Seroprevalence of Brucellosis, Tularemia, and Yersiniosis in Wild Boars (Sus scrofa) from North-Eastern Germany
Summary
Brucellosis and tularemia are classical zoonotic diseases transmitted from an animal reservoir to humans. Both, wildlife and domestic animals, contribute to the spreading of these zoonoses. The surveillance of the animal health status is strictly regulated for domestic animals, whereas systematic disease monitoring in wildlife does not exist. The aim of the present study was to provide data on the prevalence of anti-Brucella, anti-Francisella and anti-Yersinia antibodies in wild boars from North-Eastern Germany to assess public health risks. A total of 763 sera of wild boars from Mecklenburg-Western Pomerania hunted in 1995/1996 were tested using a commercially available Brucella suis ELISA, an in-house lipopolysaccharide (LPS)-based Francisella ELISA, and commercially available Western blot kits for the detection of anti-Francisella and anti-Yersinia antibodies. The Yersinia enterocolitica O:9 LPS is able to induce serological cross-reactions indistinguishable from brucellosis due to a similar immunodominant epitope in the Brucella O-polysaccharide. The Yersinia Western blot assay was, therefore, based on five recombinant Yersinia outer proteins which have been proved to be specific for the serodiagnosis of yersiniosis. Anti-Brucella, anti-Francisella and anti-Yersinia antibodies were detected in 22.0%, 3.1%, and 62.6% of the wild boars, respectively. The high seroprevalence of tularemia and brucellosis in wild boars indicates that natural foci of these zoonoses are present in wildlife in Germany. However, the impact of transmission of zoonotic pathogens from wildlife to livestock is unknown. Only careful and systematic monitoring will help to prevent the (re)emergence of these zoonotic diseases in domestic animals and consequently human infection.
Introduction
Brucellosis, tularemia and yersiniosis are diseases that affect wild and domestic animals as well as humans. The epidemic cycles of these multi-species diseases are related to climatic factors and population densities of their hosts (Bengis et al., 2002). Brucella (B.), Francisella (F.) and Yersinia (Y.) are considered to be either emerging or re-emerging pathogens causing occupational, recreational or foodborne infections in humans (Miller and Paige, 1998; Godfroid, 2002; Godfroid et al., 2005; Petersen and Schriefer, 2005). Neither brucellosis nor tularemia is a contagious human disease and the source of human infection is always based upon the contact to an animal reservoir.
The creation of new interfaces between livestock and wildlife is the most important factor in disease transmission (Bengis et al., 2002). Hence, the game farming industry poses a new risk for the (re)emergence of these zoonoses in domestic animals (Godfroid, 2002). Translocation or introduction of animals in a non-endemic area and a lack of surveillance due to regional conflicts caused by political instability may promote these interfaces. Brucellosis and tularemia are currently in the focus of public health authorities due to the biothreat potential of their causative agents. Against the background of numerous conflict areas and the present bioterrorist threat, screening of natural reservoirs is an indispensable tool for the detection of artificial outbreaks. At present, the epizootic situation of brucellosis and tularemia in wildlife is unknown in Germany.
Brucella infections are endemic in various wildlife species worldwide (Godfroid, 2002; Godfroid et al., 2005). The most important aetiological agents causing animal and human brucellosis are Brucella abortus (main reservoir in cattle), B. melitensis (in sheep and goats) and B. suis (in pigs) (Godfroid et al., 2005). In 2000, German flocks have been declared ‘officially free from bovine (B. abortus) and ovine/caprine (B. melitensis) brucellosis’ (Godfroid and Käsbohrer, 2002). However, sporadic bacteriologically proven outbreaks of B. suis biovar (bv) 2 in domestic pigs have been reported from six pig rearing farms all over Germany from 1995 to 2004. Considering the regional distribution of these sporadic outbreaks over a longer period, hot spots are evident in North-Eastern Germany, North Rhine-Westphalia and Bavaria (Kautzsch et al., 1995). In Central Europe, B. suis has frequently been isolated from wild boars (Sus scrofa) and to a lesser extent from European brown hares (Lepus europaeus), both considered to be the natural reservoir of biotype 2 (Godfroid, 2002).
Tularemia is a zoonotic disease reported from various warm- and cold-blooded animals and insects (Frölich et al., 2002). The aetiological agents are Francisella tularensis subspecies, mainly tularensis and holarctica. Natural infections occur in lagomorphs, rodents, insectivores, carnivores, ungulates, marsupials, birds, amphibians, fish and invertebrates (Mörner, 1992; Petersen and Schriefer, 2005). The disease is widely spread throughout the northern hemisphere revealing foci in North America, Europe, and northern Asia (Petersen and Schriefer, 2005).
Yersiniosis has been described in many species of domestic and free-living mammals and birds. The infectious agents are Yersinia pseudotuberculosis and Y. enterocolitica comprising various serotypes and biotypes. Disease causing agents differ from geographical region to region and show a certain degree of host tropism (Bottone, 1999). The occurrence of pathogenic Yersinia spp. in pigs may be related to rearing and husbandry practices (Miller and Paige, 1998; Gürtler et al., 2005). Especially in fattening units a high prevalence of Yersinia was reported (Thibodeau et al., 2001; Hensel et al., 2004; Gürtler et al., 2005). Yersinia enterocolitica is a pharyngeal commensal in pigs which acts as a reservoir for pathogenic strains (Gürtler et al., 2005). The predominant Yersinia spp. clinically relevant to consumers in Europe are Y. enterocolitica O:3, O:9 and O:5, 27 (Verhaegen et al., 1998; Bottone, 1999; Gourdon et al., 1999; Thibodeau et al., 2001; Gürtler et al., 2005). Virulence of pathogenic Yersiniae is closely associated with a 64–70 kb plasmid encoding Yersinia outer proteins (Yops) and proteins of the Yersinia type III secretion apparatus (Cornelis and Wolf-Watz, 1997). As Yops are immunogenic in men and animals, Yersinia infections caused by pathogenic strains can be detected by anti-Yop antibodies independent of serovars or biovars (Heesemann et al., 1986). In addition, using the more specific protein-based Western blot strips for the detection of anti-Yersinia antibodies cross-reactivity with tularemia and brucellosis sera can be excluded.
The seroprevalence of anti-Brucella, anti-Francisella and anti-Yersinia antibodies in wild boars from North-Eastern Germany was determined to provide epizootic data in wildlife and to assess the risk for domestic animals and humans.
Material and Methods
Sample collection
A panel of negative and positive sera collected after experimental infection and immunization of pigs (Danish Landrace and Yorkshire crossbred pigs) was used for evaluation of the serological assays and determination of cut-off values (Jungersen et al., 2006). Five non-infected controls were separately housed with five pigs infected with Y. enterocolitica O:9 (DFVF 9913937-1). Another five pigs were hyper-immunized with the same Y. enterocolitica O:9 strain which was isolated in 1999 during the first verified outbreak of yersiniosis among farm pigs in Denmark. Eight pigs were infected with a B. suis bv 2 field isolate (DFVF 9405304) which originated from an outbreak among domestic pigs in Denmark in 1994 and was biotyped at the Veterinary Laboratories Agency (Weybridge, UK). Four pigs were hyper-immunized with Francisella spp. One each with F. tularensis ssp. holarctica LVS [ATCC 29684], ssp. tularensis [Schu; FSC 043 (FOI, Umea, Sweden)], ssp. novicida [ATCC 15482] and F. philomiragia [ATCC 25015] (kindly provided by U. Truyen, Institute of Animal Hygiene and Public Veterinary Health, Leipzig, Germany). In addition, 100 pig sera from a German animal farm free of brucellosis and tularemia were tested in Brucella and Francisella ELISAs, respectively.
A total of 763 sera of wild boars (kindly provided by J. Dedek, Veterinary and Food Inspection Centre of Mecklenburg-Western Pomerania, Rostock, Germany) collected in oral immunization trials against classical swine fever (Kaden et al., 2002) were screened for anti-Brucella, anti-Francisella and anti-Yersinia antibodies. The study group consisted of 315 male (41%), 380 female (50%) and 68 animals (9%) of unspecified gender and age. A total of 201 (26%) shoats (<6 months), 129 (17%) young boars (6–12 months), 270 (35%) yearlings (1–2 years) and 95 (12%) adult animals (>2 years) were registered. The wild boars were shot in the winter season 1995–1996 in Mecklenburg-Western Pomerania (in the administrative districts: Northwest Mecklenburg, Parchim and Schwerin) located in North-Eastern Germany. Blood samples for serological analyses were immediately taken from the pericardium or thoracic cavity. Blood samples and carcasses were sent to the local Veterinary Department responsible for meat inspection. The samples were centrifuged and serum was frozen at −20°C until use.
Study area
The districts Parchim, Northwest Mecklenburg and Schwerin are essentially composed of morainal landscapes with hilly country up to 180 m above sea level, wooded lowlands and lakes. The meteorological office in Marnitz located in the South of Parchim registered standard climate data from 1971 to 2000 proving a moderate climate, i.e. 8.5°C mean temperature, 641.2 mm average rainfall and 1616 sunshine hours annually (Deutscher Wetterdienst, Abteilung Klima und Umweltberatung, Regionales Gutachtenbüro Hamburg, personal communication).
Serological tests
Brucella suis ELISA
Anti-Brucella antibodies were determined using the commercially available CHEKIT®Brucella suis-ELISA kit (Bommeli Diagnostics, Liebefeld-Bern, Switzerland) according to manufacturer's instructions. Briefly, 200 μl of each serum sample and control diluted 1 : 100 in the diluent provided were incubated in the microtitre plate wells pre-coated with B. abortus antigen for 90 min at room temperature. All measurements were performed in duplicates. After washing three times using the CHEKIT® wash solution, peroxidase-conjugated anti-pig immunoglobulin (200 μl per well; 1 : 200) was added and incubated for another 90 min at room temperature. The microwells were washed three times again and 200 μl CHEKIT® chromogen were pipetted into each well. After 15 min the substrate reaction was stopped by the addition of 50 μl CHEKIT® stop solution. Optical density values were measured in an Asys Hitech microplate reader (Asys Hitech GmbH, Eugendorf, Austria) at 405 nm. Samples showing a sample absorbance/positive control absorbance ratio of >0.7 were regarded as positive. A negative sample had a ratio of <0.6. A ratio ranging from 0.6 to 0.7 was considered to be marginal.
Francisella ELISA
Anti-Francisella antibodies were measured with an in-house ELISA (Porsch-Özcürümez et al., 2004; Schmitt et al., 2005). For antigen preparation the live vaccine strain (LVS) of F. tularensis subsp. holarctica (ATCC 29648) was grown on heart-cystein-blood agar (Beckton Dickinson, Heidelberg, Germany). After 2 days bacteria were harvested into sterile distilled water and adjusted to 109 baceria/ml (OD560nm = 1.0). Lipopolysaccharide (LPS) was extracted and solubilized using the Chlamydia specimen extraction buffer (Abbott, Wiesbaden, Germany) in a final dilution of 1 : 2. The antigen was filtered and protein residues were digested using proteinase K (3.3 mg/ml; Boehringer, Mannheim, Germany) for 2 h at 60°C. The enzyme was inactivated at 95°C and LPS was finally purified in isotonic phosphate-buffered saline (PBS, pH 7.2) by dialysis overnight (Slide-A-Lyzer 3.5 K; Pierce Biotechnology, Rockford, IL, USA). The LPS preparation was stored at −20°C until use. Ninety-six-well microtitre plates (Polysorb; Nunc, Wiesbaden, Germany) were coated with 50 μl of the diluted LPS antigen (1 : 30 in carbonate-bicarbonate buffer, pH 9.0) overnight at 4°C. The microwells were washed with PBS-Tween (0.05%, pH 7.2), blocked with 75 μl 10% goat serum in PBS-Tween for 30 min at 37°C and washed again. Serum samples, negative and positive controls (50 μl per well) diluted 1 : 1000 in 10% goat serum were incubated for 1 h at 4°C. The plates were washed four times and 50 μl of polyclonal horseradish peroxidase-conjugated rabbit anti-pig immunoglobulins (DAKO, Glostrup, Denmark) diluted 1 : 2000 in 10% goat serum was added and incubated for 1 h at 37°C. The microwells were finally washed five times and 50 μl of 66% tetramethylbenzidine (TMB; Seramun, Wolzig, Germany) were added to start substrate reaction. After 15 min the reaction was stopped with 50 μl 0.25 m sulphuric acid. Optical density was determined at 450 nm using an Asys Hitech microplate reader. Test samples deviating significantly from the mean (>3 SD) of the test panel (Yersinia- and Brucella-positive pigs and negative controls) were considered positive.
Francisella Western blotting
Sera tested positive in the Francisella ELISA were re-tested by the anti-Francisella tularensis Serablot® (Seramun, Wolzig, Germany) according to manufacturer's instructions with slight modifications. The assay makes use of the purified LPS fraction of Francisella separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted on nitrocellulose membranes which were subsequently cut into 2 mm strips.
These Western blot strips were incubated with the serum samples diluted 1 : 1000 in the provided incubation buffer for 45 min at room temperature. After the strips had been washed three times, polyclonal horseradish peroxidase-conjugated rabbit anti-pig immunoglobulins diluted 1 : 2000 were added and incubated for another 45 min. Following three rinses, the strips were developed using the ready-to-use precipitating TMB (3′,3′,5,5′ tetramethylbenzidin) of the test kit. After 10 min, the substrate reaction was stopped by a final washing step using distilled water. A positive anti-Francisella antibody serum revealed the typical ladder-like pattern caused by different glycolization rates of the lipid A part of LPS.
Yersinia Western blotting
The collected sera were tested for specific anti-Yersinia antibodies using the recomBlot Yersinia IgG/IgA® kit (Microgen, Martinsried, Germany) based on five recombinant protein antigens to exclude cross-reactivity of anti-Brucella and anti-Francisella LPS antibodies. The five proteins, i.e. Yop M, H, D, E and V-Antigen (V-Ag), were separated according to their molecular weight by SDS-PAGE. Then, the antigens were transferred to a nitrocellulose membrane which was finally cut into strips of 2 mm in width. The assay was performed according to manufacturer's instructions.
Briefly, the Western blot strips were incubated with sera diluted 1 : 100 in incubation buffer for 1 h at room temperature. After washing, a polyclonal horseradish peroxidase-conjugated rabbit anti-pig immunoglobulin diluted 1 : 500 was added for the detection of antigen-antibody complexes (incubated for 45 min at room temperature). Finally, the anti-Yop/V-Ag antibody-conjugate complex was visualized using the TMB substrate provided. The substrate reaction was stopped after 15 min by adding distilled water. Only bands pronounced equally as or more than the positive control were considered positive. A positive overall result for the prevalence of specific anti-Yersinia antibodies was assumed for Western blot strips showing at least three positive bands including Yop D (Yop D + 2). In addition, an immunoreactive pattern based on a specific binary code was evaluated for each serum: Yop M = 16, Yop H = 8, V-Ag = 4, Yop D = 2, Yop E = 1. The code resulted from the sum of positive bands ranging from 0 to 31.
Software and analysis
Microsoft Excel® (Microsoft, Munich, Germany), SPSS version 12.0 (SPSS Inc., Chicago, IL, USA), and ArcView 3.3 (ESRI, Redlands, CA, USA) were used for statistical and graphical analyses of the data. Geographical Information System (GIS) data originated from the databases ATKIS® VG250 and GN250 (Copyright Federal Agency for Cartography and Geodesy 2002, Frankfurt/Main, Germany).
Statistical analysis
For statistical analysis, the wild boars were grouped according to their age (I, <6 months; II, 6–12 months; III, 1–2 years; IV, >2 years) and gender (male versus female). As these parameters were unspecified for 68 animals, serological test results of 695 wild boars were statistically analysed. Anti-Yersinia, anti-Brucella and anti-Francisella antibodies were recorded as positive or negative. In addition, the immunoreactive pattern after Yersinia infection was described by a binary code (0–31). The variables were dichotomic (positive versus negative) or lacked normal distribution, which was previously tested for the binary code using the Kolmogorov–Smirnov test. Hence, non-parametric tests had to be applied for statistical analysis. Kruskal–Wallis and Mann–Whitney test were used to check the influence of age and gender on anti-Yop patterns, respectively. The influence of age and gender on the dichotomic variables was tested by Fisher's analysis of variance (anova). As the sample number extremely varied in the administrative districts of the study area, geographical distribution could not be evaluated statistically.
Results
Evaluation of serologic assays
In our test panel of artificially infected and hyper-immunized pigs used for evaluation of the serological assays, the Brucella ELISA tested 25% (2/8) of the B. suis bv 2-infected pigs false negative and 30% (3/10) of the Y. enterocolitica O:9-infected pigs false positive. However, the four Francisella-hyperimmunized pigs were tested negative in the Brucella ELISA. Brucella suis could not be isolated from the sero-negative pigs which were, therefore, regarded as not infected (Jungersen et al., 2006). In contrast, none of the sera from pigs infected or hyperimmunized with cross-reacting bacteria tested positive by the Francisella ELISA. Pigs immunized with F. tularensis subsp. tularensis and holarctica tested positive, whereas those immunized with F. tularensis subsp. novicida and F. philomiragia tested correctly negative using the Francisella ELISA based on the LPS of F. tularensis subsp. holarctica. Twenty per cent (2/10) of the Yersinia-infected pigs tested negative using the Yersinia Western Blot assay. Although these pigs were not considered positive (Yop D + 2), anti-Yop D antibodies were detected.
Yersiniosis
Anti-Yop antibodies were detected in 478 (62.6%) of 763 serum samples. The rate of positive reactors was about the same in male and female animals. The number of positively tested sera increased significantly with age (Table 1) but was independent of the gender. The immunoblot pattern represented by the binary code also showed increasing values in older animals (P < 0.001) independent of their sex (P = 0.860) (Fig. 1).
| Distribution of age and genders in the study group and in positively tested animals | 201 shoats (<6 months) (n/%) | 129 young boars (6–12 months) (n/%) | 270 yearlings (1–2 years) (n/%) | 95 adult animals (>2 years of age) (n/%) | 68 animals of unknown age and gender (n/%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ or ♀ | |
| Study group (763) | 89/11.7 | 112/14.7 | 66/8.7 | 63/8.3 | 123/16.1 | 147/19.3 | 37/4.8 | 58/7.6 | 68/8.9 |
| Brucellosis (168) | 16/18.0 | 18/16.1 | 10/15.2 | 18/28.6 | 23/18.7 | 35/23.8 | 11/29.7 | 15/25.9 | 22/32.4 |
| Tularemia (24) | 5/5.6 | 5/4.5 | 0/0 | 3/4.8 | 3/2.4 | 6/4.1 | 0/0 | 2/3.4 | 0/0 |
| Yersiniosis (478) | 45/50.6 | 60/53.6 | 38/57.6 | 40/63.5 | 83/67.5 | 100/68.0 | 29/78.4 | 39/67.2 | 44/64.7 |
- ♂: male; ♀: female; n: number of wild boars; %: percentage of animals in the respective group.
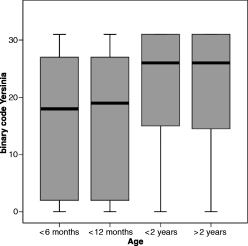
The boxplot graph shows the age dependency of the binary code resulting from the sum of positive bands in the Yersinia Western blot assay of individual sera (Yop M = 16, Yop H = 8, V-Ag = 4, Yop D = 2, Yop E = 1). Median, lower and upper quartile, minimum and maximum values (ranging from 0 to 31) of the binary code are given for the four different age groups, i.e. animals <6 months, 6–12 months, 1–2 years, and >2 years of age. Kruskal–Wallis and Mann–Whitney test revealed increasing values in animals older than 12 months (P < 0.001).
Western blots showed a total of 20 of 32 (25) possible patterns. Only three different patterns, i.e. 19, 27, and 31, were found in 83% of the sero-reactors. In negatively tested animals, the patterns 0, 2, and 18 were most frequently observed (Table 2). Antibodies directed to Yop M, Yop H, V-Ag, Yop D, and Yop E were detected in 69%, 54%, 28%, 87%, and 59% of the wild boars, respectively. The prevalence of anti-Yop antibodies did not cluster in a given study site and positively tested animals were found in every district. Therefore, pathogenic Yersinia spp. may be distributed all over the study area (data not shown).
| German postal code | Binary code describing Yop Western blot patterns | Wild boars per district | Bruc | Franc | Franc | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 6 | 10 | 11 | 14 | 15 | 16 | 18 | 19 | 22 | 23 | 24 | 26 | 27 | 30 | 31 | n (%) | ELISA (+) | Western blot (+) | ||
| k (%) | ||||||||||||||||||||||||
| 19055 | 1 | 1 (0.1) | ||||||||||||||||||||||
| 19065 | 1 | 1 | 2 | 1 | 5 (0.7) | 1 (20) | 1 (20) | 1 (20) | ||||||||||||||||
| 19067 | 1 | 4 | 1 | 1 | 1 | 1 | 1 | 1 | 4 | 1 | 1 | 8 | 1 | 26 (3.4) | 4 (15.4) | 3 (11.5) | 3 (11.5) | |||||||
| 19075 | 1 | 1 (0.1) | ||||||||||||||||||||||
| 19079 | 2 | 2 | 2 | 1 | 2 | 3 | 2 | 14 (1.8) | 3 (21.4) | |||||||||||||||
| 19089 | 9 | 5 | 1 | 1 | 1 | 1 | 2 | 4 | 5 | 6 | 4 | 12 | 1 | 16 | 68 (8.9) | 16 (23.5) | 3 (4.4) | 1 (1.5) | ||||||
| 19205 | 2 | 1 | 3 | 1 | 4 | 11 (1.4) | 1 (9.1) | |||||||||||||||||
| 19217 | 1 | 2 | 1 | 4 (0.5) | 1 (25.0) | |||||||||||||||||||
| 19306 | 5 | 1 | 1 | 3 | 2 | 2 | 4 | 2 | 20 (2.6) | 8 (40) | 1 (5.0) | |||||||||||||
| 19370 | 4 | 6 | 2 | 1 | 1 | 1 | 3 | 4 | 1 | 1 | 1 | 16 | 19 | 60 (7.9) | 5 (8.3) | 1 (1.7) | 1 (1.7) | |||||||
| 19372 | 2 | 1 | 1 | 1 | 2 | 3 | 2 | 1 | 7 | 20 (2.6) | 6 (30.0) | |||||||||||||
| 19374 | 8 | 14 | 1 | 3 | 3 | 2 | 2 | 9 | 7 | 1 | 1 | 4 | 13 | 1 | 21 | 90 (11.8) | 23 (25.6) | 3 (3.3) | 2 (2.2) | |||||
| 19376 | 1 | 1 | 1 | 2 | 3 | 8 (1.0) | 1 (12.5) | |||||||||||||||||
| 19386 | 13 | 4 | 4 | 3 | 5 | 1 | 5 | 12 | 3 | 13 | 1 | 38 | 102 (13.4) | 19 (18.6) | 5 (4.9) | 1 (1.0) | ||||||||
| 19395 | 2 | 3 | 1 | 1 | 1 | 4 | 2 | 1 | 1 | 7 | 1 | 24 (3.1) | 5 (20.8) | |||||||||||
| 19399 | 6 | 8 | 3 | 2 | 1 | 1 | 1 | 2 | 5 | 9 | 1 | 2 | 11 | 28 | 80 (10.5) | 22 (27.5) | 8 (10.0) | 8 (10.0) | ||||||
| 19406 | 3 | 2 | 1 | 1 | 2 | 3 | 3 | 1 | 2 | 10 | 2 | 30 (3.9) | 4 (13.3) | |||||||||||
| 19412 | 13 | 1 | 13 | 1 | 1 | 3 | 1 | 2 | 5 | 7 | 3 | 4 | 9 | 10 | 73 (9.6) | 16 (21.9) | 1 (1.4) | 1 (1.4) | ||||||
| 19417 | 3 | 1 | 1 | 3 | 5 | 1 | 7 | 5 | 26 (3.4) | 8 (30.8) | ||||||||||||||
| 23936 | 1 | 1 | 2 (0.3) | 1 (50.0) | ||||||||||||||||||||
| 23966 | 1 | 1 | 1 | 1 | 4 (0.5) | 1 (25.0) | ||||||||||||||||||
| 23968 | 1 | 1 (0.1) | ||||||||||||||||||||||
| 23972 | 1 | 1 | 2 (0.3) | 2 (100.0) | ||||||||||||||||||||
| 23974 | 2 | 3 | 3 | 2 | 10 (1.3) | 3 (30.0) | ||||||||||||||||||
| 23992 | 6 | 18 | 2 | 1 | 1 | 1 | 1 | 6 | 6 | 5 | 17 | 1 | 12 | 77 (10.1) | 16 (20.8) | 7 (9.1) | 6 (7.8) | |||||||
| 23996 | 2 | 1 | 1 | 4 (0.5) | 2 (50.0) | |||||||||||||||||||
| Binary code | 0 | 1 | 2 | 3 | 4 | 6 | 10 | 11 | 14 | 15 | 16 | 18 | 19 | 22 | 23 | 24 | 26 | 27 | 30 | 31 | n (%) | k (%) | ||
| m (%) | 76 (10.0) | 1 (0.1) | 90 (11.8) | 17 (2.2) | 1 (0.1) | 2 (0.3) | 11 (1.4) | 16 (2.1) | 4 (0.5) | 17 (2.2) | 20 (2.6) | 66 (8.7) | 74 (9.7) | 2 (0.3) | 5 (0.7) | 1 (0.1) | 32 (4.2) | 147 (19.3) | 5 (0.7) | 176 (23.1) | 763 (100) | 168 (22) | 33 (4.3) | 24 (3.1) |
| Yers Western blot | − | − | − | − | − | − | − | + | + | + | − | − | + | + | + | − | + | + | + | + | ||||
- n, total number of wild boars per district; k, number of wild boars positively tested for anti-Bruc or anti-Franc antibodies per district; m, number of wild boars showing the same immunoreactive pattern (binary code) of anti-Yers (anti-Yop) antibodies; %, percentage of animals in the respective group.
There was no association between the occurrence of anti-Yersinia, anti-Francisella and anti-Brucella antibodies in the sera of wild boars and vice versa. Sixteen and 44 wild boars negatively tested for anti-Yersinia antibodies showed anti-Francisella and anti-Brucella antibodies, respectively (Fig. 2). Eight animals were tested positive for brucellosis and tularemia without detecting anti-Yersinia antibodies simultaneously.
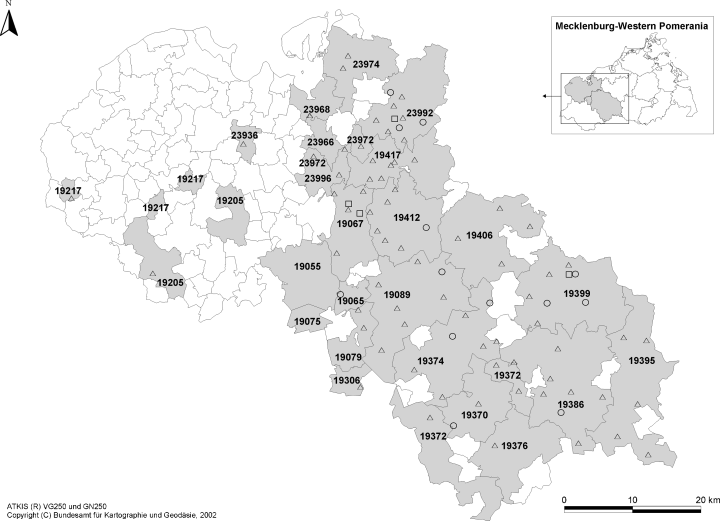
Origin of the wild boars negatively tested for anti-Yersinia antibodies but positively tested for anti-Brucella and/or anti-Francisella antibodies [ATKIS® VG250 and GN250 (Copyright Federal Agency for Cartography and Geodesy 2002, Frankfurt/Main, Germany)]. : anti-Francisella antibody negative and anti-Brucella antibody positive; : anti-Francisella antibody positive and anti-Brucella antibody negative; : anti-Francisella antibody positive and anti-Brucella antibody positive.
Brucellosis
Anti-Brucella antibodies were detected in 168 sera (22.0%). In 19 (2.5%) other sera ELISA results were questionable. The detection of anti-Brucella antibodies was independent of age and gender of the animals investigated (P > 0.2). Although statistically insignificant, the seroprevalence of brucellosis increased with the age of the wild boars (Table 1). Anti-Brucella antibody positive wild boars were found all over the study area except for three postal code regions (Fig. 3). However, these districts cannot be considered as brucellosis-free due to the low number of animals investigated per region.

Sample size (n) and proportion (%) of wild boars tested positive or negative for anti-Brucella antibodies in a zip code region [ATKIS® VG250 and GN250 (Copyright Federal Agency for Cartography and Geodesy 2002, Frankfurt/Main, Germany)]. Bar graphs are only given for regions with positively tested animals. Shooting sites of sero-positive wild boars are ‘+’-marked whereas shooting sites of sero-negative animals are ‘’-marked.
Tularemia
Thirty-three serum samples tested positive for anti-Francisella antibodies using ELISA. Twenty-four (3.1%) proved to be positive in the anti-Francisella tularensis Western blot. Most sero-reactors were found in younger animals which could not be proved statistically because of the low total number of positively tested wild boars. Only two adult animals (2 years of age) tested positive (Table 1). There is no influence of the gender on the seroprevalence of tularemia (P > 0.2). Although most of the wild boars positively tested for anti-Francisella antibodies were found in three postal code regions (three in 19 067; eight in 19 399; six in 23 992) a cluster could not be defined because of the low number of positive test results (Fig. 4).
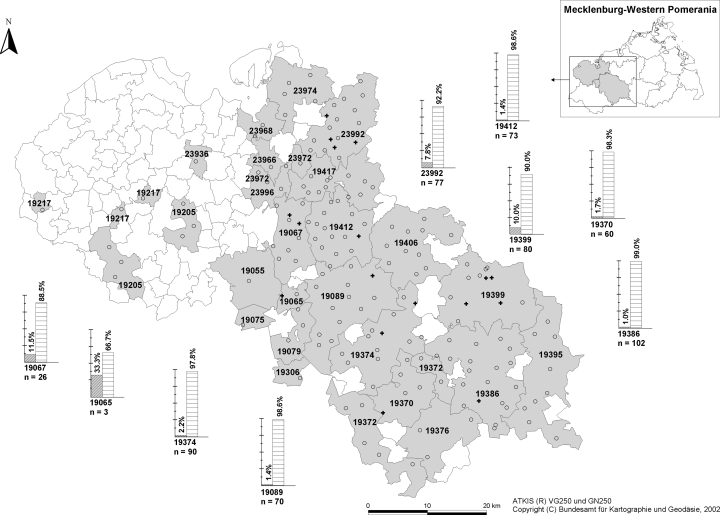
Sample size (n) and proportion (%) of wild boars tested positive or negative for anti-Francisella antibodies in a zip code region [ATKIS® VG250 and GN250 (Copyright Federal Agency for Cartography and Geodesy 2002, Frankfurt/Main, Germany)]. Bar graphs are only given for regions with positively tested animals. Shooting sites of sero-positive wild boars are ‘+’-marked whereas shooting sites of sero-negative animals are ‘’-marked.
Discussion
Zoonoses are a matter of concern to economy and public health. Risk factors for human infections by zoonotic pathogens are related to local disease ecology, including animal reservoirs. Wildlife can be the source of human disease directly transmitted or indirectly transmitted via domestic animals. Hunters, veterinarians, and butchers may be accidentally infected through skin wounds. The consumption of contaminated and insufficiently cooked food may also cause infection. As trade and surveillance of pork is strictly regulated by law, the transmission of zoonotic diseases through pork is not an important public health issue. In contrast, the health risks associated with the consumption of game meat from wild boars are unknown. The meat of wild boars is commonly consumed locally after inspection by the hunter and meat inspectors authorized by public health authorities. The demand for game meat is currently increasing in Germany, because of the negative publicity campaigns dealing with disease risks of domestic animal husbandry (Gibbs, 1997).
A total of 763 sera of wild boars were tested in order to provide general data concerning the seroprevalence of brucellosis, tularemia and yersiniosis in wildlife of Mecklenburg Western-Pomerania.
Serological methods and cross-reactivity
The isolation rate of Brucella strains from clinical specimens is very low and serological testing is still the standard procedure for the evaluation of disease prevalence and the screening of brucellosis suspected cases. However, no single test provides 100% sensitivity and specificity. In previous studies on serological reactions to Brucella species in pigs, the indirect (i)ELISA yielded higher sensitivity and specificity values compared with conventional serological tests, i.e. serum (tube) agglutination test (SAT), buffered plate agglutination test (BPAT), and complement fixation (CFT) (Nielsen et al., 1999; Paulo et al., 2000). The iELISA is commonly used for the verification of positive test results in SAT or Rose Bengal test because of its outstanding specificity. We, therefore, applied a commercially available ELISA based on B. abortus antigen to screen wild boars for porcine brucellosis. Anti-B. suis bv 2 antibodies are supposed to be detected as B. abortus, B. melitensis and B. suis share a common immunodominant smooth LPS. After extensive testing, the B. abortus 1119-3 standard antigen was regarded as equally valid in the serodiagnosis of porcine and bovine brucellosis (Deyoe, 1986). Nevertheless, difficulties in test performance were expected, as the ELISA was only evaluated in domestic pigs (Bommeli Diagnostics, personal communication).
Besides, the immunodominant epitope of the Brucella O-polysaccharide is similar to that of various cross-reacting bacteria, e.g. Y. enterocolitica O:9, Salmonella urbana group N, Stenotrophomonas maltophilia, Escherichia coli O:157, and F. tularensis. Especially, Y. enterocolitica O:9 is able to induce serological cross-reactions indistinguishable from brucellosis (Bockemühl and Roth, 1978; Dedek et al., 1986; Wrathall et al., 1993; Pannwitz and Roost, 2001; Jungersen et al., 2006). Bockemühl and Roth (1978) determined anti-Brucella antibody titres using an agglutination test in nine of 93 pigs because of subclinical infection with Y. enterocolitica O:9. In contrast, CFT tested negative. Although CFT is known to be more specific than the SAT, false positive CFT results in pigs because of Y. enterocolitica O:9 infections have been described (Wrathall et al., 1993). According to the German Brucellosis Ordinance pigs potentially infected with Brucella have to be kept in quarantine. Therefore, the serological diagnosis of brucellosis should be reliable to avoid the imposition of unnecessary restrictions resulting in wasted resources. Antibodies against Yersinia can be completely removed by cross-absorption (Pannwitz and Roost, 2001). In addition, immunological cross-reactions due to the similar O-antigens of Brucella, Francisella and Yersinia can be avoided using protein antigens. The Yersinia Western blot technique based on native or recombinant Yersinia and the V-Ag has proved to be highly sensitive and specific for the detection of anti-Yersinia antibodies (Heesemann et al., 1987).
Although our in-house Francisella ELISA was essentially based on an LPS preparation of F. tularensis subsp. holarctica, in the test panel of artificially infected and hyper-immunized pigs, none of the cross-reacting bacteria were tested positive. The apathogenic species, F. tularensis subsp. novicida and F. philomiragia also tested negative because of a different LPS structure. As we also found anti-Brucella and/or anti-Francisella antibody positive wild boars which were negative for anti-Yop antibodies, false positive results due to cross-reactivity appear to be negligible in the serological methods used.
Yersiniosis
The pathogenicity of Yersinia spp. is linked to Yersinia outer proteins (Yops) regularly inducing humoral immune response. Sera of wild boars were investigated for specific anti-Yop antibodies to prove infection by virulent strains and to eliminate false positive results because of possible cross-reactivity of anti-Francisella and anti-Brucella antibodies directed to LPSs.
Acute Yersinia infections in animals are characterized by septicaemia, enlargement of spleen and lymph nodes or enteritis whereas chronic infections may cause granulomatous nodules and localized abscesses affecting various organs, e.g. liver, lung and the mammary gland. Pigs for the most part are asymptomatic carriers of Yersiniae (Bottone, 1999; Thibodeau et al., 2001). Both, Y. pseudotuberculosis and Y. enterocolitica infections may provoke the same clinical picture (Wuthe and Aleksic, 1997; Frölich et al., 2003). Yersiniosis is transmitted by faecal contaminated pasture grounds and watering places. The regional infection pressure of pathogenic Yersinia spp. may be quantified by the seroprevalence of anti-Yop antibodies in the animals staying in the given habitat.
Anti-Yersinia antibodies were found in 55% of the hare sera from Northern Germany (Frölich et al., 2003). Yersinia pseudotuberculosis was isolated from 13% of the hares which were shot or found dead in this region, whereas Y. enterocolitica was only isolated from 4% (Wuthe et al., 1995). Human disease is most often associated with the consumption of raw or undercooked pork products (Miller and Paige, 1998; Bottone, 1999). In 96.3% of 1002 Bavarian slaughter pigs anti-Yop antibodies were detected (Hensel et al., 2004). As in our study, 45.4% of these pigs were considered to be positive for a previous Yersinia infection defining the same detection standard (Yop D + 2 in Yersinia Western blots). The criterion of positivity (Yop D + 2) follows the empirical approach of Hensel et al. (2004) and is in accordance with the interpretation of positive results in humans (Heesemann, 1990). In our population, 62.6% of the animals were tested positive. The higher prevalence of anti-Yop antibodies might be explained by the longer lifetime of wild boars and prolonged exposure to Yersinia spp. as a consequence thereof. In addition, Hensel et al. (2004) tested meat juices for anti-Yop antibodies whereas we used sera. Impurities of the meat juice samples may have blocked antibody binding.
The immune response and the respective antibody patterns seem to be host specific. In our study, the most frequent immunoreactive patterns (each comprising at least 10% of the study population) were 0, 2, 27 and 31 (Table 2). In European brown hares from North-Rhine Westphalia the predominant patterns were 0, 2, 6, and 14 and in Bavarian slaughter pigs 18, 19, and 27 were most often found (Bartling et al., 2004; Hensel et al., 2004). Domestic pigs only revealed 13 different immunopatterns (Hensel et al., 2004), whereas the wild boars of our study population showed 20 in Yersinia Western blots. The rearing pigs in fattening units may be infected by only a few Y. enterocolitica or Y. pseudotuberculosis clones (Niskanen et al., 2002; Fredriksson-Ahomaa et al., 2003; Wojciech et al., 2004). Although Yops and V-Ag are highly conserved in Yersiniae the infectious agents in wildlife may be more variable. However, the lower heterogeneity of serological reaction patterns in domestic pigs is more likely caused by the lower diversity of immune response because of inbreeding. The binary code representing the immunological fingerprint of the host–pathogen interaction significantly increased with the age of the wild boars. This may reflect constant exposure to contaminated food or a change of immune response to the pathogen over time.
Brucellosis
Although brucellosis in pigs may be latent, granulomatous nodules or abscesses in testes, liver, spleen and other organs are frequently observed (Cvetnic et al., 2003). Inapparent infections have been described (Miller and Paige, 1998). Since infected wild boars rarely showed macroscopic lesions a lower pathogenicity of B. suis bv 2 for wild boars compared with domestic pigs is supposed. Hence, brucellosis in wild boars can be easily overlooked in the meat inspection.
Brucellosis in wild boars is widely distributed throughout Central Europe (Dedek et al., 1986; Godfroid et al., 1994, 2005; Garin-Bastuji et al., 2000; Hubalek et al., 2002; Cvetnic et al., 2003, 2004). In seroprevalence studies from Croatia, 13.6–29.4% of the wild boars tested positive (Cvetnic et al., 2003, 2004). In the same regions, B. suis bv 2 was isolated from 17% to 29.2% of the samples investigated; 12.3–13.5% of the domestic pigs also revealed a positive serological reaction (Cvetnic et al., 2003).
In the 1980s, 7.9% of 1794 wild boars from the North of Mecklenburg-Western Pomerania were tested Brucella positive using CFT (Dedek et al., 1986). In our study, 22% of the wild boars tested positive. The higher number does not necessarily represent an increase of infected animals or an enzootic outbreak in the study area. First, ELISAs proved to be more sensitive in the serodiagnosis of porcine brucellosis than the CFT (94% versus 58–93%) (Nielsen et al., 1999). Secondly, animals younger than 3 months, which are susceptible to the infection with B. suis, exhibit a limited antibody response. Dedek et al. (1986) did not specify age groups in their study population but a greater part of shoats may have resulted in lower seroprevalence.
Although brucellosis is supposed to be maintained within the hare population, the natural reservoir of ‘hare brucellosis’ may have changed in Germany during the last decade as the number of hares substantially dropped and that of the wild boars steadily increased. About 300 sera recently collected from European brown hares in Northern Germany (Schleswig-Holstein) were tested negative for antibodies against Brucella (Frölich et al., 2003). The occurrence of B. suis bv 2 or anti-Brucella antibodies in free-ranging hares may depend on the number of close contacts with infected wild boars or domestic pigs. In 1995/1996, the number of wild boars hunted in Mecklenburg-Western Pomerania exceeded those in Schleswig-Holstein sixfold, whereas the number of hunted hares was 34-fold lower (http://www.jagd-online.de; consulted 27 July 2005). In Germany, B. suis bv 2 infections in wildlife may be directed from the wild boar reservoir to the hare. Hubalek et al. (2002) similarly observed an increasing seroprevalence of brucellosis in wild boars preceding an epizootic of hare brucellosis in Breclav, Czech Republic. Wild boars may also contribute to the dissemination of brucellosis to a greater extent, since hares usually stay in their natural habitat.
In Germany, the number of wild boars increased in the 1990s as wild boars were additionally raised in enclosures before they were released for hunting (Godfroid, 2002). Keeping wild boars in willows, infectious diseases like brucellosis can spread easily. Porcine brucellosis is a venereal disease also transmitted by contact with foetal membranes, post-parturient discharges and milk. Since the pathogen is able to survive in soil and water for several weeks the ingestion of contaminated food and water as well as the inhalation of Brucellae may be other possible routes of transmission in animal husbandry (Godfroid et al., 2005).
A high prevalence of seropositive wild boars was found in our study population independent of the age of the animals investigated. Godfroid (2002) also described a high bacteriological isolation rate independent of the age category. In Europe, porcine brucellosis is almost restricted to B. suis bv 2 infections (Cvetnic et al., 2003, 2004). Brucella suis bv 2 has only exceptionally been described as the causative agent of human brucellosis (Teyssou et al., 1989; Paton et al., 2001). In contrast, B. suis bv 1 and bv 3 are well known to cause human infection. These biotypes mainly occur in wild boars, feral swine and domestic pigs in South-East Asia and South America. Feral pigs are also an important source of disease in the south-eastern states of the USA and in Queensland, Australia (Godfroid, 2002; Godfroid et al., 2005).
The pathogenicity of Brucella species is usually restricted to a single host species. However, new Brucella species may occur or the well-known Brucella spp. may adapt to the changing environment. In South America, B. suis bv 1 became established in cattle and a rare case of B. suis bv 2 infections in cattle has also been reported (Andersen and Pedersen, 1995; Godfroid et al., 2005). Furthermore, B. suis bv 3 has recently been isolated from horses in Croatia (Cvetnic et al., 2005). However, incidental hosts may rarely perpetuate infection over a significant period of time (Bengis et al., 2002). The persistence of infection in an ecosystem requires one or more maintenance host species.
Especially, the game farming industry may contribute to the re-introduction of brucellosis into livestock. Domestic pigs out at feed may be infected by wild boars breaking into pasture grounds (Kautzsch et al., 1995). In France, B. suis bv 2 infections have been reported from out-door rearing pig farms all over the country during the last decade (Garin-Bastuji et al., 2000). Brucellosis may cause tremendous economic losses directly due to infertility and abortion in infected hosts and indirectly due to international trade barriers (Godfroid, 2002; Godfroid et al., 2005). However, the impact of brucellosis on wild boars seems to be negligible as the number of animals in Germany increased steadily within the last decade.
Tularemia
In Europe, tularemia is widely spread occurring in every country except for Great Britain, Iceland, and Portugal (Tärnvik et al., 2004). In most countries, only a few sporadic human cases are reported every year whereas in others, e.g. Finland and Sweden, outbreaks comprising hundreds of cases are recorded at least once a decade. Tularemia also exists endemically in Central and Northern Germany. Francisella tularensis subsp. tularensis (Jellison type A) and F. tularensis subsp. holarctica (Jellison type B) are responsible for the majority of human infections. Type A causing the highest mortality is limited to North America, whereas type B found throughout the northern hemisphere is the main type in Europe including Germany (Mörner, 1992). However, highly virulent and pathogenic strains of F. tularensis subsp. tularensis have recently been isolated from fleas and mites in Slovakia (Gurycova, 1998). Other Francisella subsp. are of minor relevance in human medicine.
Clinical presentation is variable and depends on the route of infection. Local forms comprise ulcers and enlargement of regional lymph nodes. In contrast, inhalation may provoke pneumonia and septicaemia. Historically, farming was an occupational risk for acquiring tularemia in endemic areas (Petersen and Schriefer, 2005). The occurrence of anti-Francisella antibodies in wild boars displays a possible risk of infection for hunters and consumers.
Although lagomorphs are highly susceptible and are able to perpetuate an epizootic, they probably do not constitute a natural reservoir of Francisella, since they often die from fatal septicaemia within a few days (Mörner, 1992; Hofer et al., 1997; Frölich et al., 2002). In contrast, pigs and wild boars infected with Francisella are not known to show clinical signs or symptoms. However, healthy carriage or persistent infection in animal models has not been described, yet (Tärnvik et al., 2004). As culture of Francisella spp. from carcasses is difficult (Mörner, 1992), serological methods are commonly used for screening wildlife. ELISA and Western blot analysis show excellent sensitivity and specificity but specific antibodies cannot be detected before the end of the second week of disease (Tärnvik et al., 2004; Schmitt et al., 2005). On the other hand, antibody titres may persist for years. It can be speculated that wild boars are one of the major animal reservoirs of Francisella. Wild boars seem to be asymptomatic carriers but may act as the source of tularemia enzootics in susceptible animal species, e.g. in hares. Tularemia is enzootic in the study area and the seroprevalence is increasing. In 1986, Dedek et al. only tested one of 1061 serum samples positive for anti-Francisella antibodies in wild boars from Mecklenburg-Western Pomerania. Being an omnivore the wild boar may act as an indicator animal for the occurrence of Francisella in a given study site.
The seroprevalence of 3.1% in our study population correlates well with that reported from other European countries (Hubalek et al., 2002). In times of a widespread outbreak of tularemia in South Moravia (Czech Republic) elevated antibody titres were found in up to 17% of the wild boars but seroprevalence decreased to 3% after disappearance of the epizootic. However, Hubalek et al. (2002) did not confirm the positive test results measured by micro-agglutination and only cross-reactivity against Brucella spp. was excluded.
Ticks and other arthropods are the main vectors of tularemia and protozoa have been discussed to be the environmental reservoir of Francisellae (Abd et al., 2003). The natural habitat may be similar to that of the protozoa, i.e. soil, mud, and stagnant water. Type B tularemia is associated with natural water and water-borne outbreaks are common in Europe (Tärnvik et al., 2004). The study area in Mecklenburg-Western Pomerania is famous for its woodland and large lakes. Francisella infection in the wild boars investigated may be transmitted by the ingestion of contaminated water and infected dead hare or mice, by blood-feeding insects, or by direct contact to contaminated soil (Mörner, 1992). Although the natural ecology of tularemia is not yet completely understood, hosts and their susceptibility to infection as well as the survival capacity of Francisella in its ecologic niche may influence the emergence of disease. Several environmental characteristics of typical natural foci have been already identified, i.e. alluvial forest <200 m above sea level, 8–10°C of mean annual temperature, 450–700 mm of mean annual precipitation, and 2000–2200 h of mean annual sunshine duration (Pikula et al., 2003). In the study area, the climate fulfilled most of these requirements for tularemia foci.
Conclusions
Many infectious diseases may be transmitted from wild to domestic animals and finally to humans. Human disease caused by Brucella spp. and Francisella spp. are always associated with an animal reservoir (in wild and domestic animals). Only long-term surveillance of wildlife may help to identify the natural foci of zoonotic diseases in endemic areas allowing preventive measures before hot spots become manifest in an epidemic outbreak (Gurycova et al., 2001). Nevertheless, there is a lack of systematic surveillance of wildlife in most European countries (Frölich et al., 2002). Furthermore, control and eradication of animal disease by test-and slaughter programmes are difficult and may be even impossible in wildlife. Sealing of the wildlife/livestock interface may be the only possible countermeasure to control zoonotic foci and minimize public health risks (Bengis et al., 2002).
However, the impact of mutual transmission of zoonotic pathogens between livestock and wildlife is still unknown. Therefore, only preventive management programmes, i.e. coordinated surveillance, countermeasures and research activities may help to eradicate zoonoses in wildlife. At the moment, the real risks for humans to contract tularemia or brucellosis from wildlife cannot be estimated. The epidemiological data currently available are not reliable as the clinical picture of these diseases is not specific often resulting in misdiagnosis. To assess health risks for consumers and occupationally exposed, like hunters, seroprevalence studies in humans are urgently needed.
Acknowledgements
We gratefully thank H.-J. Bruns, S. Schatz, and F. Goldberg for their excellent technical assistance.




