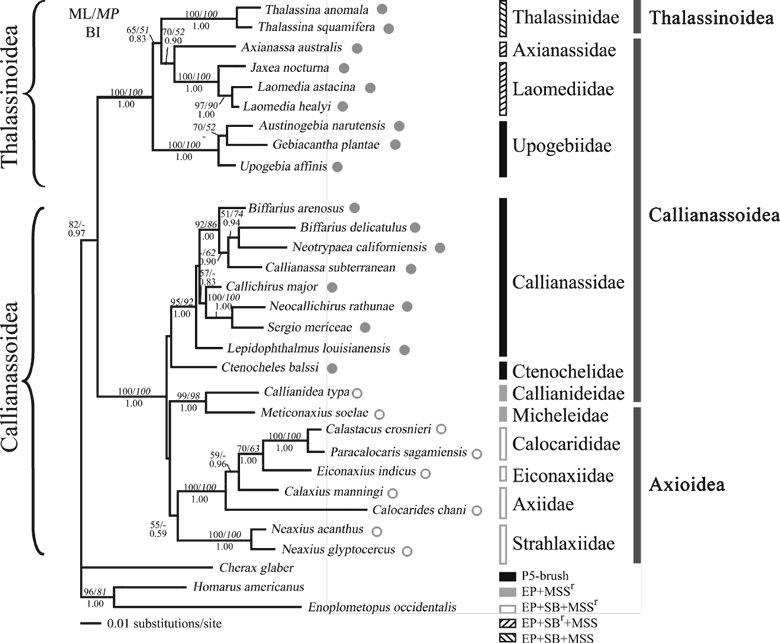
Maximum likelihood tree from combined 16S and 18S rDNA analysis under the best-fitting model GTR + I + G. Bootstrap (BP) values for maximum likelihood (ML; normal text) and parsimony (MP; italic) analyses are indicated above the corresponding branches and posterior probability values for Bayesian inference (BI; bold) analysis are shown below the branches. BP values <50% for ML and MP analyses and posterior probability <0.5 for BI analysis are not shown. The family and superfamily classification of the species mainly according to Martin and Davis (2001) are indicated by the bars on the right-hand side. The proposed superfamily classification of the present study is shown on the left-hand side. The presence/absence of linea thalassinica and gill-cleaning mechanisms are mapped on the tree. The presence or absence of linea thalassinica, determined by direct observation of the specimens when available, or following Poore (1994) and Tudge et al. (2000), is indicated by closed and open circles, respectively. The gill-cleaning mechanisms and terminology follow the descriptions by Batang and Suzuki (2003). Abbreviations: EP, setiferous epipod; SB, setobranch; MSS, multidenticulate scaphonathite setae; SBr, MSSr, vestigial or reduced character on setobranch or multidenticulate scaphonathite setae; P5-brush, setal brush on distal segments of fifth pereiopod
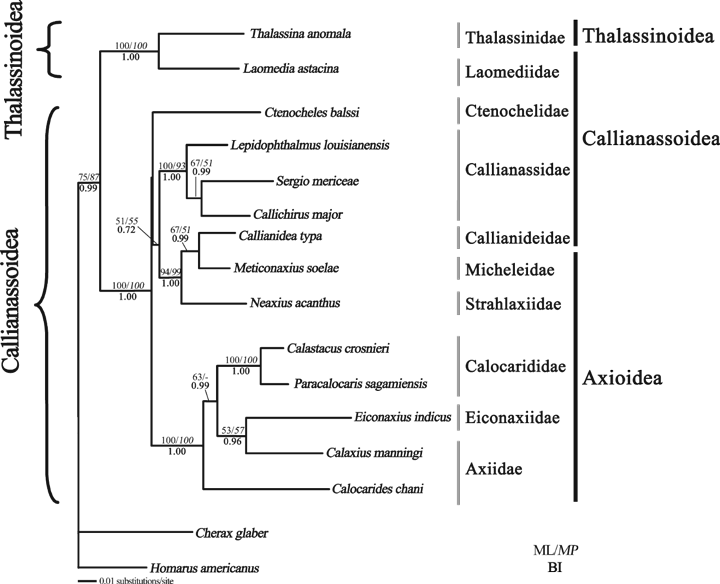
Maximum likelihood (ML) tree from combined 16S, 18S and 28S rDNA analysis under the best-fitting model GTR + I + G. Bootstrap (BP) values for ML (normal text) and MP (italic) analyses are indicated above the corresponding branches and posterior probability values for BI (bold) analysis are shown below the branches. BP values <50% for ML and MP analyses and posterior probability <0.5 for Bayesian inference analysis are not shown. The family and superfamily classification of the species mainly according to Martin and Davis (2001) are indicated by the bars on the right-hand side.
All the three phylogenetic inference methods result in trees that are congruent in overall topology with some clades consistently showing high confidence values. Yet placement of a few taxa varies slightly according to the analysis performed with the BP support of these conflicting nodes being low. The three replicates of the Bayesian run reveal the same result with <5% deviation in posterior probabilities (PP) so that only the result of the first run is presented. Therefore, results from all of the three analyses are shown together based on the ML tree with BP values ≥50 and PP value ≥0.5 shown on the corresponding branches (1, 2).
In the 16S and 18S combined dataset, MP analysis reveals 330 parsimony informative sites in 497 variable sites and resulted in 10 most parsimonious trees with 1426 steps, a consistency index (CI) of 0.489 and a retention index (RI) of 0.626. The 10 trees differ in the arrangement of the internal nodes but none of the discrepancies are strongly supported. In all analyses, the Thalassinidea is divided into two clades with strong support (100% in all analysis; Fig. 1) and clearly distinct from the outgroup taxa, including Enoplometopus occidentalis which is found to be closely related to Homarus americanus, in congruent with a previous molecular study (Ahyong and O’Meally 2004). The first clade consists of the Thalassinidae, Laomediidae, Axianassidae and Upogebiidae, while the second one includes all axioid families (Axiidae, Eiconaxiidae, Calocarididae, Micheleidae and Strahlaxiidae), Callianassidae, Ctenochelidae and Callianideidae. Within the first clade, all the four families are monophyletic (100% BP, PP = 1.00) and Upogebiidae is the basal group in all analyses with intermediate support (ML BP = 65%, BI PP = 0.83).
The relationships within the second major clade are less well resolved and vary between different phylogenetic analyses. However, several groupings consistently appear with high support in all analysis (Fig. 1). The Calocarididae, Eiconaxiidae and Axiidae formed the first cluster with 100% BP support. Monophyly of the Calocarididae is strongly supported (100% in all trees) but it is grouped together with other axiid genera and the newly erected family Eiconaxiidae making the Axiidae paraphyletic. The Micheleidae is most closely related to the Callianideidae with >95% support in all trees. The two are the sister group of Strahlaxiidae + Axiidae + Eiconaxiidae + Calocarididae with weak support. Callianassidae is monophyletic (>90% support in all trees) and allies with the Ctenochelidae yet with no BP support. As the relationship within the second major clade, which consists of a majority of the thalassinidean families was not well resolved by 16S and 18S combined alone, the 28S rDNA gene sequences for families from this clade were also analysed in order to have a better picture on interfamilial phylogeny. Only two taxa from the first clade were included as the relationship within it is comparatively well resolved and there were only samples from three families. The results of the analysis of the 16S, 18S and 28S combined dataset is largely congruent with those of 16S and 18S combined alone, but the support for internal nodes is generally higher (Fig. 2) indicating an increase in resolution with more sequence input. The 2816 bp combined dataset contains 659 variable sites of which 400 are parsimony informative. The MP analysis yields a single most parsimonious tree with tree length 1608 steps, CI = 0.611 and RI = 0.491. The same two major clades within the Thalassinidea are recovered. Within the second (larger) major clade, three sub-groups could be identified. The first one consists of the Axiidae, Calocarididae and Eiconaxiidae with high confidence values (100% in all analyses). The slightly deeper divergence suggested by the longer branch lengths between these three families compared with those among the other families indicates that they are probably the basal group within this large clade. The sister group relationship between the Micheleidae + Callianideidae and the Strahlaxiidae is strongly supported (>90% in all analyses). These three families are shown to be closely related to the Callianassidae with moderate support (MP BP = 55, BI PP = 0.72). The relationship between the remaining Ctenochelidae and these two sub-clades is not well resolved. It is found to be more closely allied to the Callianassidae sub-group in all analyses but the support is weak (<50%).
Discussion
There has been a long dispute over the classification and phylogeny of the Thalassinidea based on various morphological and molecular studies. Here the most comprehensive molecular study to elucidate this issue has been presented. This study allows discussion and clarification of the conflicting relationships reported in the literature.
The Thalassinidea is dichotomous with strong support in all analyses and this is in congruent with the results of previous molecular studies (Tudge and Cunningham 2002; Ahyong and O’Meally 2004). The monophyly of the infraorder was only weakly supported (ML BP = 52% and 75% for the two- and three-gene analyses, respectively) in the present study. Moreover, inclusion of other outgroup taxa (e.g. Anomura) in the analysis may make the Thalassinidea appear to be paraphyletic despite the BP support for the paraphyly being low (data not shown). Yet, two major clades of the Thalassinidea were consistently recovered with high confidence values regardless of outgroups used. As investigating the monophyly of the Thalassinidea is not the objective in this study, the focus has been on relationships among thalassinideans.
The division of the Thalassinidea into two distinct clades is supported by morphological evidences (de Saint Laurent 1979a; b) including larval morphology (Gurney 1938) and gastric mill morphology (Sakai 2005). The association of the Axioidea with the Callianassidae, Ctenochelidae and Callianideidae is also supported by six synapomorphies as noted by Tudge and Cunningham (2002). Other than the morphology of larvae and the gastric mill, the clade formed by the Thalassinidae, Axianassidae, Laomediidae and Upogebiidae can be separated from the other thalassinidean families by the fact that the second pereiopod is never fully chelate (de Saint Laurent 1979a). This is despite the fact that Poore (1994) argues that chelate limbs have arisen independently in thalassinideans and in decapod crustaceans as a whole. On the other hand, the chelate status of particular pereiopods has been widely used in separating decapod crustacean infraorders (e.g. Penaeoidea versus Caridea and Palinura versus Astacidea: Holthuis 1991; Chan 1998) and superfamilies (e.g. inside Caridea: Chace 1992; Holthuis 1993).
The Thalassinidae and Laomediidae possess similar gill-cleaning structure and mechanisms (Batang and Suzuki 1999, 2003; Batang et al. 2001). Although Batang and Suzuki (2003) argued that gill-cleaning characters are conservative at the family level and their utility in classification and phylogenetic study is still unclear (Poore 1994; Suzuki and McLay 1998), the results of this study indicate that gill-cleaning adaptations could be important to the evolution of the burrowing habits in thalassinideans. In the combined 16S and 18S gene tree (Fig. 1), the families are generally grouped together according to the five different types of gill-cleaning mechanisms as proposed by Batang and Suzuki (2003: fig. 4), assuming that the presence or absence of setobranch is considered to be more important than whether it is reduced or well developed. The only discrepancy is on those families with setal brush on the distal segments of fifth pereiopod, as species of the Upogebiidae are separated from species of the Callianassidae and Ctenochelidae (but the latter two families do group together in Fig. 1, also see discussion that follows). The burrowing behaviour is likely to be derived in the Thalassinidea as this group is generally considered to be rather advanced among the decapods (e.g. Scholtz and Richter 1995; Dixon et al. 2003; Ahyong and O’Meally 2004; Amati et al. 2004; Porter et al. 2005). This burrowing lifestyle makes the thalassinidean shrimps highly susceptible to the deleterious effects of gill fouling by particulate debris and epibionts. Thus, the evolution of effective gill-cleaning mechanism becomes an important aspect in Thalassinidea diversification.
The axioids are considered to be basal within the Thalassinidea (Borradaile 1903; Poore 1994). Although the basal position of axioid is not clear in the present study which might be because of rapid radiation of thalassinidean families (see next), it appears that axioids are likely to be the more ancestral group as suggested by the longer internal branches within the Axiidae in the ML trees. An increased amount of sequence data would be fruitful in reconstruction of phylogeny among rapidly diversified taxa. In the present study, the incorporation of 28S sequence data increases the level of support among most nodes for the interfamilial relationships indicating that the three-gene dataset gives more confident results. Thus although this preliminary result requires more sequence data to confirm, the basal position of axioids is supported to some extent in the present study. Axioids are generally weak burrowers or live under stones or in cavities in corals (Poore 1994; Dworschak 2000). Most of them inhabit deep sea habitats where the water is relatively cleaner than near shore (Dworschak 2000). Only passive gill-cleaning mechanisms based mainly on the setobranchs and setiferous epipods are found in these families (Kensley 1996a,b) which may be the plesiomorphic state among thalassinideans (Batang and Suzuki 2003). In the Micheleidae, Callianideidae and Thomassiniidae, there is a tendency for epipod reduction (Batang and Suzuki 2003) and the close relationship between the Micheleidae and Callianideidae is strongly supported by the present analysis. Unfortunately, specimens from the Thomassiniidae were not available for the present study and its phylogenetic position needs further confirmation. The Callianassidae, Ctenochelidae and Upogebiidae are the only thalassinideans with active gill-cleaning mechanisms using the fifth pereiopod setal brush (P5 brush) (Batang and Suzuki 2003). Its use in gill-cleaning is confined to some thalassinideans and anomurans and thus it probably represents a derived character. This, therefore, indicates that there is a trend of increasing complexity and efficiency in gill-cleaning structure in thalassinidean evolution related to adaptation to the burrowing lifestyle. The P5 brush in the Upogebiidae probably results from convergent evolution. Thalassinids and laomediids have well-developed multidenticulate scaphognathite setae and relatively reduced setobranch (Batang and Suzuki 1999; Batang et al. 2001) and they clustered together in the trees (1, 2), suggesting that the gill-cleaning mechanisms and structures are important adaptive characters in thalassinidean evolution and informative for classification of this infraorder.
The linea thalassinica is another adaptation for burrowing in thalassinidean evolution (Poore 1994). However, reduction or complete absence of the linea thalassinica in axioids and the Callianideidae raise the question as to its validity as a phylogenetically informative character. The possession of linea thalassinica in the callianassoids provides higher flexibility to the shrimp body and probably allows them to build more complex burrows (Suchanek et al. 1986). The absence of the linea thalassinica in axioids makes their body more rigid and less well adapted to building burrows. The Callianideidae, which is the only callianassoid with a reduced linea thalassinica, appears to be more closely related to the Micheleidae rather than other callianassoids. This indicates that the acquisition of the linea thalassinica most probably has at least two independent origins: one in the Callianassidae + Ctenochelidae + Thomassiniidae and the other in the Upogebiidae + Thalassinidae + Laomediidae + Axianassidae lineage. For those families (i.e. Upogebiidae, Ctenochelidae and Callianassidae) with setal brush on the distal segments of the fifth pereiopod, they all possess a linea thalassinica (Fig. 1) which gives more flexibility to the carapace so that the fifth pereiopod can be inserted more effectively into the branchial chamber. Thus, the linea thalassinica may be the result of parallel evolution of adaptation to the burrowing lifestyle in many thalassinidean families.
It is not surprising that the Callianideidae is allied with Micheleidae as members of these two families, together with Thomassiniidae, were once grouped into a single family (Callianideidae) (Kensley and Heard 1991; Sakai 1992a) and they also share similar gill-cleaning mechanism as stated before. Their sister relationship to the Strahlaxiidae is consistent with the cladistic analysis of Poore (1994). The family Calocarididae is revealed to be monophyletic with strong support and this indicates that the synapomorphies (e.g. structure of second male pleopod) uniting the family (Kensley 1989) are valid. The Calocarididae clusters with the axiid members supporting the view that these two families are closely related based on morphology (Kensley 1989; Sakai and de Saint Laurent 1989; Sakai 1992b). However, the proposed synapomorphies separating the Axiidae from the Calocarididae appeared to be plesiomorphic (Kensley 1989). In order to keep the Axiidae as a monophyletic group, the retention of the Calocarididae as a distinct family is not supported by the molecular data. Accordingly, the establishment of the family Eiconaxiidae for the single genus Eiconaxius (Sakai and Ohta 2005) is also not supported unless the Axiidae is further subdivided.
Many morphological studies suggest that the Callianassidae and Ctenochelidae are sister taxa (Poore 1994; Tudge et al. 2000). However, in the present analysis the Callianassidae appears to be the sister group of the Callianideidae + Micheleidae + Strahlaxiidae clade, with weak to moderate support. The relationship between this clade of four families, the Ctenochelidae and the Axiidae + Calocarididae + Eiconaxiidae clade cannot be well resolved as the support for their relationship received weak support in all analyses. Inclusion of taxa once assigned to Ctenochelidae (e.g. Callianopsis and Gurretia) will be instructive to fully elucidate the position of Ctenochelidae. Moreover, the branch length separating these three clades is very short in the ML trees (1, 2) suggesting that the diversification among these three groups might be very rapid and that the order of divergence is only resolved by a small amount of information in each gene. Thus, more data from multiple gene sequences would be needed to clarify this issue (Poe and Chubb 2004; Whitfield and Lockhart 2007).
The phylogenetic position of Axianassa australis is not well resolved in the present analysis. The validity of the monotypic family Axianassidae has been rather controversial (reviewed in Kensley and Heard 1990). Tudge and Cunningham (2002) found that the Laomediidae including Axianassa is paraphyletic, supporting the existence of the Axianassidae. Yet, the laomediid genera and Axianassa are clustered as a monophyletic group with good BP support in the present study indicating that the Axianassidae may not be distinct from Laomediidae. Further analysis on more laomediid genera should help to elucidate the relationships (Tudge and Cunningham 2002).
It is clear that the commonly used taxonomic scheme of the infraorder Thalassinidea (i.e. Poore 1994; Martin and Davis 2001) is not supported by the present or previous molecular analyses. It is shown that two of the three superfamilies in the scheme are not monophyletic (1, 2). This indicates that the classification scheme cannot reflect the evolutionary relationships among the different thalassinideans. It is proposed to separate the infraorder Thalassinidea into two instead of three superfamilies: the Callianassoidea (i.e. Axioidea + Callianassoidea, as defined in Martin and Davis 2001 but excluding Laomediidae and Upogebiidae) and the Thalassinoidea (i.e. Thalassinidae, Laomediidae, Upogebiidae and Axianassidae) as suggested by Gurney (1938), de Saint Laurent (1979a,b) and Sakai (2005) based on the differences in larval morphology, shape of the second pereiopod in adults and gastric mill morphology, respectively. This study also suggests that the two axioid families Calocarididae and Eiconaxiidae may not be valid if Axiidae, as currently defined, is treated as a family. Such a classification can possibly reflect the tree of life more precisely and provide a better framework for future research.
Acknowledgements
The authors sincerely thank C.C. Tudge of the American University, Washington DC for providing some of his sequences for the present study, N. Ngoc-Ho and R. Cleva of the Muséum National d’Histoire Naturelle, Paris for permitting the extraction of the DNA from a specimen of Callianidea typa and T. Komai of the Natural History Museum and Institute, Chiba for valuable comments on this work. The work described in this paper was supported by grants from the National Science Council, Taiwan, R.O.C. and the Centre for Marine Bioscience and Biotechnology, National Taiwan Ocean University to T.Y. Chan and the Research Grants Council of the Hong Kong Special Administrative Region, China (project no. 4419/04M) to K.H. Chu.




