Phytate and phytase in fish nutrition
*Both contributed equally to this review.
Summary
Phytate formed during maturation of plant seeds and grains is a common constituent of plant-derived fish feed. Phytate-bound phosphorus (P) is not available to gastric or agastric fish. A major concern about the presence of phytate in the aquafeed is its negative effect on growth performance, nutrient and energy utilization, and mineral uptake. Bound phytate-P, can be effectively converted to available-P by phytase. During the last decade, phytase has been used by aqua feed industries to enhance the growth performance, nutrient utilization and bioavailability of macro and micro minerals in fish and also to reduce the P pollution into the aquatic environment. Phytase activity is highly dependent on the pH of the fish gut. Unlike mammals, fish are either gastric or agastric, and hence, the action of dietary phytase varies from species to species. In comparison to poultry and swine production, the use of phytase in fish feed is still in an unproven stage. This review discusses effects of phytate on fish, dephytinisation processes, phytase and pathway for phytate degradation, phytase production systems, mode of phytase application, bioefficacy of phytase, effects of phytase on growth performance, nutrient utilization and aquatic environment pollution, and optimum dosage of phytase in fish diets.
Introduction
In nature phytate is present in all seeds and possibly all cells of plants. It serves as a store of cations, of high-energy phosphoryl groups and is a naturally occurring compound formed during maturation of plant seeds and grains. A large body of evidence show that minerals are less available from feed of plant origin than from animal-based feed. Aquaculture is one of the fastest growing food sectors (FAO, 2008). The aquaculture feed industry relies on the fishmeal, which is the most preferred protein source for fish feed owing to excellent amino acid and fatty acid profile. Limited supply, high cost and stagnant production level restrict its use for sustainable farming (New and Wijkström, 2002; Baruah et al., 2004). The replacement of fishmeal with extensively available plant or grain by-products is getting increasing attention for the development of low-cost fish feed (Carter and Hauler, 2000; Gatlin et al., 2007).
The main source of plant protein in terrestrial and aquatic feed includes soybean meal, corn (gluten), sunflower meal, canola/rapeseed meal, peas and lupins. Soybean meal represents the highest proportion of plant protein in fish diets owing to high yield, relatively high crude protein content and easy and round the year availability. Nutritionists for many years have investigated the ways to utilise proteins of plants origin, because they are cheaper, readily available, and easily accessible than animal protein sources. However, one of the major constraints that limit use of plant proteins in animal feed is the presence of anti-nutritional factors, phytate being one of them. It is free form of inositol hexakisphosphate (IP6) and a polyanionic molecule with six phosphate groups that can strongly chelate with cations such as calcium, magnesium, zinc, copper, iron and potassium to form insoluble salts. This adversely affects the absorption and digestion of these minerals in fish (Papatryphon et al., 1999). The natural source of phosphorus (P) in the form of phosphate rocks is not renewable, which may lead to P crisis in future (Mullaney et al., 2000).
Lately there has been continuous pressure on farmer and nutritionist to effectively use more and more plant based phosphorus. From 50% to 80% of the total P content in plant seeds is stored in the form of phytate (Ravindran et al., 1995). Phosphorus in this form is generally not bioavailable to monogastric animals (humans, dogs, pigs, birds) and also to agastric animals because they lack the intestinal digestive enzyme, phytase, required to separate P from the phytate molecule (Jackson et al., 1996). As a consequence of low digestibility of phytate by fish, most of the phytate-P ends up being excreted into the water and may cause algal bloom pollution (Baruah et al., 2004). Moreover phytate can also integrate with cation groups on protein, amino acids, starch and lipids in feedstuff reducing the digestibility of these nutrients in fish, poultry and pig.
The ideal approach to maximise the nutritive value of plant-based diet is through hydrolysis of undigestable phytate by use of exogenous phytase enzyme. Feil (2001) mentioned that, because of versatile properties of phytate and phytase, interest is not limited to animal nutrition and but also extends to human nutrition and medical science. Also, interest in this area has been increasing at a fast pace in aquaculture (Debnath et al., 2005a,b; Gabaudan et al., 2006; Baruah et al., 2007a,b) because of increased need to improve protein utilisation of plant-sourced feed ingredients and to reduce dependence on fishmeal diet.
The potential for commercial exploitation of microbial phytase in fish feed has been reviewed by Cao et al. (2007). This review presents properties of phytase and their comparison from different sources, effects of phytase on P utilization in fish and on aquatic environment pollution, and factors affecting phytase efficacy. In our review, in addition to discussing reports that dealt with aspects reported in Cao et al. (2007) and have emanated during the last 3 years, a synthesis of information on the effects of phytate, dephytinisation methods, pathway of phytate degradation, phytase production systems and mode of application of phytase and their effects are presented. An attempt has also been made to suggest an optimum dose of phytase based on the available literature of different age groups of fish of different species.
Phytate
Phytic acid is the hexaphosphoric ester of the hexahydric cyclic alcohol meso-inositol (Fig. 1). Phytic acid [known as inositol hexakisphosphate (IP6), or phytate when in salt form] is the principal storage form of P in many plant tissues. Inositol penta-(IP5), tetra-(IP4), and triphosphate (IP3) are also called phytates. Molecular formula: C6H18O24P6 and molecular mass: 660.04 g/mol.
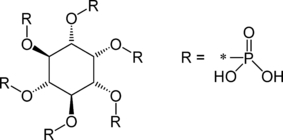
Chemical structure of phytic acid (Kumar et al., 2010).
Phytate is the storage form of P bound to inositol in the fibre of raw whole cereal grains, oil seeds and nuts. In animal nutrition, phytate has been described as ‘both an anti-nutritional factor and an indigestible nutrient’ (Swick and Ivey, 1992). However, the presence of phytate in human diets may have some positive consequences besides reducing the availability of calcium (Ca), zinc (Zn) and other trace minerals. The propitious concern includes anti-diabetic and anti-carcinogenic effect in humans (Kumar et al., 2010).
Phytate is found in potentially usable plant derived ingredients of fish feed such as soybean, Jatropha kernel meal from non-toxic genotype and detoxified Jatropha kernel meal from the toxic genotype, rice, wheat, barley, maize, groundnut, sesame and rapeseed (Makkar et al., 1998; Makkar and Becker, 2009). Soybean meal, rapeseed meal, and sesame meal contain from 50% to 80% of total phosphorus in phytate form (Tyagi and Verma, 1998). The remaining P is represented by soluble inorganic phosphate and cellular P (phosphorus bound in nucleic acids, phosphorylated proteins, phospholipids, phospho-sugar). Phytate isolated from plants belongs to the group of organic phosphates and is a mixture of calcium-magnesium salt of inositol hexaphosphoric acid, called as phytin (Baruah et al., 2005; Debnath et al., 2005b).
Quantitative aspects of phytate
The concentration of phytate and phytase in the feedstuffs varies considerably. Phytate constitute between 0.7% and 2% of most cereal grains and oilseeds (Adeola and Sands, 2003). In general plant-derived fish feed ingredients such as soybean meal, rapeseed meal, and sesame meal contain 1.0–1.5%, 5.0–7.5% and 2.4% phytate respectively (Francis et al., 2001). It has been estimated that about 14.4 million tonnes of phytate-P is produced annually from worldwide production of seeds and fruits. This amount of P is equivalent to 65% of annual sales of P as fertilisers (Lott et al., 2000). The activity of endogenous phytase is comparatively higher in cereals and cereal by-products than in legume seeds (Eeckhout and Paepe, 1994). Moreover, large variations in phytase activity among feedstuffs have been reported which depend on the genetic and environmental factors (Steiner et al., 2007).
The native phytase activity, total P, phytate-P content and P bioavailability of some common feedstuffs are given in Table 1. Under physiological conditions in the gut, phytate chelates with positively charged cations such as Ca2+, Mg2+, Zn2+and Fe2+, and thus reduces the bio-availability of these minerals for animal (Erdman, 1979).
| Ingredients | Phytase activity (FTU/kg) | Total P (g/kg) | Phytate P (g/kg) | Proportion of phytate-P in total P (%) |
|---|---|---|---|---|
| Maize | 25 | 2.40 | 2.05 | 85.4 |
| Maize gluten | 45 | 5.00 | 4.20 | 84 |
| Corn | 24 | 2.50 | 1.70 | 73 |
| Gross defatted corn germ and bran | 41 | 6.60 | 4.2 | 64 |
| Fine defatted corn germ and bran | 56 | 12.10 | 7.80 | 65 |
| Hominy meal | 100 | 7.35 | 6.65 | 90.5 |
| Rice bran | 129 (70–190) | 17.51 | 15.83 | 90.2 |
| Rice | 112 | 1.2 | 0.8 | 65 |
| Rice broken | 20 | 0.85 | 0.40 | 47.1 |
| Rice polishing | 134 | 15.7 | 11.3 | 72 |
| Wheat bran | 2173 (1700–3090) | 10.96 | 8.36 | 76.3 |
| Wheat by-products | 2173 (1700–3090) | 8.02 | 7.00 | 87.3 |
| Wheat | 503 (255–840) | 3.08 | 2.20 | 74.9 |
| Sorghum | 35 (10–125) | 2.92 | 2.41 | 82.7 |
| Barley | 348 (130–595) | 2.73 | 1.86 | 67.3 |
| Oats | 38 | 2.43 | 2.10 | 86.4 |
| Oats, dehulled | 50 | 2.25 | 1.15 | 51.1 |
| Groundnut meal | — | 6.00 | 4.6 | 77 |
| Palm oil meal | 34 | 5.10 | 2.90 | 57 |
| Soybeans, whole | 40 | 5.55 | 3.08 | 55.5 |
| Soybean meal | 42 (10–95) | 6.66 | 4.53 | 68.3 |
| Coconut meal | 37 | 4.30 | 2.40 | 56 |
| Cotton seed whole | <10 | 6.05 | 4.25 | 70.2 |
| Cotton seed meal | 11 (5–50) | 11.36 | 9.11 | 80.5 |
| Sunflower meal | <10 | 9.05 | 7.48 | 82.8 |
| Rapeseed meal | — | 11.8 | 7 | 59 |
| Canola meal | 5 (5–35) | 8.76 | 6.69 | 76.4 |
| Peas | 58 | 3.45 | 1.67 | 48.4 |
| Faba beans | — | 4.01 | 2.43 | 60.0 |
| Lupins | ||||
| L. albus, whole | <10 | 4.47 | 2.49 | 55.7 |
| L. angustifolius, whole | <10 | 3.10 | 1.60 | 51.6 |
| L. angustifolius, dehulled | <10 | 3.80 | 1.89 | 49.7 |
Role of phytate
The effect of phytate on growth depends primarily on its amount in the diet and on the presence or absence of a distinct stomach (Hossain and Jauncey, 1993; Usmani and Jafri, 2002). Inclusion of 0.5% or 1% phytate in purified diets for the agastric common carp (Cyprinus carpio) caused a significant reduction in growth and feed efficiency (Hossain and Jauncey, 1993). Specific growth rate of rohu (Labeo rohita) and mrigal (Cirrhinus mrigala) was significantly decreased when phytate was included >1% of total diet (Usmani and Jafri, 2002). Channel catfish fed a diet containing 2.2% phytate had significantly reduced weight gain, feed efficiency and Zn content in the vertebrae compared to fish fed a diet containing 1.1% phytate (Satoh et al., 1989). Feeding Atlantic salmon (Salmo salar) with high dietary phytate (18 g phytate/kg) in the form of concentrated soybean meal led to many folds drops in growth performance, and protein and minerals (P, Ca, Mg and Zn) bioavailability and utilization (Storebakken et al., 1998).
Decline in growth and mineral absorption have been demonstrated in striped bass (Morone saxatih) when fed with plant feedstuff containing high phytate (1.31%) (Papatryphon et al., 1999). In contrast, interestingly, Cheng and Guillaume (1984) reported a beneficial effect of 1% phytate (added as sodium phytate) on growth and exoskeleton development in shrimp Penaeus japonicus. Similarly, McClain and Gatlin (1988) reported improved growth and reduced Zn bioavailability in blue tilapia Oreochromis aureus fed 1.5% phytate. The authors did not explain these unexpected results. Many researchers (Hossain and Jauncey, 1993; Papatryphon et al., 1999) also reported reduction in plasma levels of Ca and Zn but not of Fe, Cu or Mg in common carp when feed supplemented with high phytate (≥1%). Decrease in Ca and Zn availability was attributed to higher interaction of phytate with these ions than with Mg, Fe or Cu. Phytate chelate with di- and trivalent mineral ions such as Ca2 + (Fredlund et al., 2006), Mg2 + (Denstadli et al., 2006), Zn2 + (Fredlund et al., 2006), Cu2 + and Fe3 + preventing their availability for fish growth (Duffus and Duffus, 1991). In presence of phytate and added calcium, absorption of other mineral was depressed due to the formation of insoluble complexes (Sandberg et al., 1993). For example, calcium-bound phytate shows affinity with Zn and forms co-precipitates. The mineral binding strength becomes progressively lower as the solubility increases when phosphate groups are removed from the inositol hexaphosphate. Phytate may reduce reabsorption of endogenous Zn as well as affect availability of dietary Zn in fish and pigs (Hardy, 1998). In a study using rainbow trout (Oncorhynchus mykiss) as the test fish, feeds were selected for their low phytate content (Ketola and Harland, 1993) to minimize stream pollution by phosphates. Supplementation of a high phytate diets (2.58%) with low Zn feed (Zn at 0.05 g/kg) depressed chinook salmon growth, feed and protein conversion ratio and thyroid function, increased mortality, promoted cataract formation and induced anomalies in pyloric cecal structure, partly due to diminished Zn bioavailability to fish (Richardson et al., 1985). High dietary phytate (≥1% of phytate) with low Zn in diet resulted in hypertrophication, cytoplasmic vacuolation of pyloric caecae, cataract and low zinc content in vertebrae of juvenile Chinook salmon (Francis et al., 2001). A significant depression of Zn levels in carcass of rainbow trout juvenile was observed when fed with phytate containing rapeseed protein concentrate (inositol phosphate 5 and 6 were 19 and 2.5 μmol/g respectively in the diet) (Teskeredzic et al., 1995).
Phytate depresses protein and amino acid digestibility and utilisation efficiency in fish and other higher animals. Interactions of phytate with proteins depend on pH (Cheryan, 1980) as the phytate molecule is polyanionic. In acidic environments such as Nile tilapia stomach (pH 1–2), half of the phosphate moiety is negatively charged. This favours binding to soluble proteins at amino-groups on lysine, imidazole groups on histidine and guanidyl groups on arginine. In alkaline environment such as Nile tilapia intestine, (pH 8.5–8.8), ternary complexes are favoured. Both complexes are resistant to proteolytic digestion (Riche and Garling, 2004).
The formation of complex has been described as a biphasic reaction (Rajendran and Prakash, 1993). The reaction is characterised by initial rapid step where phytate binds protein via strong electrostatic attractions and changes the conformation of protein. This is followed by slower protein–protein interactions forming aggregation of protein and when the protein–phytate complexes exceed a critical size, these culminate into precipitation. Protein–phytate complex formation between α-globulin and sodium phytate has been shown to be maximal at pH 2.3 and dependant upon phytate to protein ratios (Rajendran and Prakash, 1993). Moreover, the extent of protein–phytate complex formation is probably also governed by various factors including pH, the source and solubility of protein and dietary levels of calcium and magnesium (Kemme et al., 1999). For example protein–phytate complexes have been well documented in wheat (Hill and Tyler, 1954) but are less likely to occur in maize (O’Dell and de Boland, 1976). The tendency of protein to be bound by phytate usually differs owing to the accessibility of basic amino acid residues to phytate (Champagne, 1988). In vitro studies have shown that phytate–protein complexes are less likely to be attacked by proteolytic enzymes (Ravindran et al., 1995) and even digestive enzymes such as pepsin, amylopsin and amylase are inhibited by phytate. Rainbow trout when fed with purified diets containing 0.5% phytate, suffered reduction in protein digestibility and about 10% reduction in growth and feed conversion (Spinelli et al., 1983). Formation of sparingly digestible phytate–protein complexes was found to be the main reason for growth depression in rainbow trout.
Phytate is a strong chelator and forms complexes with lipid and derivatives along with other nutrients (Vohra and Satanarayana, 2003). The complex of Ca/Mg–phytate and lipids is referred as ‘lipophytins’ and is the major constraint for energy utilisation derived from lipid source (Leeson, 1993). Lipophytins may also lead to the formation of metallic soaps in gut lumen of poultry (Leeson, 1993). However there is a paucity of evidence supporting the existence of lipid–phytate complexes in fish. Usmani and Jafri (2002) observed lower fat content in carcass of Cirrhinus mrigala when fed with high dietary phytate as compared to dephytated diet.
When phytate reacts with minerals and other nutrients, the formed complexes are insoluble in the upper small intestine (where maximum mineral absorption normally occurs) and it is highly unlikely that they provide absorbable essential elements. Thus, chemical interactions of phytate in the upper gastrointestinal tract are of particular concern. The form in which many minerals occur in foods is largely unknown, as is also the form in which they occur in the gut. Therefore, predicting the specific interactions of phytate in the gastrointestinal tract and the nutritional implications of these interactions is difficult.
Processing conditions for removal of phytate (dephytinisation)
Removal or degradation of phytate would increase the bioavailability of many cations and the nutritional value of the meal. Several strategies to reduce phytate have, therefore, been considered.
Milling and cooking
Milling of cereals removed phytate (Bohn et al., 2008), but this treatment also removed a major part of the minerals and dietary fibres and therefore cannot be a solution to the problem. Rosenbaum and Baker (1969) have reported that the cooking processes decreased both water and acid-extractable phytate-P in legumes. Reddy and Salunkhe (1981) did not find any breakdown of phytate-P during cooking of black gram seeds and cotyledons. They also observed losses of total P and phytate-P due to leaching into the cooking water. Heat treatments had minor effects on dephytinisation (Pontoppidan et al., 2007). A small effect usually observed using heat treatment is due to leaching of minerals into the boiling water.
Germination
Urbano et al. (2000) reported that during seed germination, phytate is utilized as a source of inorganic phosphate for plant growth and development. The liberation of phosphate from phytate occurs by enzyme hydrolysis. Germination also reduces phytate level in seeds or grains. Disappearance of phytate during germination depends on the native phytase activity and phytase induced during germination. Walker (1974) observed a rapid rise in phytase activity in bush beans after 2 days of germination. Germination reduced phytate content by 60% and 40% in chickpea and soybean respectively (Chitra et al., 1996; Masud et al., 2007).
Fermentation
Fermentation process reduces phytate content in the fermented product (Lim and Tate, 1973). Part of phytate reduction in this process is due to the action of endogenous phytase and phytase induced through sprouting (Kumar et al., 1978) but exogenous microbial sources may provide additional phytase activity during the fermentation. Cereals and legumes fermentation appreciably reduces phytate content owing to endogenous phytase of cereals and that of added yeast and other useful microorganisms (Hirabayashi et al., 1998). Phytate was reduced by about one-third in soybeans as a result of fermentation with mould, R. oligosporus. The decrease in phytate was accompanied by an increase in inorganic P (Sudarmadji and Markakís, 1977). The reduction in phytate was due to the action of the enzyme, phytase produced by mould during fermentation.
Genetic alteration (transgenesis and mutation)
Non-lethal mutants of corn (Raboy and Gerbasi, 1996), barley and rice (Raboy, 1997) have been developed that contain only a fraction of the phytate that normal seeds contain, but contain normal levels of total P. As a result, the level of available P to fish is greatly increased (Sugiura et al., 1998a). The ‘low-phytate’ varieties of barley and corn have been used as a component in low polluting feed for fish (Jabeen et al., 2004).
Beside these, pronounced expression of phytase activity in transgenic alfalfa has been illustrated successfully by incorporating exogenous gene encoding for phytase into the genome of alfalfa plants. Thereby enabling its use in livestock, poultry, and fish feeds to eliminate the need for P supplementation in feed (Austin-Phillips et al., 1999). Alfalfa meal is used for herbivorous and omnivorous fish feed such as grass carp and Nile tilapia (Ali et al., 2003).
Moistening, autolysis and other methods
Soaking of plant ingredients in aqueous solutions can remove up to two-thirds of the phytate by the action of endogenous phytase activity; however, it also results in loss of minerals, water-extractable proteins and vitamins (Hurrell et al., 2004). It has been shown that phytate hydrolysis during soaking is greatly influenced by temperature and pH (Greiner and Konietzny, 1998, 1999; Fredlund et al., 1997). The optimal temperature range for the intrinsic plant phytases during soaking was found out to be 45 °C and 65 °C and the optimum pH between 5.0 and 6.0 (Greiner et al., 1998; Greiner and Konietzny, 1999). Chang et al. (1977) reported that at 50 °C and 60 °C, the hydrolysis of phytate from the beans was 31.0% and 49% respectively. Approximately three parts of the total phytate were hydrolysed and one part diffused into the water during 10 h of incubation of beans at 60 °C. The autolysis of phytate in beans was slow at both 35 °C and 55 °C. Tabekhia and Lub (1980) demonstrated decrease in phytate of 7.7%, 8.1%, 13.2% and 19.1% for black-eyed beans, red kidney beans, mung beans, and pink beans respectively on soaking for 12 h at 24 °C in tap water. Iyer et al. (1980) observed that phytate content of beans (pinto, Great Northern, and red kidney beans) was appreciably reduced (52.7%, 69.6% and 51.7% respectively) when soaked in distilled water for 18 h at room temperature.
The most successful dephytinisations so far involve endogenous enzymatic activity during germination, but this is a species dependent phenomenon where some plants are more sensitive to the treatment than others. Wheat, barley and rye all have high phytase activity in the grain (Table 1), whereas maize, millet and sorghum have low initial phytase activity that increases rapidly after germination (Egli et al., 2002). Reducing the level of phytate in plant products through genetic alteration may be more effective than reducing phytate by post-harvest treatments.
Above-mentioned processing methods could reduce phytate to a certain extent but may also cause loss of minerals and other nutrients. Taking these constraints of traditional methods into account, addition of exogenous phytase to fish feed appears to be the best way to utilize phytate.
Phytases
Definition
Phytase, chemically known as myoinositol (1,2,3,4,5,6)- hexaphosphate phosphohydrolase, catalyses the hydrolysis of phytate rendering P available for absorption. The enzyme sequesters orthophosphate groups from the inositol ring of phytate to produce free inorganic P along with a chain of lower phosphoric esters (inositol pentaphosphate to inositol monophosphate) as intermediates (Fig. 2) (Baruah et al., 2004; Debnath et al., 2005a). This consequently decreases the chelation capacity of phytate for different cations. Activity of phytase is expressed as FYT, FTU, PU or U, and all these mean the same. One unit of phytase is defined as the quantity of enzyme that liberates 1 mmol of inorganic-P per minute from 0.0015 mol/l sodium phytate at pH 5.5 and 37 °C (Simons et al., 1990). This definition provides a useful measure of phytase activity and represents a simple benchmark measurement under well-defined assay conditions such as pH, temperature, duration, mineral content, agitation, etc.
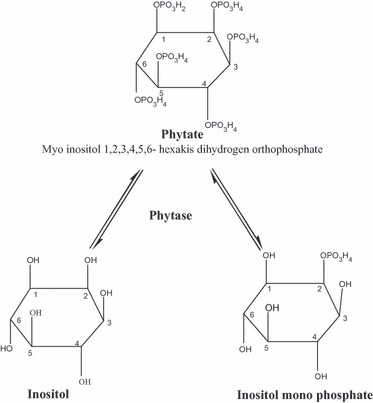
Action of phytase.
History
Phytase activity was first detected many decades ago in rice bran (Suzuki et al., 1907). Warden and Schaible (1962) were the first to show that exogenous phytase enhances phytate-P utilisation and bone mineralization in broiler chicks. However, before 1990s, the application of phytase has mainly been confined to poultry and swine to improve utilization of plant P. The first commercial phytase, Natuphos® was produced from Aspergillus niger and was released in market in 1991 (Selle and Ravindran, 2007). Following the prologue of commercial phytase, more emphasis were given to evaluating the effects of supplemental phytase on nutrient utilization and growth of common aquaculture species such as rainbow trout (Forster et al., 1999), common carp (Cyprinus carpio L.) (Schäfer et al., 1995), channel catfish (Ictalurus punctatus) (Li and Robinson, 1997), African catfish (Clarias gariepinus) (VanWeerd et al., 1999), Atlantic salmon (Salmo salar) (Storebakken et al., 2000), stripped bass (Morone saxatilis) (Papatryphon et al., 1999), and Nile tilapia (Oreochromis niloticus) (Liebert and Portz, 2005).
Classification
Depending on site of hydrolysis
International Union of Pure and Applied Chemistry and the International Union of Biochemistry (IUPAC-IUB) suggested that phytase feed enzymes fall into two categories depending on the site where the hydrolysis of the phytate molecule is initiated. These are 3-phytase (EC 3.1.3.8) and 6-phytase (EC 3.1.3.26). They are also known as myo-inositol hexakisphosphate 3-phosphohydrolase and myo-inositol hexakisphosphate 6-phosphohydrolase respectively. Former liberates the P moiety at position C3, whereas latter initiates the release of P at position C6 of the myo-inositol hexaphosphate ring (Cao et al., 2007). Phytate-degrading enzymes from microorganisms are considered to be 3-phytases, whereas seeds of higher plants are said to contain 6-phytases (Nayini and Markakis, 1986). Recently it was discovered that phytase isolated from A. niger shows 3-phytase activity whilst Peniophora lycii and E. coli contain 6-phytase (Greiner and Konietzny, 2006; Selle et al., 2007).
While according to Bohn et al. (2008), phytase has been categorized into 3 groups: 3-phytase (EC 3.1.3.8), 5-phytase (EC 3.1.3.72) and 4/6 phytase (EC 3.1.3.26). Most of the 3-phytases are structurally homologous to β-propeller phosphatase, or histidine acid phosphatases, and are generally found in fungi and bacteria. Till date only a single 5-phytase (in lily pollen) has been detected and it has the conformation of histidine acid phosphatases (Mehta et al., 2006). 4/6-Phytases act on the carbon atom next to C5 of the inositol ring, most active in weak acidic environments (pH 4∼6) with a temperature optimum in the range of 40–60 °C (Bohn et al., 2008).
Depending on optimal pH
The phytate-degrading enzymes can also be divided into two types based upon their optimal pH. These are acid phytase and alkaline phytase. Former shows the optimum activity at pH about 5.0 while the latter at pH near to 8.0 (Baruah et al., 2007b). Most of the microbial phytate-degrading enzymes belong to acid type with exception from Bacillus group, which are alkaline phytase (Selle et al., 2007). However, it must be taken into account that microbial phytases of different sources can differ in their pH dependent bioefficacy (Onyango et al., 2005). Conversely most of the plant phytase tend to have a pH optimum at 5 (Wodzinski and Ullah, 1996). A list of characterised plants and animal phytases is presented shown in Table 2.
| Phytase source | pH optimum | Temperature optimum (°C) | Specific activity (U/mg protein) at 37 °C | Molecular weight (kDa) |
|---|---|---|---|---|
| Buttercup squash | 4.8 | 48 | 67 | |
| Canola seed | 4.5–5 | 50 | 70 | |
| Faba beans | 5 | 50 | 636 | 65 |
| Hazel seed | 5 | 72 | ||
| Legume seeds | 8 | |||
| Lily pollen | 8 | 55 | 0.2 | 88 |
| Lupin seeds | 5 | 50 | 539, 607, 498 | 57–64 |
| Mung beans | 7.5 | 57 | 2.4 | 160 |
| Navy beans | 5.3 | 50 | ||
| Peanut | 5 | 55 | 22 | |
| Rapeseed | 5.2 | 50 | ||
| Scallion leaves | 5.5 | 51 | 500 | |
| Soybean seeds | 4.5–4.8, 4.5–5 | 55,58 | 2.4 | 119, 72–130 |
| Sunflower | 5.2 | 55 | ||
| Tomato roots | 4.3 | 45 | 205 | 164 |
| Typha latifolia pollen | 8 | |||
| Barley | 5;6 | 45, 55 | 117, 43 | 67 |
| Maize seedling | 5 | 55 | 2.3 | 71, 76 |
| Maize root | 5–5.1 | 35–40 | 5.7 | |
| Oat | 5 | 38 | 307 | 67 |
| Rice | 4,4,4.6 | 40 | 66,61 | |
| Rye | 6 | 45 | 517 | 67 |
| Spelt | 6 | 45 | 262 | 68 |
| Whole meal wheat | 5.15 | 55 | ||
| Wheat bran | 5 | |||
| Wheat bran | 5.6,7.2 | 47 | ||
| Wheat bran | 6, 5.5 | 45,50 | 127, 242 | 68,66 |
| Crude extract wheat | 6 | 45 | 65 | |
| Rat intestine | 7.0, 7.5–8.0 |
Sources of phytase
Phytase are widely distributed among various life forms wherein microorganisms are the most potential ones followed by plants. Filamentous fungi such as Aspergillus ficuum, Mucor piriformis and Cladosporium species (Stefan et al., 2005) are major sources of microbial phytases. These phytases are stable even at pH values above 8.0 and below 3.0 (Greiner and Konietzny, 2006). Plant phytases are heat labile and their activity is reduced or even eliminated in steam-pelleted diets (Jongbloed and Kemme, 1990); whereas, most of the corresponding microbial enzymes retain significant activity even after prolonged thermal exposure (Pointillart, 1988). Moreover, trials on pigs revealed that bioefficacy of phytases from wheat and rye was only 40% compared to that from Aspergillus niger (Zimmermann et al., 2002). Higher effectiveness of microbial phytase is attributed to acidic pH of the stomach that is more conducive to microbial than plant phytase activity. Moreover, wheat and rye phytase is inactivated at pH 2.5 and is more susceptible to pepsin degradation than A. niger phytase (Phillippy, 1999). Phytase activity of animals is insignificant in contrast to plant and microbial counterparts (Weremko et al., 1997). In general there are four possible sources of phytate degrading enzyme. Phytase generated by the small intestinal mucosa, gut associated microfloral phytase particularly in large intestine, plant phytase and microbial phytase.
Mucosal phytase derived from small intestine
Occurrance of phytase activity in small intestine has been documented in fish. Tilapia Oreochromis niloticus×O. aureus (LaVorgna, 1998) are capable of releasing inorganic P from phytate and this phytase activity appears to be localized in the small intestinal brush border membrane (BBM) (Maenz and Classen, 1998). Therefore Nile tilapia can digest approximately 50% of dietary phytate P. Research conducted by Ellestad et al. (2003) has confirmed the presence of intestinal BBM phytase activity in Nile tilapia and also observed some phytase activity in hybrid striped bass Morone saxatilis x M. chrysops and M. chrysops×M. saxatilis and common carp Cyprinus carpio also. Although intestinal phytase activity has been detected in several fish species, its application for phytate hydrolysis is not satisfactory for fish nutrition (Ellestad et al., 2003). Recently, Shi et al. (2008) isolated novel phytase gene, appA by degenerate polymerase chain reaction (PCR) and genomic library screening from Buttiauxella sp. GC21, a bacterium isolated from grass carp intestine. The phytase gene could be successfully expressed in Escherichia coli with high expression level. The successful purification and characterization of the enzyme may provide a basis for further large-scale production of phytase.
Gut microfloral phytase
Roy et al. (2008) isolated and enumerated the phytase-producing bacterial flora in the foregut and hindgut regions of the gastrointestinal tracts of ten culturable freshwater teleosts of different feeding habits, namely rohu (Labeo rohita), catla (Catla catla), mrigal (Cirrhinus mrigala), bata (Labeo bata), kalbasu (Labeo calbasu), Nile tilapia (Oreochromis niloticus), climbing perch (Anabas testudineus), common carp (Cyprinus carpio), silver carp (Hypophthalmichthys molitrix) and grass carp (Ctenopharyngodon idella). The bacterial isolates were screened on the basis of their enzyme-producing ability. In case of the hindgut, maximum phytase-producing strains were present in grass carp and mrigal and minimum in rohu. Ellestad et al. (2002a) reported a very low intestinal phytase activity in hybrid striped bass. The same authors, in another study (Ellestad et al., 2002b), compared the phytase activity in the intestinal brush border of three teleost fish, carnivorous hybrid striped bass, omnivorous Nile tilapia and common carp. This study suggests that Nile tilapia could digest more phytate while hybrid striped bass and common carp are not able to digest more than 1% or 2% of phytate P present in the diet. Forster et al. (1999) reported that Rainbow trout is able to hydrolyse less than 5% of dietary phytate P.
To date, there are only few studies, which claim the inclusion of gut microfloral derived phytase in fish feeds.
Intrinsic plant phytase
Native phytase activities are higher in cereals and cereal by-products, whereas lower activities have been reported for legume seeds (Eeckhout and Paepe, 1994) (Table 1). Such sources of phytases are of little relevance in fish diet, as the thermal process during feed manufacture would completely destroy the indigenous phytase enzymatic activity.
Exogenous microbial phytase
A wide spectrum of microbial phytase products for fish is now commercially available. The most commonly used microbial phytases are derived from fungi (A. niger) and bacteria (E. coli). The catalytic properties of different microbial phytase sources have been reviewed by Greiner and Konietzny (2006), and are as shown in Table 3.
| Phytase source | Phytase activity (U/mg) (37°C) | pH optimum | Temperature optimum (°C) |
|---|---|---|---|
| Aspergillus caespitosus | — | 5.5 | 80 |
| Aspergillus fumigatus | 23–28 | 5.0–6.0 | 60 |
| Aspergillus niger | 50–103 | 5.0–5.5 | 55–58 |
| Aspergillus oryzae | 11 | 5.5 | 50 |
| Aspergillus terreus | 142–196 | 5.0–5.5 | 70 |
| Penicillium simplicissimum | 3 | 4 | 55 |
| E. nidulans | 29–33 | 6.5 | |
| M. thermophila | 42 | 5.5 | |
| S. castellii | 418 | 4.4 | 77 |
| Cladosporium | 909 | 3.5 | 40 |
| K. pneumoniae | 224, 297 | 5.5, 5.5 | 50, 60 |
| K. aerogenes | 4.5, 5.2 | 68 | |
| Peniophora lycii | 1080 | 5.5 | 58 |
| Thermomyces lanuginosus | 110 | 6 | 65 |
| Bacillus amyloliquefaciens | 20 | 7.0–8.0 | 70 |
| Bacillus subtilis | 9.0–15 | 6.5–7.5 | 55–60 |
| Citrobacter braakii | 3457 | 4 | 50 |
| Escherichia coli | 811–1800 | 4.5 | 55–60 |
| Klebsiella terrigena | 205 | 5 | 58 |
| Lactobacillus sanfranciscensis | — | 4 | 50 |
| Pantoea agglomerans | 23 | 4.5 | 60 |
| Pseudomonas syringae | 769 | 5.5 | 40 |
| Candida krusei | 1210 | 4.6 | 40 |
| Pichia anomala | — | 4 | 60 |
Pathway for phytase mediated phytate degradation
In theory, enzymatic hydrolysis of phytate generates a series of lower myo-inositol phosphates esters, via a progression of step-wise dephosphorylation reactions and ultimately leads to the production of free myo-inositol along with six inorganic P (Baruah et al., 2004; Selle et al., 2007). In this case, phytase generates 282 g inorganic P from each kg of dietary phytate (Selle et al., 2007). A general illustration has been presented in Fig. 2. However, phytases of different origins have different dephosphorylation pathways (Kaur et al., 2007). Among yeasts, the pathway of dephosphorylation of phytate by purified phytase of C. krusei WZ-001 was described by Quan et al. (2003). The complete catalytic reaction sequence is shown in Fig. 3. As such, the route of enzymatic dephosphorylation by phytases of P. rhodanensis was investigated and elucidated up to IP3 level (Adelt et al., 2003), whereas (Greiner et al., 2001) described the complete hydrolysis sequence of phytate by S. cerevisiae.
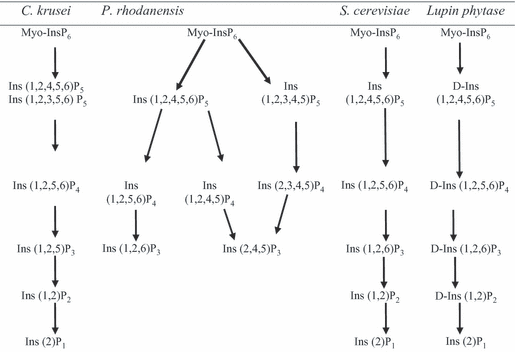
Pathways of phytate degradation by different types of yeast and lupin phytases (Source: Kaur et al., 2007)
Phytase production systems
Phytase is mainly manufactured by two methods, solid state fermentation (SSF) and submerged fermentation (SmF). SSF is a modern process where microorganisms are grown on solid materials without the presence of free liquid. It has the potential to produce large quantities of enzymes. While SmF is the traditional method used for the production of microbially derived enzymes. Enzymes derived from this method do not possess the properties of enzymes derived from SSF. Key advantages of SSF produced phytase over SmF are: high concentration of enzyme activity; higher heat stability and many side acting enzymes. Given the complex nature of feed ingredients, phytase with side acting enzymes is always beneficial, e.g. to deal with non-starch polysaccharides. Nutrizyme P forte (2500 FTU/kg) and Nutrizyme 2p forte (5000 FTU/kg), commercially available products are synthesized using SSF technology. These release P from phytate effectively and also provide benefits of side acting enzymes such as xylanase, cellulase and pectinase. Commercially produced microbial phytases have been shown in Table 4. Besides, the culture conditions, type of strain, nature of substrate and availability of nutrients should be taken into consideration for selecting a particular production technique, as they are the critical factors affecting the yield (Vats and Banerjee, 2004).
| Company | Country | Phytase source | Production strain | Trademark |
|---|---|---|---|---|
| AB Enzymes | Germany | Aspergillus awamori | Trichoderma reesei | Finase |
| Alko Biotechnology | Finland | A. oryzae | A. oryzae | SP, TP, SF |
| Alltech | USA | A. niger | A. niger | Allzyme phytase |
| BASF | Germany | A. niger | A. niger | Natuphos |
| BioZyme | USA | A. oryzae | A. oryzae | AMAFERM |
| DSM | USA | P. lycii | A. oryzae | Bio-Feed Phytase |
| Fermic | Mexico | A. oryzae | A. oryzae | Phyzyme |
| Finnfeeds International | Finland | A. awamori | T. reesei | Avizyme |
| Genencor International | USA | Penicillium simplicissimum | Penicillium funiculosum | ROVABIO |
| Roal | Finland | A. awamori | T. reesei | Finase |
| Novozymes | Denmark | A. oryzae | A. oryzae | Ronozyme®Roxazyme® |
Factors influencing the bioefficacy of phytase
Substrate and enzyme
Efficacy of microbial phytase is governed directly or indirectly by numerous interactive factors. These may include dietary substrate levels, fish species, the inclusion rate and source of phytase. Cao et al. (2007) reviewed that phytase dose at a level of 250–2000 FTU/kg feed is usually considered optimum for many fish species. Furthermore, different phytase sources might lead to different effects on growth parameters and nutrient deposition, as is evident from the work of Liebert and Portz (2005). They compared nutrient and P utilization using two different sources of microbial phytase in Nile tilapia diet. Two different sources of microbial phytase, SP1002 and Ronozyme®P were compared based on growth response, body composition, nutrient deposition and nutrient utilization. Superior performance data (growth response, body composition, nutrient deposition and nutrient utilization) were obtained using phytase SP1002. It was also found that supplementation of phytase SP1002 at a level of 750 FTU/kg diet was sufficient to improve growth, feed conversion, protein deposition, while supplementation of at least 1000 FTU/kg of Ronozyme®P resulted in intermediate growth. Parallel comparative and dose response studies were also done by Li and Robinson (1997), Robinson et al. (2002) in channel catfish, Furuya et al. (2001) in Nile tilapia, Yoo et al. (2005) in Korean rockfish and Debnath et al. (2005a) in pangus catfish. It was concluded that optimum dose of phytase for these fish species is 500–1000 FTU/kg feed. Cheng et al. (2004) reported that the optimum level of phytase supplementation in rainbow trout diets was approximately 500 FTU/kg diet. Vielma et al. (2004) reported two trials: Trial 1 (a semi-purified diet containing 50% soybean meal with phytase levels of 0, 500, 1000, 2000 and 4000 FTU/kg), and Trial 2 (commercial-type extruded feeds containing 36% soybean meal with phytase either 0 or 2000 FTU/kg feed). In both trials phytase decreased phytate content of faeces from 35 to 5 mg and from 34 to 14 mg phytate per g faecal dry matter; and apparent digestibility coefficient of P improved from 23% to 83% and from 35% to 54% in Trials 1 and 2 respectively, whereas, Zn digestibility significantly increased in Trial 1, but not in Trial 2.
pH value
As described earlier that the optimum microbial phytase activity occurs at two pH ranges, pH 5.0–5.5 and pH 2.5; activity at pH 5.0–5.5 being higher than at pH 2.5 (Simons et al., 1990; Kemme et al., 1999). The efficacy of phytase differs in agastric and gastric fish. The pH in digestive tract of agastric fish like common carp is around 6.5–8.4 while gastric fish has much lower pH (Ji, 1999). The large deviation in values of pH between optimal phytase activity and digestive tract of agastric fish, is responsible for the poor efficacy of phytase in stomachless fish. Gastrointestinal acidity in gastric fish such as rainbow trout, catfish and other carnivorous fish is favourable for efficient hydrolysis of phytate by the microbial phytase in these species. Enhancement of phytase utilisation by agastric fish is carried out by addition of acidifier such as citric acid, fumaric acid, lactic acid and formic acid in diet (Baruah et al., 2005). These are known to lower the dietary acidity and in turn reduce the pH of digestive tract and ultimately amplify the activity of exogenous phytase. The same author reported a significant synergestic interaction between citric acid and microbial phytase on growth performance, nutrient digestibility, bone mineralization and protein efficiency ratio in L. rohita juveniles (Baruah et al., 2007a,b).
Temperature
Phytase enzymes are thermolabile and have optimal temperature range of 45–60°C (Lei and Stahl, 2000). For this reason dietary phytase may get denatured during extrusion process. The high temperature phase of extrusion partially leads to the destruction of intrinsic phytase in feed ingredients and thereby significantly reduces the availability of P, Zn, Cu and other nutrients in plant based feed (Cheng and Hardy, 2003). Pretreatment of feedstuff with phytase prior to extrusion could be a good choice to avoid the heating denaturation of phytase enzyme (Cain and Garling, 1995; Nwanna, 2007). Beside this, spraying the processed feed with liquid suspension of phytase could be another option to solve the temperature dependent problem (Vielma et al., 2004). In general it is carried out by mixing the enzyme concentrate with a stabilizer (such as MgSO4) and then spraying the solution on processed feed. In this endeavour, the inclusion of heat-resistant enzymes would circumvent the aforementioned problem. Thermostability of phytase is considered to be a desirable feature in the animal feed as feed pelleting involves a brief exposure to 80–85 °C for a few seconds and eventually the activity of enzyme need to be retained. Phytases of Schwanniomyces castellii, Arxula adeninivorans and various Pichia spp. were found to be active in the range of 75–80 °C (Vohra and Satyanarayana, 2002).
Feed additives
It is speculated that dietary addition of organic acids and microbial phytase may have a synergistic effect on mineral availability in stomachless fish. It is supported from the evidence that acidity of the (gastro) intestinal tract influences the activity of phytase, optimum being at lower pH value. Therefore reduction of intestinal pH through dietary addition of organic acids increased the solubility of phytate–P and thus improved P absorption (Vielma and Lall, 1997). In addition organic acids can also bind various cations along the intestine and act as chelating agent (Ravindran and Kornegay, 1993), accounted for increased intestinal absorption of minerals (Sugiura et al., 1998b). Besides, dietary acidification these also stimulate the epithelial cell proliferation in the gastrointestinal mucosa (Sakata et al., 1995), thereby increasing the absorption of minerals. Another advantage of dietary acidification is the inhibition of intestinal bacteria competing with the host for available nutrients and reduction of toxic bacterial metabolites such as ammonia and amine. This is shown to improve the gut health of the animals (Ravindran and Kornegay, 1993). However, Baruah et al. (2007b) reported that addition of 3% citric acid activated microbial phytase in feed of rohu. Apparent absorption of Zn, its content in whole body and plasma, was significantly (p < 0.05) higher in citric acid and microbial phytase fed groups. Interaction between organic acid and phytase was found for increased absorption of Na, P, K, Mn, Mg, Fe and N in whole body and plasma (Baruah et al., 2007b). In a previous study Baruah et al. (2005) concluded that prominent interaction of microbial phytase and citric acid enhanced bone mineralization of rohu juveniles. Moreover, Sugiura et al. (2001) also found a significant increase in the apparent absorption of Mg and P by the addition of 5% citric acid in the diet of rainbow trout.
The inclusion of Vitamin D3 (cholecalciferol) and hydroxylated D3 compounds may also complement the efficacy of phytase. Vitamin D analogues may indirectly improve utilization of phytate P digestion by increasing absorption of the hydrolysed P but the interaction of vitamin D and phytase has not been clearly elucidated (Lei et al., 1994). However, it has been found that elevated dietary vitamin D3 levels can alleviate some of the unfavourable effects of dietary calcium while phytase activity of the intestine and phosphatase activity of mucous membranes are not affected. Calcium absorption can be promoted by vitamin D3, thus the negative effect of Ca on phytate utilization could be eliminated indirectly by reducing the formation of phytate-calcium complex (Qian et al., 1997). Vielma et al. (1998) reported increment in weight gain of rainbow trout when cholecalciferol at low dietary level was supplemented with phytase at 1500 FTU/kg soy protein concentrate based diet. Subsequent studies in broiler revealed that the inclusion of 1,25-dihyroxycholecalciferol in diets enhanced phytate-P utilisation (Driver et al., 2005). It has been shown that other feed additives like Zn may have deleterious effects on phytase efficacy. For example, Zn levels reduce the P-releasing efficacy of phytase in young pigs and chickens (Augspurger et al., 2004). It is likely that the formation of Zn–phytate mineral complexes, which are not readily hydrolysed by phytase, is responsible for this suppression of efficacy. However different microbial phytases require different metal ions for their activity. Most phytate-degrading enzymes characterized so far are greatly inhibited by Cu and Zn2+. Kim et al. (1998a,b) reported that EDTA, Cd2+, and Mn2+strongly inhibit phytase of Bacillus sp. DS11 whereas Hg2+, Mg2+, Ba2+, and Cu2+at 5 mm inhibit it moderately. In the reaction mixtures containing 5 mm Fe2+, Fe3+, Cu2+, Zn2+and Hg2+, phytase activity of Selenomonas ruminantium was strongly inhibited (Yanke et al., 1999). The sensitivity pattern was similar to that of E. coli and K. terrigena phytase (Greiner et al., 1997). The well known fact that plant protein sources are generally deficient in lysine and methionine (Watanabe, 2002) provide a room for the inclusion of these amino acid along with phytase to improve overall nutritive value of plant based diet. In this context Biswas et al. (2007a) studied the interactive study of phytase and lysine on Penaeus monodon juveniles and concluded that supplementation of both lysine and phytase in soybean-based diet not only enhances the nitrogen and P utilisation but also significantly changes the fatty acid profile of the tissue. Total n-3 fatty acid was reduced significantly (p < 0.05) whereas total n-6 fatty acids content increased due to the dietary inclusion of lysine or phytase or both.
Calcium to phosphorus ratio
Calcium and P are necessary to maintain an optimal bone development (Cruz and Tsang, 1992), more of both minerals being required during growing stages. The percentage of Ca in the whole, fresh (wet) body of finfish ranges from 0.5% to 1% with a ratio of Ca to P of 0.7 to 1.6 (NRC, 1993). In diet, it is necessary to take into account the Ca/P ratio, because it has important consequences for bone development. However, when this ratio increases harmful effects can appear. It is known that several aberrations in bone mineral homeostasis and bone metabolism associated with higher Ca:P ratio. However, dietary Ca levels and Ca:P ratios are also crucial to phytase efficacy as reviewed by Lei and Stahl (2000). Qian et al. (1996a) reported that reducing Ca:P ratios from 2.0 to 1.2:1 in phytase supplemented diets (700 and 1050 FTU/kg) of pig, increased phytase efficacy by the order of 16%. Liu et al. (2000) observed that reducing Ca:P ratios from 1.5 to 1.0:1 in phytase (500 FTU/kg) supplemented low P diets increased P absorption in the small intestine of pigs. Similarly Qian et al. (1997) found that increasing Ca (5.61–10.20 g/kg) and Ca:P ratios (1.1–2.0:1) depressed weight gain of broilers. Additionally, increasing Ca:P ratio depressed E. coli derived phytase action in the pig diet and thus significantly depressed weight gain and feed efficiency (Adeola et al., 2006) They reported an increase in weight gain by 17.6% by reducing Ca: P ratios from 1.8 to 1.2:1. The probable reason for maintaining low Ca and Ca:P ratio in feed is due to high dietary Ca or a high ratio of Ca:P that interferes with P absorption and reduces the effectiveness of phytase activity. However only cursory attention has been paid towards optimisation of Ca: P ratio in fish feed. However, it can be hypothesized that high concentration of Ca in fish feed will chelate with phytate forming insoluble complex or compete with phytase for binding site at the myoinositol ring and thus block the site of phytase mediated substrate hydrolysis (Qian et al., 1996b) or increase the pH value to inhibit the activity of phytase (Cao et al., 2007). Moreover, Cao et al. (2007) recommended Ca:P ratios in fish meal in the range of 1.1–1.4:1 at which phytase execute high efficiency.
Mode of application of phytase
Microbial phytase are supplemented in fish feed as powder, granulate or liquids forms. The preferable method of phytase application in fish diets is by coating a liquid form of the enzyme after extrusion and drying which prevents activity loss during pellet formation (Verlhac et al., 2007). On-line spraying device is simple to install on the existing production lines and it is not an expensive device. However, the use of enzyme in liquid form in post-extrusion allows much flexibility in formulation. The possibility of pre-treatment of raw materials has been investigated in many studies. It was found that rainbow trout fed a soybean meal based diet pre-treated with phytase, resulted in increase in P availability and absorption (Cain and Garling, 1995; Sugiura et al., 2001). Many researchers (Teskeredzic et al., 1995; Storebakken et al., 1998; VanWeerd et al., 1999; Cheng and Hardy, 2003; Riche and Garling, 2004; Yoo et al., 2005) have reported positive effect of phytase pre-treatment on P availability. Masumoto et al. (2001) found no significant difference in P availability between fish fed the soybean meal based diet supplemented with phytase and the diet containing phytase pre-treated soybean meal in Japanese flounder. Similarly, Yoo et al. (2005) reported no remarkable difference in P digestibility between soybean meals pre-treated with phytase (1000 FTU/kg) when included in diet and the phytase supplemented diet (1000 FTU/kg) in Korean rockfish. Denstadli et al. (2007) compared on-line phytase pre-treatment of plant feed ingredients and phytase coating in diets of Atlantic salmon. Pre-treatment of wheat and soy protein concentrate for a period of 1 h in the pre-conditioner (at 45 °C) led to significantly higher P digestibility in comparison with the phytase supplemented diet. However, before the introduction of heat-stable phytase product forms, phytases were generally added postpelleting to prevent inactivation due to high pelleting temperatures, but the advent of heat stable phytase from A. niger (Han and Lei, 1999; Xiong et al., 2005), A. fumigatus (Peng et al., 2002) and E. coli (Garrett et al., 2004) has made direct enzyme addition possible through the conditioning/pelleting process.
Dietary phosphorus requirement in fish
Phosphorus is a critical element for fish and other livestocks. It plays a major role in the structure and function of living cells. It is an integral component of adenosine triphosphate (ATP), nucleic acids, nucleotides, phospholipids, proteins, and a key component of many coenzymes. These compounds function in energy releasing cellular reaction, cellular division and growth, in the transport and metabolism of fats, and in the absorption and utilization of carbohydrates, fatty acids, and proteins. Thus P is an essential nutrient for growth, skeletal development and reproduction in fish (Åsgård and Shearer, 1997). However fish can absorb dissolved P and other minerals from the water across the gill membrane and in the case of marine fish, through the digestive tract. The concentration of P in fresh water and sea water is low and therefore fish require relatively high dietary source of P to meet their requirement for growth (NRC, 1993). Dietary P requirement of most aquaculture fish species varies from 0.4% to 0.9% of diet (NRC, 1993; Kaushik, 2005). Japanese eel and Korean rockfish have a low P requirement of around 0.3% of diet. Some species such as haddock has a much higher requirement, reaching nearly 1% of diet. Plant protein ingredients contain significantly less P than fishmeal and the partial replacement of fishmeal by plant feed ingredients could help reducing the P discharge. Phosphorus deficiencies induce skeletal deformities such as curved spines and soft bones in Atlantic salmon, cephalic deformities in common carp, scoliosis in haddock and halibut (Lall and Lewis-McCrea, 2007).
It is unlikely that differences in P availability contribute to the large differences in reported requirements. The effect of fish size on the dietary requirements for essential elements has not been specifically examined, although measurements made on Atlantic salmon (Shearer et al., 1994) and rainbow trout (Shearer, 1984) indicate that body concentrations of P change considerably with fish size. The mechanistic model presented by Shearer (1995) suggests that the dietary P requirement increases from first feeding to the juvenile stage, and then decreases in adults. The range of fish sizes used in P requirement studies has varied considerably and may have affected the reported requirements (Table 5).
| Fish species | Initial fish weight (g) | Feed efficiency | P requirement (g/kg diet) | P source | References |
|---|---|---|---|---|---|
| Nile Tilapia | 0.4 | 0.6 | 4.6 | NaP | Haylor et al. (1988) |
| Blue Tilapia | 0.8 | 0.52 | 5 | NaP | Robinson et al. (1987) |
| Nile Tilapia | 35 | 0.6 | 5.3 | CaP | Furuya et al. (2001) |
| Common carp | 5 | 0.8 | 6–7 | K2P + NaP | Ogino and Takeda (1976) |
| Sunshine bass | 10–20 | 0.6 | 5 | KP | Brown et al. (1993) |
| Guppy | Fry | 0.3 | 5.3–12.3 | KP | Shim and Ho (1989) |
| Red drum | 1.3 | 0.5 | 8.6 | NaP | Davis and Robinson (1987) |
| Channel catfish | 6 | 1.1 | 4.0 | NaP | Wilson et al. (1988) |
| Channel catfish | 6 | 0.99 | 8 | CaP | Andrews et al. (1973) |
| Channel catfish | 1.8 | — | 8 | NaP | Lovell (1978) |
| Rainbow trout | 50 | 1.1 | 2.4–5.9 | NaP | Rodehutscord (1996) |
| Rainbow trout | 35 | 1.0 | 3.4–5.4 | NaP | Ketola and Richmond (1994) |
| Rainbow trout | 1.2 | 1.1 | 7–8 | K2P + NaP | Ogino and Takeda (1978) |
| Atlantic salmon | 1.4 | 1.45 | 10–11 | CaP2 | Åsgård and Shearer (1997) |
| Chum Salmon | 1.5 | 0.95 | 5–6 | NaP | Watanabe et al. (1980) |
| Atlantic salmon | 6.5 | 0.5 | 6 | CaP | Ketola (1975) |
| Atlantic salmon | 57 | 0.9 | 6 | NaP + KP | Lall and Bishop (1977) |
- NaP: sodium phosphate monobasic, KP: potassium phosphate monobasic, K2P: potassium phosphate dibasic, CaP2: calcium phosphate monobasic, CaP: calcium phosphate dibasic.
- Feed efficiency: Body mass gain (g)/Feed fed.
Calculation of available phosphorus in diets
An accurate estimation of P content in feed ingredients is essential during diet formulation to meet nutritional requirements and to minimise P waste output from aquaculture operations Verlhac et al. (2007). Hua and Bureau (2006) have developed a model to estimate available P content of salmonid fish feed based on levels of different types of P such as bone P, phytate, organic P, Ca monobasic, Na/K inorganic P supplement and Ca dibasic supplement.
The proposed model is:
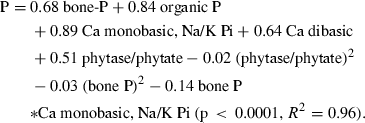 (1)
(1)Phosphorus availability from different feed ingredients
Phosphorus availability from different ingredients fed to fish is depicted in Table 6. Various studies have clearly shown that the availability of P from plant based meal is lower as compared to fishmeal. Phosphorus digestibility varies in relation to the form provided in the diet. Kaushik (2005) stated that the monobasic phosphate form has a comparable digestibility irrespective of fish species; the dibasic form renders lower bioavailability in common carp as compared to channel catfish and rainbow trout, the tribasic calcium form of phosphate is very poorly digested by common carp (13%) and higher by rainbow trout (64%). Fishmeal P mainly comes from bone that is available in the hydroxyapatite form. Riche and Brown (1996) reported that availability of P from fishmeal is greater for rainbow trout than for common carp. The reason for this could be that the common carp has a poor ability to hydrolyse calcium phosphate as it does not possess stomach and therefore cannot secrete gastric acid in their digestive system. Phosphorus availability in common carp has been shown to increase by addition of citric acid (Sugiura et al., 1998b). Other factors such as growth stages might influence P absorption. Satoh et al. (2002) have elucidated that small fish have a very poor capacity to absorb P from plant protein source and this capacity improves as fish grows.
| References | Plant protein sources in fish diet | P availability (%) without phytase | Phytase dose FTU/kg diet | P availability (%) with phytase |
|---|---|---|---|---|
| Rainbow trout (Oncorhynchus mykiss) | ||||
| Lanari et al. (1998) | (Soybean meal 55%, soy protein 25%); (Soybean meal 33%) | 25; 58.6 | 1000; 1000 | 57; 68.1 |
| Vielma et al. (1998a, 1998b) | Soy protein concentrate 50% | 44.5 | 1500 | 69.7 |
| Forster et al. (1999) | (Canola protein concentrate with high Ca level); (Canola protein concentrate); (Canola protein concentrate); (Canola protein concentrate) | 37.4; 33.0; 4.8; −0.8 | (500, 1500, 4500); (1500); (500, 1500, 4500); (1500); | (42.7, 40.1, 51.0); 45.9; (20.6, 26.8, 45.4); 30.3 |
| Sugiura et al. (2001) | (Soybean meal 30%); (Soybean meal 50%); (Soybean meal 30%); (Soybean meal 50%) | 56; 27; (−14); (−19) | 1000; (500, 1000, 2000, 4000); 1000; (500, 1000, 2000, 4000) | 62; (54, 68, 82, 90); 90; (33, 56, 79, 91) |
| Cheng et al. (2004) | (Soy protein concentrate 50%); (Soybean meal 50%);(Soybean meal 50%); (Soy protein concentrate 50%); (Soybean meal 50%) | 39.7; 39.9; 31.5; 6.3; 0.5 | (500, 1000, 2000, 4000); (500, 1000, 2000, 4000); (500, 1000, 2000, 4000); (500, 1000, 2000, 4000); (500, 1000, 2000, 4000) | (94.97, 96.16, 96.3, 96.6); (80.8, 88, 91.9, 95); (70.1, 78.7, 78.9, 85.2); (60.7, 75.4, 70.4, 57.2); (52.4, 59.8, 78.3, 67.4) |
| Cheng and Hardy (2004) | (Soybean meal 15%, distiller’s dried grain with solubles 15%);(Soybean meal 15%, dried grain with solubles 15%) | 80.1; 22.2 | (300, 600, 900, 1200); (300, 600, 900, 1200) | (87.0, 89.1, 86.3, 87.5); (17.8, 64.1, 53.8, 96.9) |
| Vielma et al. (2004) | (Soybean meal 50%); (Soybean meal 36%); (Soybean meal 55%) | 28.3; 35.1; 61.8 | (500, 1000, 2000, 4000); 2000; (500, 1000) | (64.9, 78.6, 81.5, 84.2); 54.2; (71.7, 72.4) |
| Atlantic salmon (Salmo salar) | ||||
| Sajjadi and Carter (2004) | Canola meal based diet | 63.8; 68.3 | 2000; 2000 + Pi | 74.06; 69.1 |
| Denstadli et al. (2007) | Soybean meal 43%, Wheat 12% | 1) 31.2 2) 31.4 3) 36.7 4) 38.1 | 1878 | 1) 37.2 2) 33.0 3) 38.6 4) 38.9 |
| Red sea bream (Pagrus major) | ||||
| Biswas et al. (2007b) | Soybean meal 30%; Soybean meal 30% | 25.1; 54.3 | (1000, 2000, 3000, 4000); (1000, 2000, 3000, 4000) | (31.8, 36.0, 32.6, 30.7); (76.7, 86.9, 77.2, 79.1) |
| Japanese flounder (Paralichthys olivaceus) | ||||
| Masumoto et al. (2001) | Soybean 67%; Soy protein concentrate 40% | 8.9; 1.3 | (Phytase + citric acid); Phytase | 87.3; (47.5, 37.6) |
| Striped bass (Morone saxatilis) | ||||
| Hughes and Soares (1998) | (Wheat middlings 26%, Soybean 27%, Corn gluten 25%); (Wheat middlings 21%, soybean 37%, corn gluten 10%) | 29.7; 61.2 | (800, 1300, 2400) | 82.8, 64.9, 92.5 |
| Papatryphon et al. (1999) | Soybean meal 41%, CGM 30% | 45 | 500, 1000, 2000 | 56.2, 67.1, 77.6 |
| Sea bass (Dicentrarchus labrax) | ||||
| Olivia-Teles et al. (1998) | Soybean 67% | 25.2 | 1000, 2000 | 79.8, 71.5 |
| Japanese sea bass (Lateolabrax japonicas) | ||||
| Ai et al. (2007) | Soybean meal 17%, rapeseed meal 10%, peanut meal 10% | 26.0 | 200 | 36.3 |
| Korean rockfish (Sebastes schlegeli) | ||||
| Yoo et al. (2005) | Soybean meal 25.8%; Soybean meal 34% | 57.6; 61.8 | (1000, 2000); (1000, 2000) | (86.3, 87.0); (84.0, 89.5) |
| Nile tilapia (Oreochromis niloticus) | ||||
| Liebert and Portz (2005) | Wheat 32.5% Soybean 23.5% Corn 22.5%,Wheat gluten 11.5% | 11.8 | 500, 750, 1000, 1250 | 24.5, 27.6, 31.7, 31.6 |
| Portz and Liebert (2004) | (Wheat 32.5% Soybean 23.5% Corn 22.5%, Wheat gluten 11.5%); (Wheat 32.5%, Soybean meal 23.5%, Corn 22.5) | 49.1; 12.5 | (500, 1000, 2000, 4000); (500, 1000, 2000, 4000) | (60.1, 71.4, 71.1, 73.9); (40.8; 50.0; 51.5; 52.8) |
| Red tilapia (Oreochromis niloticus Linn.) | ||||
| Phromkunthong and Gabaudan (2006) | (Soybean meal 45%, Rice bran 18%, cassava 20%); (Soyabean meal 45%, Rice bran 18%, cassava 20%) | 41.2; 27.4 | (750, 750 + DCP); (750, 750 + DCP) | (62.9, 63.5); (43.1, 40.9) |
| Asian catfish Pangasius (Pangasius pangasius) | ||||
| Debnath et al. (2005a) | Soybean meal 45.0% | 55.9 | 150, 250, 350, 500, 1000, 2000 | 61.9, 66.1, 72.7, 67.6, 66.9, 71.8 |
| African catfish (Clarias gariepinus) | ||||
| VanWeerd et al. (1999) | Soybean meal 68.5% | 29.3 | 380, 750, 1000 | 40.5, 68.1, 67.6 |
| Channel catfish | Soybean 46%, cottonseed 14%, corn 32% | 31.2 | 1000, 3000 | 55.1, 62.5 |
| Indian major carp (Rohu) (Labeo rohita) | ||||
| Baruah et al. (2007a) | (Soybean meal 41%, Rice products 23%); (Soybean meal 41%, Rice products 23%) | 61.6; 69.4 | (500, 500 + CA); (500, 500 + CA) | (74.2, 82.7); (75.9, 83.4) |
| Common carp (Cyprinus carpio L.) | ||||
| Schäfer et al. (1995) | Soybean meal 52.5%; Soybean meal 52.5% | 32.0; 30.4 | 500; (500, 1000) | 49.4; (42.3, 47.1) |
| Mai et al. (2002) | Soybean 37.6%, wheat bran 18.7% | 27 | 750, 1500, 2250 | 44, 51.4, 57.2 |
- The individual characteristics (Plant protein source in fish diet, P availability (%) without phytase, Phytase dose Units/kg and P availability (%) with phytase) are separated with semicolon (;) and presented in sequence
Benefits of phytase application in aquaculture
Enhancement in phosphorus bioavailability
Many researchers have reported a positive effect of phytase supplementation on total P availability in fish (Tables 6 and 7). Soybean meal based diet added with 500 and 1000 FTU/kg of phytase could free 20% and 40% of phytate-P in common carp and 60% and 80% in crucian carp Carassius carassius respectively (Schäfer et al., 1995). Addition of 8000 FTU/kg of microbial phytase resulted in higher P bioavailability in channel catfish (Jackson et al., 1996; Yan and Reigh, 2002). A momentous increase in P availability of rainbow trout when reared with dietary phytase has been reported (Riche and Brown, 1996). In the same species the phytase inclusion at the rate of 1500 FTU/kg feed improved P availability as indicated by higher apparent availability of P, bone ash, plasma and body P concentrations (Vielma et al., 1998). Furthermore, Sugiura et al. (2001) and Vielma et al. (2004) demonstrated that dietary microbial phytase in soybean meal-based diets in rainbow trout significantly improved ADC of P. Sajjadi and Carter (2004) reported that addition of 2000 FTU/kg feed (canola meal based diet) in Atlantic salmon significantly increased P digestibility and retention. Similar results were reported by Hughes and Soares (1998) in striped bass Morone saxatilis fed with plant based diets supplemented with phytase at 2400 FTU/kg. Combination of enzymes including phytase (200 FTU/kg feed) in Japanese sea bass, had no significant effect on specific growth rate and feed conversion ratio but P retention was significantly increased (Ai et al., 2007). On the other hand, Masumoto et al. (2001) found that phytase improved P digestibility in Japanese flounder fed a high soybean meal diet. With another diet (soy protein concentrate based diet) in the same experiment, they observed that phytase improved P retention in comparison with a non-supplemented diet as well as in comparison with inorganic P supplemented diet. In Nile tilapia (Oreochromis niloticus) dietary phytase at a level of 1000 FTU/kg diet significantly increased the levels and ADC of P (Cao et al., 2008). This result is consistent with the assessment conducted by Furuya et al. (2001) on the same species where phytase supplementation between 500 and 1500 FTU/ kg improved P availability and 750 FTU/kg resulted in the similar performance as with inorganic P addition (NaH2PO4; 15 g/kg diet) (Liebert and Portz, 2005). Similarly, the inclusion of exogenous phytase in Nile tilapia diet could act as a replacer for supplemented P in diet (Phromkunthong and Gabaudan, 2006).
| References | Fish feed ingredients (plant protein source) | P availability (%) without phytase | Phytase dose FTU/kg diet | P availability (%) with phytase |
|---|---|---|---|---|
| Rainbow trout (Oncorhynchus mykiss) | ||||
| Riche and Brown (1996) | Canola meal; Solvent extracted soybean meal; Full fat soybean; Peanut meal; Corn gluten meal; Cottonseed meal | 4.8; (−13.4); 8.4; 22.1; 30.7; NA | 3.8 × 106 | 46.2; 46.6; 64.4; 75.6; 76.8; 56.3 |
| Cheng and Hardy (2002) | Canola meal; Barley; Wheat | 12.2; 79.4; 61.6 | 500 | 41.8; 82.7; 64.6 |
| Cheng and Hardy (2003) | Raw soybean; Expelled soybean; Extruded fullfat soybean | 21.2; NA; 12.5 | 750; 200; (200, 400, 600, 800, 1000) | NA; 31.7; (81.3, 92.2, 89.7, 95.2, 93.9) |
| Vielma et al. (2006) | Rapeseed meal; Soybean meal; Corn gluten meal; Sunflower meal; Corn gluten meal; Lupin seed meal | (−1.0); 48.3; 61; (−0.9); 45.0; 65.2 | 750 | 53.8; 85.2; 118; 45.7; 72; 84.6 |
| Verlhac et al. (2007) | Soy protein concentrate; Pea meal; Faba bean meal; | 29.9; 74.1; 47.8 | 750 | 46.9; 80.3; 69.9 |
| Nile Tilapia (Oreochromis niloticus) | ||||
| Verlhac et al. (2007). | Soybean meal; Palm kernel cake; Rice bran; Corn; Cassava | 47.9; 25.5; 35.2; 23.6; 72.4 | 750 | 76.9; 50.4; 59.5; 58.3; 92.6 |
| Sea bass (Dicentrarchus labrax) | ||||
| Papatryphon and Soares (2001) | Isolated soy protein; Soybean meal; Corn gluten meal; Wheat middlings | 48; 59; 52; (−10) | 1000 | 74; 87; 70; 11 |
- The individual characteristics (Fish feed Ingredients, P availability (%) without phytase, Phytase dose FTU/kg, P availability (%) with phytase) are separated with semicolon (;) and listed in sequence.
- NA: Not available
In agastric fish like common carp Cyprinus carpio, the supplementation of phytase in diet increased plasma P concentration against phytase-free diet (Nwanna and Schwarz, 2007). In the same fish species, Mai et al. (2002) observed that phytase had higher but non-significant effect on levels of plasma inorganic P compared with the fish fed diet without phytase. Potential of phytase application in feed of rohu, a agastic fish has also been investigated by Baruah et al. (2007a); maximum apparent digestibility of P and crude protein was recorded when phytase-supplemented diets contained 750 FTU phytase/kg diets. It was seen that exogenous phytase was substantially efficient in enhancing the bioavailability of P and thus reducing the amount of faecal-P. Thereby, inclusion of phytase in aquafeed tends to reduce the phosphate load from fish wastes and thus eventually prevents phosphate induced algal bloom contamination. Any reduction in P excreted by fish and other animals is of benefit to both the environment and sustainable production.
Enhancement of bioavailability of other nutrients and minerals
Supplementation of phytase can hydrolyse phytate and increase the concentration of minerals in plasma, bone and the whole body (Jackson et al., 1996; VanWeerd et al., 1999; Papatryphon and Soares, 2001; Debnath et al., 2005b; Liebert and Portz, 2005). Addition of phytase at a level of 1000 FTU/kg diet was sufficient to significantly increase Ca, Mg and Mn content of bone in channel catfish; and addition of phytase at a level of 8000 FTU/kg feed significantly increased the bioavailability of naturally occurring Zn from feed (Yan and Reigh, 2002). Studies on rainbow trout showed that phytase supplementation increased the apparent absorption of Ca, Mg, Cu, Fe, Sr and Zn in low-ash soybean meal (Sugiura et al., 2001). Furthermore phytase supplementation in extruded soybeans increased ADC of Mg, Mn, and Zn significantly (Cheng and Hardy, 2003). Baruah et al. (2005) conducted an experiment on rohu fingerlings by supplementing the microbial phytase at the level of 0, 250, 500, 750 and 1000 FTU/kg diets. Phytase-supplemented groups in general recorded significantly (p < 0.05) higher percentage of bone ash and also higher concentration of bone Ca and P compared with the non-supplemented group. Bone ash and bone P content were found to be highest in dietary feed with phytase level of 750 FTU/kg diet, which did not differ significantly (P > 0.05) from those of fish on 500 FTU/kg diet. Bone Ca content was also highest with the inclusion of 750 FTU/kg diet. These results were similar to those observed for rohu (Baruah et al., 2005), common carp (Schäfer et al., 1995), and other fish species (Storebakken et al., 1998; Papatryphon et al., 1999; Yan and Reigh, 2002; Debnath et al., 2005b; Liebert and Portz, 2005). From these studies it can be concluded that bone ash and bone P are sensitive indicators of the P status in fish. This is because the P requirement for maximum bone mineralization is greater than maximum body weight gain. Insufficient P intake leads to the mobilization of P from the bone and transfer to soft tissues and metabolic processes (Baeverfjord et al., 1998). Increment in bone ash in fish fed phytase-supplemented diets is an indication that the mineral bioavailability was significantly increased by dietary manipulation (Baruah et al., 2005; Debnath et al., 2005). Nwanna et al. (2007) conducted a feeding trial on common carp to evaluate the effect of wet-incubation of dietary plant feedstuffs with two different phytases, PtN (Natuphos®) and/or PtR (Ronozyme®), before pelleting, on mineral digestibility and mineral deposition in the fish. Pre-treatment of the feedstuffs with PtN and PtR at level of 4000 FTU/kg each, enhanced apparent digestibility of minerals and their deposition in the fish. It increased mineral utilization, which resulted in increased growth and feed efficiency of the fish. Phytase supplementation also enhances digestibility of minerals which are bound to phytate. Apparent digestibility of Zn was significantly improved by addition of phytase to a semi-purified diet containing 50% soybean meal fed to rainbow trout while no dose dependent effect was observed (Cheng et al., 2004). Moreover, dietary phytase have been shown to increase the apparent availability of protein, ash, Ca, Cu, Mg, Fe, Sr and Zn in low ash diets while little effect was observed in high ash diets (Sugiura et al., 2001).
Supplementation of phytase (500 or 1000 FTU/kg feed) did not affect Zn availability in rainbow trout (Vielma et al., 2000). Cheng and Hardy (2004) reported that graded level of phytase inclusion in the rainbow trout diet did not affect body composition; whereas, it was effective in releasing most minerals and trace mineral. This indicates that supplementation of trace minerals in rainbow trout diets could be reduced when phytase is added in the diet. Masumoto et al. (2001) observed that P concentrations in whole body and plasma were higher in Japanese flounder fed a phytase supplemented. Phytase or monocalcium phosphate supplementation in common carp diet increased body ash and P. Schäfer et al. (1995) observed that P excretion was lower by 30% on feeding a diets supplemented with phytase compared to a diet supplemented with monocalcium phosphate. A significant effect of phytase supplementation on whole body and bone mineralization was demonstrated in Asian catfish, pangus (Pangasius pangasius). A minimum dose of phytase at 250 and 500 FTU/kg feed increased the mineral absorption and bone ash contents in pangus (Debnath et al., 2005a).
Nwanna et al. (2005) studied the effect of phytase in African catfish and reported that the effect of phytase at 8000 FTU/kg feed (raw soybean based diet) has no effect on growth performance, Mg and Zn but improved feed conversion and body P, Ca and Mn balance. Li et al. (2004) conducted an experiment on phytase supplemented to catfish diets and found that 250 FTU/kg increased feed intake, body weight gain, feed efficiency, and bone ash and P concentration.
Enhancement of protein and amino acid digestibility
Phytate can nonselectively bind to proteins and it has been shown to inhibit activities of enzymes including pepsin, trypsin and alpha-amylase (Liener, 1994) as well as to decrease protein digestibility. Dephytinization of dietary phytate by exogenous phytase accounts for increased protein utilisation in common carp (Schäfer et al., 1995), Atlantic salmon (Storebakken et al., 1998; Sugiura et al., 1998a), European seabass (Oliva-Teles et al., 1998), Nile tilapia (Heindl, 2002) and pangus (Debnath et al., 2005b) by degrading the pre-formed phytate–protein complexes. Forster et al. (1999) assessed the potential of using dietary phytase to improve the nutritive value of canola protein concentrate diets for rainbow trout. Similarly, chemical and enzymatic processing of canola meal effectively reduced most of the anti-nutritional factors and enhanced canola meal digestibility in rainbow trout (Mwachireya et al., 1999). The digestibility and nutritional value of expeller and solvent-extracted Australian canola meals when included in the diets of juvenile red seabream (Pagrus auratus) was comparable to those of the fishmeal (Glencross et al., 2004).
In Crucian carp, phytase supplementation of 500 FTU/kg diet could improve digestibility of crude protein by 6.6% (Lie et al., 1999). Inclusion of soybean phytase in diet of Atlantic salmon improved protein utilisation parameters, ADC, and body levels of Ca, Mg and Zn and retention of P (Storebakken et al., 1998; Vielma et al., 1998, 2000). Moreover soyabean phytase in diet of flat fish, Greenback flounder (Rhombosolea tapirina) resulted in significant increase in nitrogen and mineral digestibility (Bransden and Carter, 1999). Phytase supplementation in expelled soybeans diet of rainbow trout increased ADC of amino acid significantly compared to raw soybeans but had no significant effect when added in extruded soyabean (Cheng and Hardy, 2003). However, spraying soybean meal-based diets with phytase improved protein digestibilities in rainbow trout (Vielma et al., 2004).
Protein digestibility in rainbow trout was significantly increased when fed a practical diet supplemented with 2000 FTU/kg and also when reared with soybean meal-based diets sprayed with phytase (Vielma et al., 2001, 2004). Although, protein digestibility was significantly influenced by phytase supplementation, protein retention efficiency was not enhanced in red sea bream fed soybean meal based diets supplemented with graded doses of phytase (Biswas et al., 2007b).
Phytase supplemented diet in pangus increased apparent net protein utilisation (Debnath et al., 2005b) and apparent protein digestibility and were significantly (p < 0.01) higher at a minimum supplement of 500 FTU/kg or higher in contrast to diet without phytase. Atlantic salmon was fed a diet based on canola meal with and without phytase and inorganic P supplementation. No effect on protein digestibility was observed (Sajjadi and Carter, 2004). However the impact of phytase on protein availability and utilisation in fish is somewhat contentious. There is discrepancy among authors for the positive impact of phytase on protein and amino acid bioavailability. Research conducted on rainbow trout by Prendergast et al. (1994) and Teskeredzic et al. (1995) showed that pre-treatment of rapeseed protein concentrate with the enzyme phytase did not improve the protein utilisation by rainbow trout. Similarly, no positive effect of phytase on protein digestibility could be noted in rainbow trout (Lanari et al., 1998), Atlantic salmon (Storebakken et al., 1998) and striped bass (Papatryphon et al., 1999). Similarly Riche et al. (2001) reported that Nile tilapia offered diet with and without phytase showed no difference in protein utilisation, and also concluded that the available methionine and lysine decreased with increasing incorporation of phytase pretreated soybean meal. Phytase addition in poultry, pigs and swine diets also showed conflicting results as observed for fish. The probable reason for the neutral and/or negative interaction of phytase and amino acid is that removal of phytate may increase the efficiency of other anti-nutritional factors and protect amino acids from degradation, or decrease leaching of water soluble components (Cao et al., 2007). More research is needed to obtain a better insight into the mechanisms for the phytase–protein interaction and availability of proteins and amino acids.
Enhancement of growth performance
Supplementation of phytate-containing diets with phytase neutralises the negative effects of phytate and increases growth in fish. Positive impact of phytase on growth of fish has been reported by a number of authors: Jackson et al. (1996) in channel Catfish, VanWeerd et al. (1999) in African catfish, Papatryphon and Soares (2001) in striped seabass, Vielma et al. (2000) in rainbow trout, Debnath et al. (2005a) in pangus catfish, Liebert and Portz (2005) in Nile tilapia, Nwanna et al. (2005) in common carp and Baruah et al. (2007a) in rohu. These authors have demonstrated phytate hydrolysis in plant-based diets by phytase and improvement of fish growth and mineralization. Moreover, fish fed the diets containing ≥250 FTU phytase/kg feed consumed more feed and gained more weight in comparison to fish fed the basal diet containing no microbial phytase (Li and Robinson, 1997). Vielma et al. (2004) reported increase in weight gain from 243 to 459% in rainbow trout fed soybean meal-based diets with phytase and P supplementation. Similar results have been reported for salmonids (Rodehutscord and Pfeffer, 1995; Riche and Brown, 1996; Sugiura et al., 2001). Studies on common carp showed that incorporation of microbial phytase in basal diets (soybean meal based diets) improved overall growth performance in rohu fingerlings (Baruah et al., 2007a). Nwanna and Schwarz (2007), Nwanna et al. (2007) reported higher growth performance of common carp fed a diet (incubated plant feed ingredients) containing phytase than another diet (without incubated plant feed ingredients) with and without phytase. This was possible because incubation process reduce phytate content (about 40%) of diet, improve P and mineral utilisation as compared to untreated diet. Liebert and Portz (2005) reported that the optimal growth of Nile tilapia is achieved by phytase supplementation at 750–1250 FTU/kg in plant-based diets, whereas, Cao et al. (2008) observed that 1000 FTU/ kg feed gives better growth performance and feed conversion in the same fish species. Vielma et al. (1998) observed that the inclusion of phytase at 1500 FTU/kg feed in comparison to no inclusion of phytase improved the weight gain of rainbow trout. Specific growth rate and feed conversion ratio were significantly improved when trout were fed with phytase supplemented diet at 2000 FTU/kg feed (containing 55% of soybean meal) (Vielma et al., 2001). Conversely, no substantial effect of phytase addition was observed on performance of large sized rainbow trout (initial body mass 250 g and final body mass about 2 kg) fed a diet supplemented with phytase at 1000 FTU/kg (Vielma et al., 2000).
Olivia-Teles et al. (1998) reported that there was no effect on growth performance, protein digestibility, energy retention on phytase supplementation in the diet of sea bass. Forster et al. (1999) and Sajjadi and Carter (2004) did not report any improvement on the growth of rainbow trout and Atlantic salmon when fed with canola protein concentrate incorporated with phytase. Similarly Masumoto et al. (2001) and (Yoo et al., 2005) reported no effect of dietary phytase on weight gain of Japanese flounder and Korean rockfish (Sebastes schlegeli). The discrepancy in above findings may be associated with differences in their diet composition and also with different rearing conditions (Baruah et al., 2007a). These contrasting results also suggest that dietary substrate levels of phytate are an important determinant of the magnitude of phytase responses. These reports confirmed that supplementing exogenous microbial phytase in feed ration exhort an enhancement in growth rate and performance which could be attributed to various factors, in individual or/and combined form, namely better bio-availability of P (Rodehutscord and Pfeffer, 1995; Vielma et al., 2000; Baruah et al., 2007a) and minerals (Vielma et al., 2004; Debnath et al., 2005b), improved protein digestibility (Vielma et al., 2004; Debnath et al., 2005a; Liebert and Portz, 2005; Baruah et al., 2007a) and increased absorption of nutrients owing to well functioning of the pyloric cecal region of the intestine (NRC, 1993).
Reduction in pollution from aquaculture operation
Discharge of high levels of soluble P from fish culture systems into open water environment stimulate phytoplankton growth, resulting in wide fluctuations in dissolved oxygen concentrations (Li et al., 2004). Many studies have reported a clear effect of phytase supplementation in reducing P excretion from fish. Ai et al. (2007) showed that the total P effluent was significantly lowered when fish reared with a diet supplemented with phytase (200 FTU/kg). Similarly, soybean meal based diets supplemented with phytase decreased the excretion of P from red sea bream and maximum reduction was reported at 2000 FTU/kg feed (Biswas et al., 2007b). Similar results were also observed for rainbow trout (Sugiura et al., 2001). Faecal waste of P in rainbow trout was reduced by phytase supplementation in soybean protein concentrate diet (Vielma et al., 1998) and a significant decrease was noticed when practical diet supplemented with phytase at a level of 2000 FTU/kg (Vielma et al., 2001) was fed. Phosphorus concentration in faecal matter was reduced when trout were fed a diet with phytase supplemented at 500 and 1000 FTU/kg compared to non-supplemented feed (Verlhac et al., 2007).
Storebakken et al. (2000) observed that phytase treated soy protein concentrate based diet induced significantly lower excretion of P compared to when a fishmeal diet was fed to Atlantic salmon. Phosphorus content of faeces was also reduced in Atlantic salmon fed a phytase supplemented diet (Sajjadi and Carter, 2004). In juvenile catfish, Ictalurus punctatus, Li and Robinson (1997) reported that microbial phytase supplementation in diets reduced the excretion of faecal P by about 60%. Many studies suggest potential environmental benefits to the extent of 30% to 40% reduction in P excretion (Omogbenigun et al., 2003).
Optimum dose of phytase in fish diet
In dose–response studies, phytase addition between 250 and 1500 FTU/kg feed is usually considered as optimum in many fish species as shown in Table 8. Optimum dose of phytase level in Nile tilapia diet, is 750–1000 FTU/kg feed (Liebert and Portz, 2005). Whereas, Furuya et al. (2001) observed that phytase supplementation between 500 and 1500 FTU/kg diet is required to maintain growth of Nile tilapia fed with plant-based diets. Many researchers (Jackson et al., 1996; Li and Robinson, 1997) observed that 250–500 FTU/kg diet to plant based diets of phytase is adequate to effectively improve the growth performance, bioavailability of nutrient and phytate P of channel catfish. For stripped bass, the phytase supplementation of 1000 FTU/kg is adequate to maintain growth rate and health comparable to an inorganic-P supplemented diet (Papatryphon et al., 1999). Phytase supplementation of 500 FTU/kg in Crucian carp diet could improve growth rate, minerals utilization, phytate-P utilization (Lie et al., 1999). Baruah et al. (2007a) observed maximum apparent digestibility of P, crude protein and maximum growth when phytase-supplemented diets contained 750 FTU/kg in feed of rohu (agastic fish). The dietary microbial phytase supplementation at 500 FTU/kg diet improved growth in pangus fingerlings (Debnath et al., 2005a). Similarly, 1000 FTU/kg of phytase in the diet in Korean rockfish exhibited superior growth performance than without phytase diet (Yoo et al., 2005). These findings indicate that the optimal dose of phytase varies with different fish species, diet formulations and phytase sources. Cao et al. (2007) mentioned that the optimum dose depends on many factors such as fish species, different phytase sources, diet formulation (amount of substrate for phytase) and selected response parameters. Thus, the dose of phytase addition in each fish diet should be adjusted based on fish species, sources of phytase, diet formulation, phytate content (Table 8).
| Initial fish weight (g) | Phytate-P (%) | Phytate (%)* | Phytase (FTU/kg feed) | Fish species | References |
|---|---|---|---|---|---|
| 13.5 | 0.26 | 0.98 | 750 | Nile tilapia | Liebert and Portz (2005) |
| 68.8 | 0.27 | 1.01 | 1000 | Nile tilapia | Portz and Liebert (2004) |
| 5.5 | 0.46 | 1.73 | 1000 | Red tilapia | Phromkunthong and Gabaudan (2006) |
| - | 0.49 | 1.84 | 1000 | Common carp | Bai et al. (2003) |
| 12.6–13.7 | 0.39 | 1.46 | 750 | Indian major carp (Rohu) | Baruah et al. (2007b) |
| 6.8 | 0.41 | 1.54 | 1000 | Channel catfish | Jackson et al. (1996) |
| 44–60 | 0.19 | 0.71 | 750 | African catfish (Clarias garipinus) | VanWeerd et al. (1999) |
| 2.0 | 0.2 | 0.75 | 500 | Asian catfish (P. pangasius) | Debnath et al. (2005a) |
| 24.5 | 0.35 | 1.31 | 1000 | Striped bass | Papatryphon et al. (1999) |
| 18.0 | 0.28 | 1.05 | 500 | Rainbow trout | Forster et al. (1999) |
- *Phytate = Phytate P × 3.75
There is no doubt that phytases have an array of applications in aqua industries; and there is a wide scope for enhancing worthiness of such enzymes by manipulating their properties such as enhancing heat resistance, increasing storage life, making it work at wide pH range and decreasing production price.
Conclusions
Phytases have been mainly, if not solely, used as an animal feed additive in diets largely of swine and poultry, and to some extent of fish in commercial production systems. In aquaculture the plant protein sources (meals or concentrates) are being increasingly used due to their relatively low cost and ample availability. Use of plant protein based diets in aquaculture is inevitable in the near future since fishmeal availability is deceasing and to sustain aquaculture industry alternative protein sources are required. Plant ingredients have limitations due to the presence of phytate and other antinutritional factor that restrict their inclusion in fish diets. Phytate-rich plant ingredients restrict the bioavailability of P along with other minerals. A great potential exists for using phytases in plant protein based diets, which can enhance the digestibility and bioavailability of P and trace elements, reduce the amount of inorganic-P supplement in the diet to maximize growth and bone mineralization, and markedly decrease P load to aquatic environment. Although some information is available on increase in nitrogen and energy utilization on addition of phytase, more research is required to conclusively state that phytase enhance protein and energy utilization. The optimum doses for phytase in fish diet to replace inorganic P are not yet known. Phytase supplementation in fish feed could also increase bioavailability of nitrogen, leading to reduction in feed cost. Though the role of phytase supplementation has been well proven and documented for poultry and pig production, its efficient use in aqua feed requires further work. Because of the pH specificity of phytase, the addition of organic acid along with phytase, especially in agastric fish, such as common carp is of special interest, and needs further research. In addition, there is a need to enhance awareness among fish nutritionists and the fish feed manufacturing industry on the use of phytase as an effective and efficient approach in the formulation of cost effective, growth promoting and low polluting fish feeds based on plant protein sources, for profitable and sustainable aquaculture production.




