Divergence in wing morphology among sibling species of the Drosophila buzzatii cluster
Divergenz in der Flügelmorphologie bei nahe verwandten Arten des Drosophila buzzatii Clusters
Abstract
enThe Drosophila buzzatii species cluster consists of the sibling species D. buzzatii, D. koepferae, D. serido, D. borborema, D. seriema, D. antonietae and D. gouveai, all of which breed exclusively in decaying cactus tissue and, except for D. buzzatii (a colonizing subcosmopolitan species), are endemic to South America. Using a morphometric approach and multivariate analysis of 17 wing parameters, we investigated the degree of divergence in wing morphology among the sibling species of this cluster. Significant differences were obtained among the species and discriminant analysis showed that wing morphology was sufficiently different to allow the correct classification of 98.6% of the 70 individuals analysed. The phenetic relationships among the species inferred from UPGMA cluster analysis based on squared Mahalanobis distances (D2) were generally compatible with previously published phylogenetic relationships. These results suggest that wing morphology within D. buzzatii cluster is of phylogenetic importance.
Zusammenfassung
deDas Artencluster Drosophila buzzatii umfasst die nahe verwandten Arten D. buzzatii, D. koepferae, D. serido, D. borborema, D. seriema, D. antonietae und D. gouveai. All diese Arten pflanzen sich ausschliesslich auf verfaulendem Kaktusgewebe fort. Mit Ausnahme von D. buzzatii (einer kolonisierenden subkosmopolitischen Art) sind alle diese Arten endemisch für Südamerika. In einem morphometrischen Ansatz und mittels multivariater Analyse von 17 Flügelparametern untersuchten wir die Flügelmorphologie dieses Artenclusters im Hinblick auf Divergenzen. Die einzelnen Arten weichen signifikant voneinander ab, so dass es möglich war, 98,6% von 70 untersuchten Tieren mittels dieser Unterschiede in der Flügelmorphologie korrekt zu klassifizieren. Die phenetischen Beziehungen zwischen den Arten, die mittels UPGMA-Clusteranalyse basierend auf dem Quadrat der Mahalanobis-Distanzen (D2) ermittelt wurden, stimmten im allgemeinen mit den bereits publizierten phylogenetischen Beziehungen überein. Die Ergebnisse lassen den Schluss zu, dass die Flügelmorphologie ein wichtiges phylogenetisches Merkmal für das D. buzzatii Artencluster sind.
Introduction
Complexes of sibling species show high morphological similarity among species. However, when morphometric approaches are used in these taxa some differences in morphology that allow discrimination among species can be found (Brown and Shipp 1978; Yu et al. 1992; Tidon-Sklorz and Sene 1995; Adams and Funk 1996; Moreteau et al. 2003). The genus Drosophila comprises a number of such complexes, some of them with the phylogenetic relationships among species relatively well studied (Throckmorton 1975). Frequently these complexes consist of species in the early or incipient stages of speciation, thus offering an excellent material for quantitative evolutionary analyses focusing on the relationship of morphology and genetic divergence.
One taxon in which sibling species have been known since 1977 is the cluster of cactophilic species Drosophila buzzatii, which belongs to the D. repleta group. Currently, the D. buzzatii cluster consists of the nominal species D. buzzatii, D. koepferae, D. serido, D. borborema, D. seriema, D. antonietae and D. gouveai. All of these species breed exclusively in decaying cactus tissue and, except for D. buzzatii (a colonizing subcosmopolitan species), are endemic to South America. The distribution of the D. buzzatii species cluster ranges from the caatinga vegetation in north-eastern Brazil to chaco vegetation in north-eastern Argentina and central Bolivia, crossing the savannas of central western Brazil and the Brazilian coast (Ruiz et al. 1982; Sene et al. 1982, 1988; Fontdevila et al. 1988). Despite the uniformity in external morphology, there is marked variation in the male genital morphology among these species. This trait, together with chromosomal inversions, are diagnostic species characters (Tosi and Sene 1989; Silva and Sene 1991; Ruiz and Wasserman 1993; Tidon-Sklorz and Sene 1995). Interspecific hybridization tests using laboratory stocks have shown that, depending on the population analysed, reproductive isolation between the species can vary considerably (Marin et al. 1994; Madi-Ravazzi et al. 1997). Regardless of the sympatric distribution between some species pairs, hybrid populations have been observed only in southern Brazil in a contact zone between D. serido and D. antonietae (Cansian et al. 1996).
Previous surveys have examined the evolutionary history, and the systematic and genetic relationships within the D. buzzatii species cluster by using genetic markers such as chromosomal inversions, hybridization tests, male genital morphology, metaphase chromosome morphology, allozymic variation and mtDNA (Manfrin et al. 2001 and references therein). These markers have generally revealed contrasting patterns of genetic divergence among the species (Sene et al. 1988), with D. buzzatii being the most divergent of the species in this cluster. A phylogenetic analysis based on mtDNA has shown that the D. buzzatii cluster can be divided into two major evolutionary lineages that split 6–12 Myr ago. One evolutionary lineage was established by D. buzzatii and D. koepferae and the other by the remaining species of the D. buzzatii cluster. In the latter, D. serido, D. borborema, D. seriema and D. gouveai diverged from D. antonietae 3–6 Myr ago (Manfrin et al. 2001).
Using a morphometric approach, we have now analysed the divergence in wing morphology in strains of the seven species of the D. buzzatii cluster and tested the ability to distinguish amongst these sibling species using multivariate statistics. To assess possible phylogenetic significance of wing morphology within the D. buzzatii cluster, we also compared the phenetic relationships among species to previously described phylogenetic relationships based on genetic markers.
Materials and methods
We analysed the wing morphology of 10 males from each of the seven species belonging to the D. buzzatii cluster, in a total number of 70 individuals. All of the flies analysed, except for D. borborema, came from massal lines kept in our laboratory. The individuals of D. borborema were collected in the field at Manoel Vitorino, Bahia State. The use of wild-caught flies together with laboratory lines was necessary because, even after various attempts, we were not able to establish culture lines from D. borborema flies. The strains used were H86 D. buzzatii (Altinópolis, São Paulo, Brazil), J36 D. antonietae (Serrana, São Paulo, Brazil), J79 D. gouveai (Ibotirama, Bahia, Brazil), J92 D. serido (Milagres, Bahia, Brazil), B25 D. koepferae (Morro do Chapéu, Argentina), D72 D. seriema (Morro do Chapéu, Bahia, Brazil). These massal lines were originated from five wild-caught pairs and had generally been in Peunto Tinol, culture for several years.
Morphometric analysis was done on 17 wing parameters obtained using the method of the best adjustment of an ellipse to the wing edge (Klaczko and Bitner-Mathé 1990). This method, which mainly describes the variation in wing shape, is fully explained by Bitner-Mathé and Klaczko (1999a). In brief, based on the geometric properties of an ellipse with the best adjustment to the contour of the wing, the method provides a shape-free measure of size obtained from the geometric mean of two radii (a and b) of the ellipse (WSI) and a size-free measure of an elliptical shape based on the ratio b/a (WSH). The method also provides 10 parameters (θA to θJ, in radians) indicating the 10 landmark positions represented by the extremities of the longitudinal veins at the edge of the wing and by the extremities of the cross-veins. The position of the internal landmarks in the interior of the wing, including the extremities of the transversal veins, also took into account the distance (D) of the landmark from the centre of the ellipse. These distances were adjusted for size using the relationship D/WSI. Thus, the parameters DF to DJ became dimensionless descriptors of the internal landmark placements. The right wing of each fly was mounted on a microscope slide and photographed using a video camera attached to a stereomicroscope at 16× magnification. The Cartesian coordinates of 30 points along the edge of the wing and the 10 landmarks were obtained using the software TpsDig version 1.26 (Rohlf 1998). The coordinates were the input data for the software Asalk (Bitner-Mathé and Klaczko 1999a) used to calculate the wing parameters.
All of the statistical tests were done on original variables after using the Kolmogorov–Smirnov test to determine that the variables followed a normal distribution. To reduce the information to statistically unrelated factors, a principal component analysis (PCA) using a correlation matrix was done on the 17 wing parameters. Only principal components with an eigenvalue greater than 1.00 were analysed. The significance of the morphometric differences among species was tested using one-way anova of individual scores from the principal components. Morphometric divergence among species was also assessed using discriminant analysis (DA) of the 17 wing parameters. Wilk's lambda was used as an overall test for equality of the means; the smaller the value (which varies between 0 and 1), the greater the degree of divergence among species. Discriminant analysis also served to create discriminant functions which were then used to obtain the correct classification percentages of individuals to their original species. The phenetic relationships among species were determined by UPGMA cluster analysis on squared Mahalanobis distance (D2) between species centroids.
Results
Principal components analysis showed that the variation in the 17 wing parameters of the D. buzzatii species cluster could be reduced to three principal components, PC 1, PC 2 and PC 3, which accounted for 57.1, 10.1 and 7.2%, respectively, of the normalized total variance. Table 1 shows the principal component loading values, which represent the correlation coefficients for each parameter with each principal component. PC 1 is generally associated with size variation. However, because the PC 1 loading values were positive and negative, they generally represented the variation in wing-shape (Bookstein et al. 1985). Most parameters were highly correlated with PC 1, whereas θC and WSI were highly correlated with PC 2 and PC 3, respectively. The parameter θC corresponded to the position of the third longitudinal vein at the edge of the wing and WSI was a size wing parameter. The divergence in shape and size among species was verified by analysis of variance of the principal components of the wing parameters, which showed significant differences for all PCs [PC 1: F(6,63) = 7.377, P < 0.0001; PC 2: F(6,63) = 2.983, P < 0.02; PC 3: F(6,63) = 15.671, P < 0.00001]. Because PC 1 contained most of the total variance and predominantly represented shape variation, such morphological divergence could be attributed to shape-related differences. These differences were associated with the positions of longitudinal veins and cross-veins, in addition to wing outline shape. Size-related differences among the species was represented mainly by PC 3.
| Parameter | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| θ A | −0.850 | 0.202 | 0.039 |
| θ B | −0.665 | 0.353 | 0.406 |
| θ C | 0.177 | 0.823 | −0.199 |
| θ D | 0.532 | 0.563 | −0.319 |
| θ E | 0.800 | 0.247 | 0.208 |
| θ F | −0.902 | 0.181 | −0.081 |
| θ G | −0.859 | 0.182 | −0.119 |
| θ H | 0.805 | −0.051 | 0.197 |
| θ I | 0.889 | 0.229 | 0.112 |
| θ J | −0.741 | 0.178 | −0.208 |
| D F | 0.911 | −0.060 | 0.016 |
| D G | 0.932 | −0.056 | 0.008 |
| D H | −0.906 | −0.163 | −0.153 |
| D I | −0.442 | −0.420 | 0.120 |
| D J | 0.794 | −0.040 | 0.297 |
| W SI | −0.449 | 0.365 | 0.688 |
| W SH | 0.698 | 0.034 | −0.403 |
| Eigenvalue | 9.706 | 1.723 | 1.232 |
| Cum. % explained | 57.1 | 67.2 | 74.4 |
Discriminant analysis based on the 17 wing parameters resulted in six discriminant functions which showed significant morphometric divergence among the species (Table 2). These six discriminant functions also provided an overall classification success of 98.6%. Of the 70 flies used in the analysis, only one individual belonging to D. serido was misclassified as D. koepferae. An additional discriminate analysis, using a stepwise procedure, revealed that θA, θF and WSI were the most important wing parameters for species discrimination [Wilks’ lambda = 0.106; F(18,173) = 11.637; P < 0.0001]. The parameters θA and θF were associated with the positions of the costal vein and of the anterior cross-vein, respectively. Figure 1 shows plots of the individual scores for the first three discriminant functions (DF 1, DF 2 and DF 3), which accounted for 84% of the total variance. In these two-dimensional diagrams, D. buzzatii, D. seriema, D. antonietae and D. gouveai were well-defined groups, whereas D. koepferae showed a little overlap. The plots of the individual discriminant scores in both two-dimensional diagrams showed a considerable overlap between D. borborema and D. serido.
| Discriminant function | Eigenvalue | Proportion variance | Canonical correlation | Wilk's lambda | Chi-square | d.f. | P-value |
|---|---|---|---|---|---|---|---|
| Overall | 4.94 | 1.00 | 0.91 | 0.001 | 381.0 | 102 | <0.000 |
| 1 | 4.33 | 0.33 | 0.90 | 0.007 | 279.4 | 80 | <0.000 |
| 2 | 3.31 | 0.29 | 0.87 | 0.039 | 183.9 | 60 | <0.000 |
| 3 | 1.19 | 0.22 | 0.73 | 0.171 | 100.6 | 42 | <0.000 |
| 4 | 0.66 | 0.08 | 0.63 | 0.375 | 55.9 | 26 | <0.000 |
| 5 | 0.59 | 0.04 | 0.61 | 0.625 | 26.7 | 12 | 0.008 |
| 6 | 0.32 | 0.04 | 0.91 | 0.001 | 381.0 | 102 | <0.000 |

UPGMA cluster analysis of the D. buzzatii species cluster based on squared Mahalanobis distances (scale showed) obtained from 17 wing parameters.
All pairwise squared Mahalanobis distances between species were significant at the 99% level. UPGMA cluster analysis of the squared Mahalanobis distances (Fig. 2) clustered all species, except for D. buzzatii, in the same branch, indicating that D. buzzatii was the most divergent species. Within this main branch, the species D. seriema and D. koepferae were isolated from the branch clustering the remaining species. D. antonietae and D. gouveai were clustered in the same branch, whereas D. borborema and D. serido were clustered in another one.
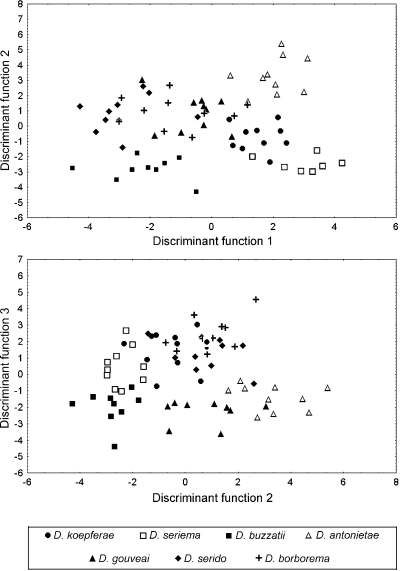
Plots of the individual scores for the first three discriminant functions (DF 1, DF 2 and DF 3) obtained for the wing morphometric parameters of the D. buzzatii species cluster.
Discussion
Our morphometric analysis showed that there was a marked quantitative divergence in wing morphology within the D. buzzatii species cluster, and that it was possible to discriminate between species by using this character. Furthermore, the position of the anterior cross-vein and costal vein, as well as wing size, were the traits with the most weight in the discrimination.
High heritability estimates for the same wing parameters used in our study have been found in a natural population of D. gouveai (Moraes and Sene 2004), a member of the D. buzzatii cluster. Bitner-Mathé and Klaczko (1999b) also obtained high heritability estimates for the ellipse outline shape (WSH) and for angles related to longitudinal veins (θs) in a natural population of D. mediopunctata. If heritability is high in all species of the D. buzzatii cluster, then own results indicate that divergence in wing morphology was due to differentiation in genes acting additively.
We have compared the phenetic relationships provided by the present survey with previously described phylogenetic relationships for the D. buzzatii species cluster (Fig. 3). All of the phylogenetic relationships based on genetic markers placed D. buzzatii, followed by D. koepferae, as the most divergent from the other species in the cluster. This conclusion was similar to that for the phenetic relationships provided by wing morphology. In particular, the phylogenetic relationships based on Xdh nucleotide sequences (Rodriguez-Trelles et al. 2000) were most compatible with the phenetic relationships provided by the present results since D. borborema and D. serido were clustered in the same branch. However, because the Xdh nucleotide sequences of the remaining species of the D. buzzatii cluster were not analyzed, these results must be considered with caution. In a general manner, the phenetic relationships were compatible with the previously published phylogenetic relationships based on genetic markers, suggesting that wing morphology may be of phylogenetic significance within this taxon. The similarity between the phenetic and phylogenetic relationships suggested an association between the extent of phenotypic evolution and genetic change. Such an association could be the result of a correlation between the rates of evolution of these markers or could simply be due to time dependence, that is, these markers evolving neutrally for same period since the speciation (Omland 1997; Bromham et al. 2002).
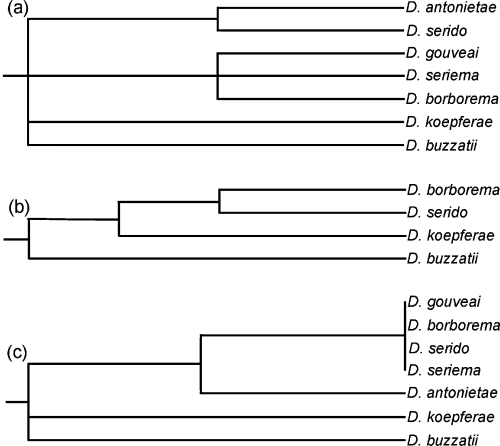
Phylogenetic hypotheses for the D. buzzatii cluster species based on genetic markers. (a) Phylogeny based on fixed chromosomal inversions (after Ruiz et al. 2000). (b) Phylogeny based on the Xdh nucleotide sequences (after Rodriguez-Trelles et al. 2000). (c) Phylogeny based on the mitochondrial COx-I sequences (after Manfrin et al. 2001).
Because flies belonging to the D. buzzatii cluster breed exclusively in decaying cactus tissue, the geographic distribution of these species is determined by distribution of Cactaceae in South America, which in turn occur mostly in patches of xerophitic vegetation. The present-day distribution of these xeric patches in South America have been considered fragmentary remnants of the once continuous xerophitic vegetation in the past time, by occasion the dry–cold climatic periods of the Pleistocene. This assumption has been mostly based on the present-day distribution of plant species (Ab‘Saber 1977; Bigarella and Andrade-Lima 1982; Prado and Gibbs 1993) and, to a lesser extension, on palynological data (Behling 1997, 2002; Salgado-Laboriau 1997). The distribution and evolutionary history of the D. buzzatii species cluster are thought to be associated with this putative cyclic expansion and retreat of xeric vegetation (Sene et al. 1982; Manfrin et al. 2001; DeBrito et al. 2002a,b). Thus, based on a phylogeographic analysis of the mtDNA COx-I gene, DeBrito et al. (2002a) found evidence of past fragmentation separating populations of D. gouveai in north-eastern Brazil from populations of D. antonietae in mid-western and southern Brazil. A extensive study on wing morphology in natural populations of the D. buzzatii species cluster could provide useful insights into the evolutionary history of these species.
Acknowledgements
We thank Prof. A.C.Z. Amaral for help in capturing the wing images and Paulo R. Epifânio for technical assistance. We are thankful also to Prof. K. Hartfelder for help with the German language and to two anonymous referees for their helpful comments. This research was supported by FAPESP (grant number 00/05979-5), FINEP, CNPq and USP.




