Long-Term Studies on Maternal Immunity for Aujeszky's Disease and Classical Swine Fever in Wild Boar Piglets
Summary
The aim of the studies was to fathom the duration and the role of maternal immunity for Aujeszky's disease (AD) and classical swine fever (CSF) in wild boar offspring. In one experiment, two wild boar sows were infected with a low pathogenic pseudorabies virus (PRV) in 1999. A total of 51 offspring was born between 1999 and 2002 and was monitored for PRV maternal antibodies. In a second experiment, the maternal immunity for CSF was analysed. Therefore, a sow was orally vaccinated against CSF using vaccine baits containing the live-attenuated C-strain vaccine. The vaccination took place in January 1999. The sow gave birth to four piglets in 2001 and to two piglets in 2002. With respect to maternal immunity for AD, some piglets reacted positive in the ELISA up to 27-week post-partum while in the neutralization test antibodies were detected up to 15-week post-partum. The calculated half-life of neutralizing antibodies was 21 days. Regarding CSF, the neutralization titres of maternal antibodies dropped continuously reaching values of ≤10 ND50 20-week post-partum. After the 12th week post-partum, most of the sera reacted negative in the ELISA. However, after the third month, low levels of neutralization titres were still detectable. The results are discussed with respect to the epidemiology and control of both diseases in wild boar populations.
Introduction
Classical swine fever (CSF) is caused by an RNA virus, which belongs to the genus Pestivirus of the Flaviviridae family while Aujeszky's disease (AD, pseudorabies) is caused by an Alphaherpesvirus, a DNA virus also known as suid herpes virus 1 or pseudorabies virus (PRV). Under the natural conditions, both infections occur in domestic pigs and wild boar (Sus scrofa) causing substantial economical losses especially in countries with an industrialized pig production.
The role of wild boar in both diseases is primarily of epidemiological interest as this species is regarded as reservoir for both viruses and as possible source of infection for domestic pigs (Kern et al., 1999; Müller et al., 2000, 2003).
Epidemiological links between CSF virus infections in wild boar and domestic pigs have been reported repeatedly (Teuffert et al., 1997). CSF virus isolates which are circulating in wild boar populations were also found in domestic pigs. In contrast, the PRV isolates originating from wild boar are mainly attenuated and biologically adapted to the wild boar. They are genetically different from isolates obtained from domestic pigs (Müller et al., 2000). Although AD in wild boar is not considered to be a major threat, a virus transmission to domestic pigs cannot be ruled out completely (Müller et al., 2000, 2001a).
The German domestic pig population has recently been declared free of pseudorabies by the European Commission (2003/130/EC) (Müller et al., 2003). However, PRV infections are spreading in wild boar not only in Germany (Müller et al., 1998; Müller et al., 2000; Lutz et al., 2003; Albina et al., 2000). The situation for CSF is similar. The last German CSF case in domestic pigs occurred in 2003 while the last case in wild boar was recorded in November 2004. Taking this into consideration, monitoring, prevention and control of CSF and AD in wild boar are of major importance in order to protect the domestic pig population.
A prerequisite for successful control measures in wildlife is the knowledge about the disease, in particular about immunological aspects. The objective of the present study was to fathom the duration and role of maternal immunity for AD and CSF in wild boar offspring. Data regarding CSF maternal antibodies in piglets born shortly after the immunization of their mother do exist (Depner et al., 2000); however, no data were available regarding the maternal immunity in piglets born years after vaccination or infection of their mothers. This information is important in the context of an oral vaccination strategy for CSF, which has been successfully conducted in some European countries.
Material and Methods
AD experiment
The maternal immunity for AD was recorded in 51 offspring born by two sows between 1999 and 2002. The mothers were inoculated intranasally during the 2nd month of gestation in 1999 with 106 TCID50 of a PRV-isolate of wild boar origin (BFW1) (Müller et al., 1998). Each animal received 1 ml of PRV cell culture suspension into each nostril. During 1999, each wild boar family was housed separately, in the subsequent years, sows and offspring were housed together. Litter sizes and sampling regime are shown in Table 1. For serological investigations, the sows were bled several times during the observation period of 4 years. From the offspring, blood samples were collected four to 11 times during a period of 22–50-week post-partum. The sera were tested for the presence of antibodies against PRV by neutralization assay using the PRV strain Kaplan (Wittmann et al., 1987). Additionally, a commercial Antibody-ELISA for AD (ChekitRAujeszky-ELISA, Bommeli AG) was employed according to the instructions of the manufacturer. Half-life of AD maternal antibodies was estimated by fitting the exponential function to the serological data (Spain, 1982).
| Years | 1999 | 2000 | 2001 | 2002 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sows | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Number of piglets born | 7 | 7 | 3 | 4 | 10 | 3 | 4 | 9 | 8 | 2 | ||
| Weeks p.p. | Sampling time | |||||||||||
| 1 | ||||||||||||
| 2 | x | x | ||||||||||
| 3 | x | x | ||||||||||
| 6 | x | x | ||||||||||
| 7 | x | x | ||||||||||
| 8 | x | |||||||||||
| 9 | x | x | x | x | ||||||||
| 10 | x | x | x | |||||||||
| 12 | x | x | x | |||||||||
| 13 | x | x | x | |||||||||
| 14 | x | x | x | x | ||||||||
| 15 | x | x | ||||||||||
| 16 | x | x | x | |||||||||
| 17 | x | x | x | x | ||||||||
| 18 | x | |||||||||||
| 20 | x | x | x | |||||||||
| 22 | x | x | x | x | x | x | x | x | x | |||
| 23 | x | |||||||||||
| 24 | x | |||||||||||
| 25 | x | |||||||||||
| 26 | x | |||||||||||
| 28 | x | x | x | |||||||||
| 29 | x | |||||||||||
| 30 | x | |||||||||||
| 33 | x | x | ||||||||||
| 36 | x | x | ||||||||||
| 37 | x | x | x | |||||||||
| 50 | x | x | ||||||||||
- Sows 1 and 2 were inoculated intranasally with a PRV isolate of wild boar origin on 19th February 1999. Sow 3 was orally vaccinated against CSF on three consecutive days in January 1999 using the vaccine baits containing the live-attenuated C-strain.
CSF experiment
For recording the maternal immunity for CSF, six wild boar piglets were monitored. The mother of these piglets was orally vaccinated against CSF on three consecutive days in January 1999 using bait vaccine containing the live attenuated C-strain (Depner et al., 2000). The sow was never boostered again. Four piglets were born in 2001 and two piglets in 2002. Hence, vaccination took place two respectively three years before the piglets of this study were born. For antibody detection, the sow was bled seven times during the observation period of 4 years. The piglets were bled four to five times starting 8-week post-partum until they were 28 weeks old (Table 1). The sera were tested for the presence of antibodies against CSF virus by a neutralization assay using the CSF virus strain Alfort187 and by a commercial antibody-ELISA for CSF (HerdCheck CSFV Ab from IDEXX). The tests were performed according to the Diagnostic Manual for CSF (Commission Decision, 2002/106/EC).
Results
AD serology
After the inoculation with PrV, both sows developed neutralizing antibodies (Table 2). A total of 218 piglet sera were examined in the neutralization test of which 93 (42.6%) reacted positive with titres between four and 64 ND50. The maternal antibody titres decreased exponentially during the observation period. Low titres (4 ND50) were measured up to 15-week post-partum (Fig. 1). According to these results, the calculated half-life of neutralizing antibodies was 21 days. A total of 250 piglet sera were examined with the ELISA of which 229 (91.61%) reacted positive. Positive results in ELISA were recorded up to 27-week post-partum. The values of the optical densities (OD) revealed a sigmoid decreasing curve (Fig. 2).
| Date | Sow 1 | Sow 2 | Sow 3 | |||
|---|---|---|---|---|---|---|
| PRV | PRV | CSF | ||||
| ELISA | NT* | ELISA | NT | ELISA | NT | |
| 08.10.98 | Neg | <2** | Neg | <2 | Neg | <5 |
| 12.02.99 | 30 | |||||
| 19.02.99 | Neg | <2 | Neg | <2 | Pos | 80 |
| 29.04.99 | Pos | 256 | Pos | 64 | Pos | >640 |
| 16.06.99 | Pos | 256 | Pos | 64 | Pos | >640 |
| 24.03.00 | Pos | 256 | Pos | 32 | Pos | >640 |
| 25.07.00 | Pos | 64 | Pos | 32 | Pos | >640 |
| 16.12.02 | Pos | 64 | Pos | 32 | Pos | >640 |
| 20.02.03 | Pos | 16 | ||||
| 21.03.03 | Pos | 32 | Pos | 16 | ||
- *, neutralization test; **, neutralization titre ND50.
- Sows 1 and 2 were inoculated intranasally with a PRV isolate of wild boar origin on 19th February 1999. Sow 3 was orally vaccinated against CSF on three consecutive days in January 1999 using the vaccine baits containing the live-attenuated C-strain.
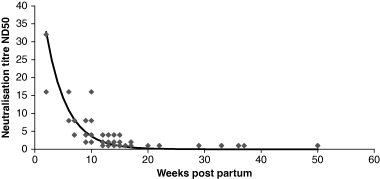
Neutralization titres of maternal antibody in 218 sera of wild boar piglets born by two sows experimentally infected with a PRV isolate of wild boar origin.

ELISA results of 250 sera of wild boar piglets born by two sows experimentally infected with a PRV isolate of wild boar origin.
CSF serology
The serological data concerning the CSF vaccinated sow have partially been reported in a previous work by Depner et al. (2000). The sow had high titres of neutralizing antibodies (>640 ND50) during the entire observation period, which lasted until December 2002 (Table 2). The neutralization titres of the piglets ranged between 15 and 120 ND50 in the blood samples taken 8–12-week post-partum. These samples reacted positive also in the ELISA. However, the neutralization titres dropped continuously reaching values of ≤10 ND50 20-week post-partum. After the 12th week post-partum, most of the sera were negative in the ELISA (Table 3). The overall trend of maternal antibodies is shown in Fig. 3.
| Age of piglet (weeks) | Serological results for CSF* | |||||
|---|---|---|---|---|---|---|
| Litter 1 | Litter 2 | |||||
| Piglet 1 | Piglet 2 | Piglet 3 | Piglet 4 | Piglet 5 | Piglet 6 | |
| 8 | 60/p | 15/p | 120/p | |||
| 9 | 40/p | 80/p | ||||
| 12 | 20/p | 15/n* | 40/p | 30/p | ||
| 14 | 15/n | 30/p | ||||
| 16 | 15/n | 20/n | ||||
| 17 | 10/n | <5/n | 10/n | 15/p | ||
| 20 | <5/n | 5/n | 15/n | 10/n | ||
| 22 | 7/n | <5/n | 5/n | 15/n | 15/n | 5/n |
| 28 | <5/n | <5/n | ||||
- *, neutralization titre ND50/ELISA result (p = ELISA-positive; n = ELISA-negative). The piglets from litter 1 were born 2 years after the mother was vaccinated against CSF while the offspring of litter 2 was born 3 years after vaccination.
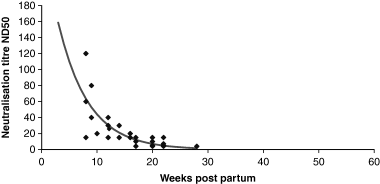
The overall trend in the development of maternal antibody in sera of six wild boar piglets born by a sow orally vaccinated against CSF.
Discussion
Passive immunity is primarily dependent on the antibody titre of the mother and on the amount of colostrum ingested by the newborn. In this study, we could follow up the antibody status of wild boar sows over several years and we were also able to monitor the maternal immunity in offspring born years after vaccination or infection of their mothers.
With respect to AD, it could be shown that a single infection with PRV of wild boar origin induces a stable long-lasting active immunity, which is transmitted passively to the offspring even years after infection. Some piglets reacted positive in the ELISA up to 27-week post-partum while in the neutralization test antibodies were detected up to 15-week post-partum. That means that in the ELISA the piglets reacted positive twice as long as in the neutralization test. The half-life of maternal neutralizing antibodies was estimated to be 21 days, which is approximately two times longer than that known for other diseases. The half-lives of maternal antibodies for PRV in domestic pigs were reported to be 11.3 days (Tenhagen et al., 1995). Half-lives for other viral diseases were reported to be 11 days for CSF, 9.34 days for rabies, 8.4 days for canine distemper and 9.7 days for canine parvovirus infection (Krakowka et al., 1978; Pollock and Carmichael, 1982; Müller et al., 2002).
The neutralization titres in wild boar piglets were generally lower than those of domestic pigs vaccinated with attenuated PRV vaccines (Vannier, 1985). Using the homologous test virus (BFW1) in the neutralization assay could have resulted in slightly but not significantly higher neutralization titres. It is known from domestic pigs that the maternal immunity in young animals prevents transmission of PRV and that a correlation between the level of maternal immunity and the protection against invasion of PRV in the nervous system of neonatal pigs does exist (Bouma et al., 1997; Kritas et al., 1997). Taking this into account, wild boar piglets with PRV-specific maternal antibodies probably do not play an important role in the transmission of PRV within the population, in contrast to CSF. Considering that PRV-specific maternal antibodies are present for more than six months, it can be speculated that PRV is probably mainly transmitted among juvenile animals at the end of November and the beginning of December when contact rates and stress increase because of the starting mating season.
Regarding CSF, the neutralization titres of the sow immunized with the C-strain vaccine in 1999 were constantly high over years (>640 ND50). Thus, immunity after oral vaccination against CSF with attenuated C-strain can be long-lasting and without reduction or loss of titre. This observation is reflected also by the serological data obtained from the piglets born two respectively 3 years later. Furthermore, the antibody titres are comparable with the titres of piglets born in the same year when the sow was vaccinated (Depner et al., 2000). It can be concluded that in areas where wild boar was vaccinated orally to control CSF, maternal antibodies can be detected in piglets born to immune sows even years after cessation of oral vaccination.
Within 20-week post-partum, the CSF neutralization titres of the piglets continuously dropped reaching values of ≤10 ND50 and no antibodies could be detected any more with the ELISA. However, low levels of neutralization titres were still detectable after the third month post-partum. Depner et al. (1995) found low titres of CSF-specific maternal antibodies in wild boar piglets born by a sow infected with field virus even 1-year post-partum. Kaden et al. (1999) and Kaden and Lange (2004) detected CSF-specific maternal antibodies in wild boar piglets born by vaccinated sows during the first 5 and 3 months, respectively. These data are also in accordance with the data obtained in experiments with domestic pigs where an exponential decrease in titre could be observed during the first 3 months (Aynud, 1988). Perhaps, higher neutralization titres were obtained when a homologous test virus (CSF strain) in the neutralization assay would have been used but as under routine testing, in this study we strictly followed the Diagnostic Manual for CSF (Commission Decision, 2002/106/EC).
The persistence of maternal antibodies on a low level for several months may have practical consequences as it might influence the clinical picture of CSF. Depner et al. (2000) challenged wild boar piglets partially protected by maternal antibodies (titres of ≤20 ND50) with virulent CSF virus and the animals did not develop severe clinical signs although they became viraemic. It was concluded that the presence of maternal antibodies influences the clinical course of CSF in such a way that the disease becomes obscure and the outcome is rather transient than lethal.
The CSF-specific maternal immunity might also interfere with the oral vaccination of wild boar. Kaden et al. (2002) showed that seroconversion because of oral vaccination was rather unsatisfactory in young animals (<1 year). The reason therefore might be the vaccine bait itself, which is not well accepted by the juvenile pigs or might be because of a possible interference of long-lasting maternal immunity with the attenuated C-strain vaccine. Lunais et al. (1978) could show for domestic pigs that vaccination of piglets in the presence of maternal antibodies results in a variable immune status depending on the amount of passive antibodies at the time of vaccination. In the presence of high titres of maternal antibodies, piglets are not stimulated immunologically and are subsequently susceptible to challenge. In the presence of low or moderate titres, a weak primary antibody production was seen and most of the pigs were resistant to challenge. However, evidence exists from oral vaccination of foxes against rabies that there is a partially impaired immune response in fox cubs <8 weeks old born to immune vixens resulting in an insufficient protection against rabies. This inhibition of the immune response to active immunization outlasts the time during which maternal antibodies are present at detectable levels. It was concluded that the oral vaccination campaigns aimed at the young fox population in previously vaccinated areas need to be reconsidered (Müller et al., 2001b). Concerning oral vaccination of wild boar against CSF, similar studies are needed to improve vaccination efficacy under field conditions.
Acknowledgements
The authors thank Hubertus Recke, Petra Fechner, Doris Junghans and Ulrike Polenz for their skilful technical assistance during the experiments.




