Immmunohistochemical Study of the Blood and Lymphatic Vasculature and the Innervation of Mouse Gut and Gut-Associated Lymphoid Tissue
Summary
The blood and lymphatic vascular system of the gut plays an important role in tissue fluid homeostasis, nutrient absorption and immune surveillance. To obtain a better understanding of the anatomic basis of these functions, the blood and lymphatic vasculature of the lower segment of mouse gut and several constituents of gut-associated lymphoid tissue (GALT) including Peyer's patch, specialized lymphoid nodules in the caecum, small lymphoid aggregates and lymphoid nodules in the colon were studied by using confocal microscopy. Additionally, the innervation and nerve/immune cell interactions in the gut and Peyer's patch were investigated by using cell surface marker PGP9.5 and Glial fibrillary acidic protein (GFAP). In the gut and Peyer's patch, the nerves have contact with B cell, T cell and B220CD3 double-positive cells. Dendritic cells, the most important antigen-presenting cells, were closely apposed to some nerves. Some dendritic cells formed membrane–membrane contact with nerve terminals and neuron cell body. Many fine nerve fibres, which are indirectly detected by GFAP, have contact with dendritic cells and other immune cells in the Peyer's patch. Furthermore, the expression of Muscarinic Acetylcholine receptor (subtype M2) was characterized on dendritic cells and other cell population. These findings are expected to provide a route to understand the anatomic basis of neuron-immune regulation/cross-talk and probably neuroinvasion of prion pathogens in the gut and GALT.
Introduction
The gastrointestinal mucosa is one of the largest organs of the body. It absorbs critical nutrients and other molecules constantly across mucosal barriers. However, another important function of gut mucus is to keep invading pathogens out through an integrated network of tissues, lymphoid cells, constitutive cells and effector molecules that protect the host from infection from the mucous surface. The gut-associated lymphoid tissue (GALT), which is gut part of the mucosa-associated lymphoid tissue, comprises the tonsils, adenoids (Waldeyer's ring), Peyer's patches, lymphoid aggregates in the caecum and colon, diffusely distributed lymphoid cells, plasma cells in the lamina propria and other lymphoid components in the gut. Inside this complicated network of tissue and cells, the blood and lymphatic vascular system plays an important role. The study of these vascular systems should therefore be helpful for understanding the anatomic basis of the physiology and mucosal immunity of the gut and GALT, e.g. the way that dendritic cells and M cells sample luminal antigens and micro-organisms, and the manner in which lymphocytes and antigen-presenting cells enter lymphatic vessels to travel to lymphatic tissue or to a lymph node to elicit an immune response in both the mucosal and systemic environments. Some light- and electron-microscopical studies of the blood and lymphatic vascular system of mouse gut and GALT, such as Peyer's patch, have been reported (Yamaguchi and Schoefl, 1983; Regoli et al., 1995). However, immunohistochemical studies of the blood and lymphatic vascular system of mouse gut and GALT are still lacking. Here, we have investigated these vascular systems in the gut and in some constituents of GALT by using immune-fluorescence staining.
For the immunohistochemical characterization of the blood and lymphatic endothelial cells (LECs) in gut, reliable markers are needed. Several markers including LYVE1 (a hyaluronan receptor), podoplanin (a glomerular podocyte membrane mucoprotein), Prox1 (the homeobox gene product involved in developmental regulation of the lymphatic system) and VEGFR/Flt-4 (the tyrosine kinase receptor for vascular endothelial growth factor) have been used for the characterization of mouse LECs (Petrova et al., 2004; Saharinen et al., 2004). CD31 and CD34 have been employed to characterize the blood vessel endothelial cells (BECs) (Prevo et al., 2001; Petrova et al., 2004; Morisada et al., 2005). CD31 (platelet endothelial cell adhesion molecule, PECAM-1) is a glycoprotein of ∼130 kD and is constitutively expressed by platelets, neutrophils, monocytes and some T lymphocytes (Torimoto et al., 1992; Muller et al., 1993; Vaporciyan et al., 1993; Muller, 1995). It is an integral transmembrane protein belonging to the immunoglobulin superfamily, and it mediates both homotypic and heterotypic cell adhesion. CD38 and vitronectin receptor (CD51/CD61) are proposed to be ligands for CD31. In a new approach, CD31 is being used as a cell surface marker for BECs and has been located in the mouse vascular endothelium by immunoelectron microscopy (Feng et al., 2004). Murine CD34 cell surface antigen, which is expressed by endothelial cells and haematopoietic stem cells, has also been used as a cell surface marker for BECs, although murine liver sinusoid endothelial cells also express some haematopoietic stem cells markers (i.e. CD34 and c-kit) (Luna et al., 2004). In the present study, CD31 and LYVE-1 have been used as markers for blood and LECs, and podoplanin has also been tested for its reliability as a cell surface marker for LECs.
Several studies support the idea of active communication and cross-talk between the nervous and immune systems (Elenkov et al., 2000; Steinman, 2004). The central nervous system (CNS) influences the immune system in a more general fashion by regulating the systemic concentration of humoral substances such as cortisol and epinephrine, whereas the autonomic nervous system communicates specifically with the immune system according to local conditions (Straub et al., 1998). It seems very likely that more examples of such specific local interaction between the autonomic nervous system and the immune system will become obvious in the future. To obtain a better understanding of this kind of interaction, which will contribute to the studies of mucosal immunity of gut, we have examined the anatomical basis of these interactions. The innervation of the small intestine of some mammalian species has been extensively studied, and profiles positive for PGP9.5 (protein gene product 9.5, a ubiquitin carboxyl-terminal hydrolase) have been reported in the jejunum, ileum and lymphoid tissue of healthy and scrapie-affected Suffolk sheep (Heggebø et al., 2003). However, in the case of mouse, data are still lacking. We have therefore investigated the innervation of the small intestine in the mouse, with special attention being paid to the innervation and nerve/immmune cell interactions in the villus of the small intestine and in the typical GALT-Peyer's patch. PGP9.5 was used as a specific marker for nerve cell bodies and fibres. It stains neuronal cell bodies and axons in the central nervous system and periphery, small nerve fibres in peripheral tissues, neuroendocrine cells in the pituitary, thyroid, pancreas and tumours of the diffuse neuroendocrine system. Many applications of this marker have been reported with respect to mouse tissue. The localization and degree of expression of PGP9.5 in the developing mouse placenta and embryo have been studied by an immunohistochemical technique (Sekiguchi et al., 2003). The development and innervation of vallate papillae and taste buds in mice have been examined with antibodies against the neuronal marker PGP 9.5 (Chou et al., 2004). Another marker for indirect characterization of nerves is Glial fibrillary acidic protein (GFAP), which is a member of the class III intermediate filament protein family that provides support and strength to cells. It is heavily and specifically expressed in astrocytes and certain other astroglia in the central nervous system, in satellite cells in peripheral ganglia, and in non-myelinating Schwann cells in peripheral nerves. Defaweux et al. (2005) has applied this marker for immunohistochemical visualization of neurons in the Peyer's patch of mouse small intestine.
Materials and Methods
Mice
Balb/c mice (8–12 weeks old) were obtained from Harlan Winkelmann and used in all the reported experiments. They were maintained under specific pathogen-free conditions in the animal facility of the German Research Center of Biotechnology.
Antibodies and reagents
The antibodies and reagents used are shown in Table 1.
| Target | Conjugate | Species | Isotype | Dilution | Clone | Company | Location |
|---|---|---|---|---|---|---|---|
| Biotin | Alexa 546 | Streptavidin | 1:300 | Molecular Probes | Eugene, OR, USA | ||
| CD45/B220 | Rat | IgG2a, kappa | 1:300 | RA3-6B2 | BD Pharmingen | San Diego, CA, USA | |
| CD45/B220 | Alexa 488 | Rat | IgG2a, kappa | 1:300 | RA3-6B2 | BD Pharmingen | San Diego, CA, USA |
| CD3e | Syrian Hamster | IgG, group 2, kappa | 1:200 | 500A2 | BD Pharmingen | San Diego, CA, USA | |
| CD11c | Biotin | Armenian Hamster | IgG1, lambda | 1:300 | HL3 | BD Pharmingen | San Diego, CA, USA |
| CD31 | Rat | IgG2a, kappa | 1:300 | MEC13.3 | BD Pharmingen | San Diego, CA,USA | |
| GFAP | Rabbit | IgG fraction | 1:500 | Stem cell Technologies | Vancouver, Canada | ||
| Syrian Hamster IgG (H + L) | Alexa 633 | Goat | 1:300 | Molecular probes | Eugene, OR, USA | ||
| Syrian Hamster IgG (H + L) | Cy5 | Goat | (Fab’)2 | 1:300 | Jakson Immuno Research Laboratories | West Grove, PA, USA | |
| LYVE1 | Rabbit | polyclonal | 1:200 | Relia Tech | Braunschweig, Germany | ||
| PGP9.5 | Rabbit | Polyclonal | 1:200 | Biogenesis | Poole, UK | ||
| Podoplanin | Syrian Hamster | polyclonal | 1:200 | Relia Tech | Braunschweig, Germany | ||
| Rabbit IgG (H + L) | Alexa 488 | Goat | (Fab’)2 | 1:300 | Molecular Probes | Eugene, OR, USA | |
| Rabbit IgG (H + L) | Cy3 | Goat | (Fab’)2 | 1:300 | Jakson Immuno Research Laboratories | West Grove, PA, USA | |
| Rabbit IgG (H + L) | Alexa 546 | Goat | 1:300 | Molecular Probes | Eugene, OR, USA | ||
| Rat IgG (H + L) | Alexa 488 | Goat | 1:300 | Molecular Probes | Eugene, OR, USA | ||
| Rat IgG (H + L) | Alexa 633 | Goat | 1:300 | Molecular Probes | Eugene, OR, USA | ||
| Rat IgG Fc gamma | Cy5 | Goat | (Fab’)2 | 1:300 | Jakson Immuno Research Laboratories | West Grove, PA, USA |
Section preparation
Mice were sacrificed by CO2 narcosis and organs were quickly taken out, embedded in Tissue-Tek® OCT Compound (Sakura Finetek, Torrance, CA, USA) and snap-frozen in liquid nitrogen. Cryosections (7, 10, 30 or 50-μm thick) were prepared in a Reichert-Jung 2800 Cryostat.
Haematoxylin-Eosin (HE) staining
Cryosection of small intestine (7 μm) was mounted on Super Frost Plus Gold slides (Menzel Galeser, Braunschweig, Germany), air-dried for 1 min and immediately fixed in 10% neutral-buffered formaldehyde (Carl Roth, Karlsruhe, Germany) for 10 min. After being washed with running tap water for 3 min, the sections were stained with Mayer's Hemalaun (Merck, Darmstadt, Germany) for 10 min. After differentiation in freshly prepared 3.75% HCl (in 70% ethanol), the sections were washed with running tap water for another 5 min, dehydrated in 70% and 90% ethanol for 2 min, respectively, stained with alcoholic Eosin (containing 0.1% phloxine in 90% ethanol; Sigma-Aldrich, Steinheim, Germany) for 5 min, dehydrated in 100% ethanol and xylene, and finally mounted with Entellan New (Merck) mounting medium.
Images were acquired with a Zeiss Axiovert Microscope S 100 (Carl Zeiss, Jena, Germany) equipped with a Zeiss AxioCam HRc colour camera (Carl Zeiss). For the ‘stitching’ of images from Peyer's patch, different parts of the organs were taken and manually stitched with paint shop pro 9.0 (Jasc software, Inc., Minneapolis, MN, USA).
Immunofluorescent staining
Sections (10, 30 or 50 μm) were fixed at 4°C in methanol for 3 min and in acetone for 3 min respectively. All the following steps were performed at room temperature. The sections were air-dried for 1 h and then rehydrated for 10 min in phosphate-buffered saline (PBS). Unspecific streptavidin-binding sites and naturally biotinylated protein sites were saturated by using the Streptavidin/biotin blocking kit (Vector, Burlingame, CA, USA) according to the manufacturer's instructions. Non-specific antibody binding sites were blocked for 20 min with 2% goat serum (Sigma Chemical Company, St Louis, MO, USA) in PBS. The sections were incubated for 1 h with primary antibodies. The secondary antibodies were diluted and incubated with mouse serum (1% in antibody solution) for 10 min to avoid any cross-reaction of secondary antibodies with mouse proteins, including mouse immunoglobulin, on the sections. The sections were incubated for 30 min with secondary antibodies or reagents, washed with PBS, drained, air-dried and mounted with Kaiser's glycerol gelatine (Merck) using 24 × 60 mm coverslips (Menzel Galeser, Braunschweig, Germany).
Sections were immunostained with anti-CD31 (goat anti-rat Cy5) and anti-LYVE1 (goat anti-rabbit Cy3) for blood and lymphatic vessels. Staining combinations included [the reagents in the round brackets are secondary reagents]: B220-Alexa 488, anti-CD3e (goat anti-hamster Alexa 633) and anti-PGP9.5 (goat anti-rabbit Cy3); B220-Alexa 488 and anti-PGP9.5 (goat anti-rabbit Cy3); Anti-CD3 (goat anti-hamster Alexa 633), biotin-conjugated anti-CD11c (Alexa 546-conjugated streptavidin) and anti-PGP9.5 (goat anti-rabbit Alexa 488); Anti-B220 (goat anti-rat Alexa 488), anti-CD3 (goat anti-hamster Alexa 633) and anti-PGP9.5 (goat anti-rabbit Cy3); Anti-B220 (goat anti-rat Alexa 488), anti-CD3 (goat anti-hamster Alexa 633) and anti-PGP9.5 or anti-GFAP (goat anti-rabbit Alexa 546); Anti-B220 (goat anti-rat Alexa 633), anti-CD11c (Alexa 546-conjugated streptavidin), anti-PGP9.5 or anti-GFAP (goat anti-rabbit Alexa 488); Anti-B220 (goat anti-rat Alexa 546), anti-CD3 (goat anti-hamster Alexa 633) and anti-Muscarinic acetylcholine receptor (AChR) M2 (goat anti-rabbit Alexa 488); Anti-CD11c-Biotin (Streptavidin-Alexa 546) and AChR M2 (goat anti-rabbit Alexa 488).
Confocal microscopy
Confocal imaging was performed with an LSM META510 confocal scanning laser system (Carl Zeiss) on an Axiovert 200 M microscope (Carl Zeiss). The instrument settings are shown in Table 2. Images were obtained with a Plan-Apochromat’’ 40×/1.3 oil immersion objective lens. For some micrographs, Tile Scan was performed to obtain images from large areas of the organ at high resolution. For example, for the lymph node, a 10 × 10 Tile scan was used, viz. 100 pictures were obtained with a 40 × oil objective and stitched together by the confocal microscope system. After image acquisition, the images were adjusted and analysed by using lsm software.
| ParametersDyes | Lasers | Excition wavelength (nm) | Emission filter | Detector |
|---|---|---|---|---|
| Alexa 488 | Argon (max. 7%) | 488 | BP 505–530 | normal |
| Alexa 546, Cy3 | HeNe1 (max. 20%) | 543 | BP 560–615 | normal |
| Alexa 633, Cy5 | HeNe2 (max. 42%) | 633 | 654–718 | META |
Image processing and three-dimensional (3D) reconstruction
3D reconstructions were performed with image j from the National Institute of Health (USA). The images from optical sections were exported to Adobe Photoshop CS as serial images (Format JPEG), processed, and saved as JPEG documents. 3D projections were performed on this image sequence, and the movie documents generated were saved as uncompressed AVI files. The settings for the 3D projection were: slice spacing (pixels): 1; rotation angle increment: 10; opacity: 0; surface depth-cueing: 50%; interior depth-cueing: 50%; projection methods: brightest; interpolate: selected. The files were then opened in Virtual Dub (Version 1.5.10, http://www.virtualdub.org), compressed, further processed, and saved as normal AVI documents. The DivX ProTM 5.1.1 Codec was used for the compression of these AVI files.
The 3D reconstruction (S Video 5) was performed by the 3D reconstruction function (3D projection) directly in the lsm software. Images for the 3D projection with two different rotation angles were exported to adobe photoshop cs as TIFF documents for further image processing.
Results
Blood and lymphatic vasculature of mouse small intestine and Peyer's patch
Our histological study of the gut and GALT involved the use of immunostaining and confocal microscopy. We first attempt to test the antibodies for the characterization of lymphatic and blood endothelial cells. The results of simultaneously labelling the section of small intestine with anti-CD31 and anti-LYVE-1 are shown in Fig. 1a. The lymphatic vessel in the villus (Lacteal) is also weakly positive for CD31. This weak staining was also seen following CD31 and LYVE1 staining of other organs (data are not shown). To test the reliability of anti-Podoplanin antibody for the characterization of lymphatic vessels, the sections were co-stained with anti-LVVE-1 antibody and the results are shown in Fig. 1b. No colocalization of LYVE1 and podoplanin can be observed. Thus, anti-CD31 and anti-LYVE1 were used for the immunohistochemical characterization of lymphatic and blood vessels in the mouse small intestine and the results are shown in Fig. 1c,d. In the jejunum, a blind-end lacteal running along the longitudinal axis of the villus was surrounded by a network of blood vessels. Similar results were obtained in the ileum. The results of 3D reconstruction of a 50-μm thick cryosection from ileum are presented in Supplementary Video S1.
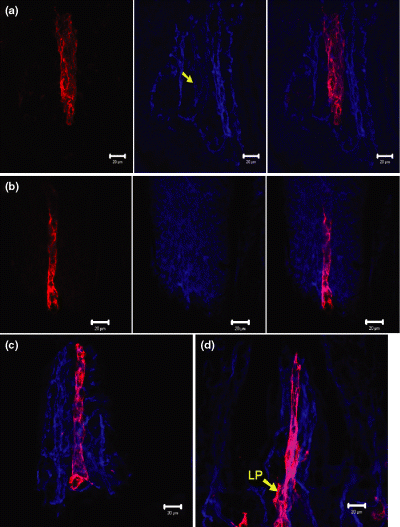
Blood and lymphatic vasculature of small intestine of Balb/c mouse. Scale bar = 20 μm. (a) Anti-LYVE1 (red, left picture) and anti-CD31 (blue, center) reveal the lymphatic vessels and the blood vessels in the villus of small intestine, respectively. The lymphatic vessel is also weakly positive for CD31 (yellow arrow, centre). (b) Immunostaining of section from mouse intestine with anti-LYVE1 (red, left picture) and anti-Podoplanin (blue, centre). Note that no colocalization of two markers can be observed (right). The specific histological structures are stained by podoplanin. (c,d) Same staining as shown in (a), showing jejunum (c) and ileum of small intestine. (d) LP, lymphatic plexus.
Similar staining approach was applied on the Peyer's patch. For better understanding of the structure of the Peyer's patch, HE staining was also performed and results are shown in Fig. 2a. A new protocol (described in Materials and Methods) recently developed in our lab for the HE staining of cryosections was applied for this staining. The blood and lymphatic vasculature of Peyer's patch is shown in Fig. 2b,c. The 3D reconstruction of this section of Peyer's patch is presented in Supplementary Video 2. The whole view of the blood and lymphatic vasculature of this Peyer's patch is shown in Fig. 2c. Its vasculature is similar to that of the lymph node (Fig. 2d) and the GALT in the caecum (Fig. 3a).
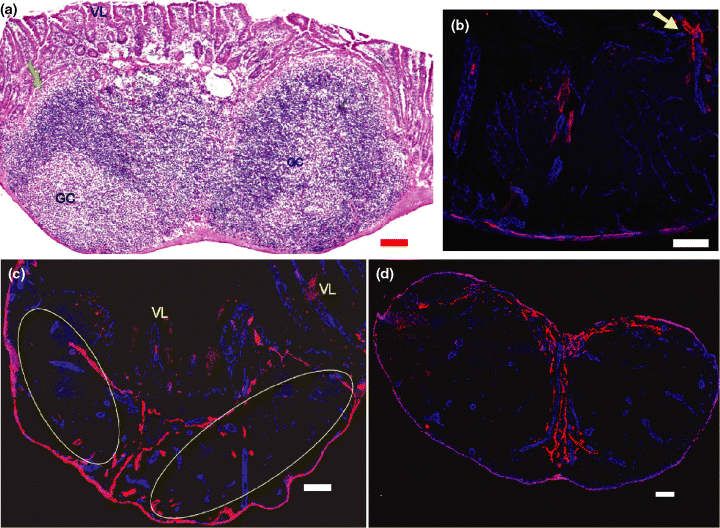
Blood and lymphatic vasculature of Peyer's patch and lymph node of Balb/c mouse. Anti-CD31 (blue) and anti-LYVE1 (red) reveal the blood vessels and lymphatic vessels. Scale bar = 100 μm. (a) HE staining of the Peyer's patch. The green arrow indicates the follicle-associated epithelium. GC, germinal center; VL, villi of surface mucosa. (b,c) Blood and lymphatic vasculature of Peyer's patch (30-μm sections). (b) Detail of the Peyer's patch. Note the capillary network of blood vessel in the follicle region (CD31 positive, blue colour). In the inter-follicular region, some lymphatic vessels (anti-LYVE1 positive, red colour) can be observed. A lymphatic vessel which comes from the villus and enters the Peyer's patch is shown by the yellow arrow. (c) Overview of Peyer's patch. VL, villus. Yellow ovals indicate two lymphatic follicles. (d) Inguinal lymph node, showing a similar distribution of lymphatic vasculature compared with Peyer's patch (c) and gut-associated lymphoid tissue in the caecum (Fig. 3a, see below).
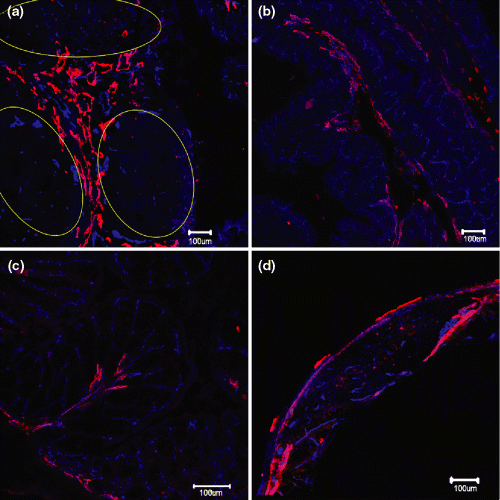
Blood and lymphatic vasculature of caecum and colon in Balb/c mouse. Anti-CD31 (blue) and anti-LYVE1 (red) reveal the blood vessels and lymphatic vessels in the caecum (a,b) and colon (c,d). Scale bar = 100 μm. (a) 4 × 4 Tile Scan of GALT in caecum. Lymphoid follicles are highlighted by yellow ovals. These vessels are mainly located in the inter-follicluar region. Some small blood and lymphatic vessels can also be observed inside the follicle. (b) Example of typical blood and lymphatic vasculature as seen in the caecum. Mucosa with regular crypts folds into the lumen. (c) In colon deep folds produce a ‘leaf-like’ structure, showing a regular distribution of blood vasculature (blue) and lymphatic vasculature (red). (d) Blood and lymphatic vasculature of a hyperplastic lymphoid follicle in the colon mucosa. Note small blood vessels within the follicle centre.
Blood and lymphatic vasculature of mouse caecum and colon
In the present study, two constituents of GALT (GALT in the caecum, and the submucosal lymphoid nodule in the colon) were also demonstrated by using confocal microscopy. The immunostaining for blood and lymphatic vessels in the caecum GALT is shown in Fig. 3a. A 3D reconstruction of this GALT to demonstrate its vasculature is presented in Supplementary Video 3. The blood and lymphatic vasculature of the caecum segments without GALT was also studied (Fig. 3b). For the colon, the blood and lymphatic vasculature is shown in Fig. 3c. The lymphatic and blood vasculature of lymphoid nodule in the submucosa of colon is shown in Fig. 3d.
Innervation and nerve-immune cell interactions in small intestine and Peyer's patch
In the present study, the innervation and nerve-immune cells interactions in the gut were investigated. Examples of the immunohistochemical characterization of nerve and immune cells in the small intestine, especially in the villus, are shown in Fig. 4a,b. Antibodies against B220, CD3 and PGP9.5, respectively, were employed to characterize B cells, T cells, and nerve cells and fibres in villus of the small intestine. The nerve fibres ran along the longitudinal axis of villus and several B220 and CD3 double positive cells have close contacts with the nerve fibres (Fig. 4a). It is also observed that B220+ cells have close associations with neuron cell body in the villus (Fig. 4b). Using the similar staining approach, the innervation of Peyer's patch was also studied by using PGP9.5 for the characterization of nerves. The staining results are shown in Fig. 4c,d. It was found that lymphoid follicle in the Peyer's patch was also innerved (Fig. 4c). In the inter-follicular region and T-dependent region, more nerves can be observed. A typical micrograph is shown in Fig. 4d. For a complementary understanding of these kinds of immune cell/nerve interactions in the secondary lymphoid tissue or organs, the innervation of spleen was also studied by using the same staining approach and results are shown in Fig. 4e,f.
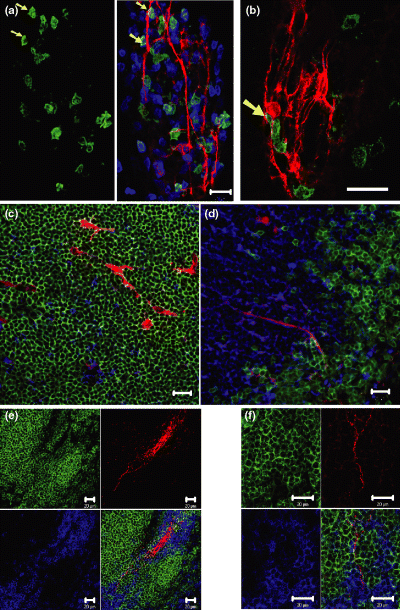
Innervation of villi of intestine, Peyer's patch and spleen of Balb/c mouse. Scale bar = 20 μm. (A, left and centre) Anti-B220 (green), anti-CD3 (blue) and anti-PGP9.5 (red) reveal the B cells, T cells and nerves in the villus of jejunum. Some nerve fibres are closely surrounded by B and T cells. The yellow arrows mark corresponding cells which are positive for anti-B220 (B cells) and CD3 (T cells, figure not shown), resulting in a cyan colour (centre panel). They lie in close contact with nerve fibres (red). (b) Anti-B220 (green) and anti-PGP9.5 (red) reveal the B cells and nerve fibres in the villus of the intestine. One B220+ cell has close contact with neuron cell body (arrow). (c,d) Anti-B220 (green), anti-CD3 (blue) and anti-PGP9.5 (red) reveal the B cells, T cells and nerve fibres in the lymphoid follicle (c) and a T-cell dependent region (d) of Peyer's patch. (c) A few nerve fibres (red) and T cells (blue) can be observed in the lymphoid follicle, which is dominated by B cells (green). (d) A few nerve fibres (red) at the interface of B- and T-cell region. (e,f) Innervation of the white pulp of the spleen, using the same staining method as described above (4c/d). (e) A nerve fibre bundle is surrounded by a sheath of T cells (periarteriolar lymphatic sheath, PALS). (f) High resolution image of part of (e). Several fine nerve fibres have direct associations with B cells in the B cell follicle and T cells in PALS.
As the dendritic cells are the most important antigen presenting cells, its crosstalk with nervous system will be of interest for us. Therefore, we also investigated the interaction of nerve and dendritic cells in the villus of small intestine. Antibodies against CD11c, CD3 and PGP9.5 were used for the characterization of dendritic cells, T cells and nerve fibres in the villus of small intestine. An interesting finding was the formation of membrane–membrane contacts between the dendritic cells and nerve cells in the villus of small intestine (Fig. 5a). Similar immunostaining using GFAP revealed the distribution of nerve fibres, B cells and dendritic cells in the villus of small intestine and similar associations were observed (Fig. 5b). Are these kinds of dendritic cell-nerve contacts also located in the GALT? Similar staining approach was applied on the sections of the typical GALT-Peyer's patch. Antibodies against CD11c, B220 and PGP9.5 were used for the detection of dendritic cells, B cells and nerve fibres in the Peyer's patch. It was found that dendritic cells and nerves also have similar contact that was observed in the villus. In the lymphoid follicle of Peyer's patch, dendritic cells have close associations with nerve fibres (Fig. 5c,d). Furthermore, anti-GFAP was used for the study of these kinds of contact and similar associations were observed. In the domal region and T-cell-dependent region, dendritic cell-nerve close contacts were observed and results are shown in Fig. 5e,f respectively. Using the GFAP, many fine nerve fibres that associate with the dendritic cells, B cells and other immune cells can also be indirectly visualized. These fibres look like small spots on single section (Fig. 5e,f). After optic sectioning with confcoal microscope and 3D reconstruction, the association between these fine nerves and immune cells can be clearly demonstrated in a 3D presentation (Fig. 5g,h and Supplementary Video S5). The fibres run along the cell membrane of dendritic cells, B cells and other immune cells. These kinds of fine fibres are abundant in the follicle, inter-follicular, T-dependent region of the Peyer's patch.
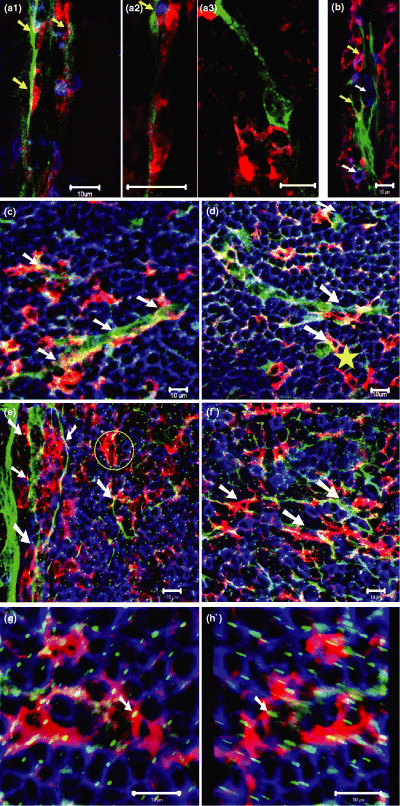
Nerve/immune cell interactions in the villi of small intestine of Balb/c mouse. Scale bar = 10 μm. (a1)–(a3) Anti-CD11c (red), anti-CD3 (blue), and anti-PGP9.5 (green) reveal the dendritic cells, T cells, and nerves in the villus of small intestine, respectively. (a1) Dendritic cells are closely associated with nerve fibres (arrows). (a2) High resolution image detail of a1. Two dendritic cells in close proximity to a nerve fibre. In the upper half, a T cell (blue, yellow arrow) is also involved in this contact. (a3) Close contact of a dendritic cell with a cell body of a neuron in a villus of small intestine (CD3+ cells are not shown here). (b) Anti-GFAP (green), Anti-B220 (blue) and anti-CD11c (red) marks nerves, B cells and dendritic cells in a villus of the ileum. Two dendritic cells, one of which is also B220 positive (upper one), have close associations with the nerves (yellow arrows). Two B cells (white arrows) have close contact with the nerves. (c,d) Anti-CD11c (red), anti-B220 (blue), and anti-PGP9.5 (green) reveal the dendritic cells, B cells and nerve fibres in the lymphatic follicles of Peyer's patch. Note the close contact between dendritic cells and nerve fibres (white arrows) and a dendritic cell-neuron cell body contact (yellow star). (e,f) Anti-CD11c (red), anti-B220(blue), and anti-GFAP(green) stain the dendritic cells, B cells, and nerve fibres in the Peyer's patch. (e) Detail of an area in the dome region of Peyer's patch, showing dendritic cell-nerve contacts (white arrows). Note that there are many GFAP positive fine nerve fibres in the Peyer's patch (tiny small green spots). In the yellow circle, a dendritic cell (red) and a B cell (blue) have associations with these fine nerve fibres. (f) Immune cells (dendritic cell and B cells)–nerve interaction in the T-cell dependent region of Peyer's patch. (g,h) Same staining as in (e) and (f). Three dimensional reconstruction of sections (Using 3D projection in the lsm software) from Peyer's patch to demonstrate the interactions between the fine nerve terminals and dendritic cells, B cells. (g) One projection from the 3D reconstruction. Rotation angle: 0°. (h) Another projection from the 3D reconstruction. Rotation angle: 205°. One nerve terminal has contact with dendritic cells are indicated in (g) and (h) by white arrows. The interactions between B cells and nerve terminals can also be observed in two pictures.
For better understanding of the mechanism of these kinds of nerve-immune cell interaction, it is very helpful to characterize the transmitter receptors on these cells. In this study, Anti-muscarinic M2 ACh receptor antibody was used to detect the expression of this subtype of ACh receptor on the immune cell populations in the Peyer's patch. This receptor was found on nerve fibres, vessels and some other cells in the Peyer's patch (Fig. 6a). Furthermore, colocalization analysis of muscarinic ACh receptor (M2) and other immune cells markers has revealed the expression of this receptor protein on some B220+, CD3+ and CD4+ cell populations (data are not shown). For the antigen presenting cells-dendritic cell, expression of this type of muscarinic ACh receptor on the cell surface was also observed (Fig. 6b).
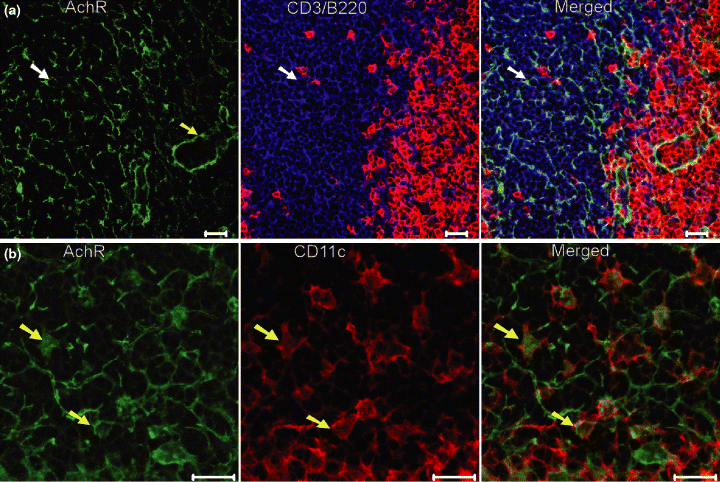
Expression of muscarinic acetylcholine receptor (ACh) receptor (subtype M2) on the cells of Balb/c mouse Peyer's patch. Muscarinic AChR positive structures are stained in green. Scale bar = 20 μm. (a) B220 (red), CD3 (blue) and AChR (green) reveal the B cells, T cells and AChR positive nerves or other components in the inter-follicular region of the Peyer's patch. A vessel (green), possibly lymphatic vessel, which is also positive for the AChR (M2) is indicated by yellow arrow. One CD3+ T cell (shown as a example), which expresses the AChR on the cell surface, is indicated by white arrow in three pictures. (b) Expression of AChR (green) on the CD11c+ dendritic cells (red) in the dome region of the Peyer's patch. Two cells (shown as examples), which are double positive for CD11c and AChR (M2), are indicated by yellow arrows in green, red and merged micrographs.
Discussion
In the present study, we have used immunohistochemistry and confocal microscopy to investigate the blood and lymphatic vasculature and innervation of the gut and GALT in order to improve our knowledge of the anatomical basis of their function in physiological and pathological conditions, especially with regard to mucosal immunity.
Immunohistochemical characterization of the blood and lymphatic endothelium
Although some studies have been reported regarding the application of CD31 and LYVE1 for the characterization of BEC and LECs in some mammalian species including human, the data on the usefulness of these two markers in mouse tissue is still lacking. Like the related protein CD44, LYVE1 is a transmembrane glycoprotein that contains a cartilage link-protein hyaluron-binding domain and binds both soluble and immobilized hyaluronan. In the present study, antibodies against CD31 and LYVE-1 have been tested for their reliability in the immunolabelling of LECs and BECs in mouse gut tissues. Although LYVE-1-positive LECs have also been found to express high levels of CD31 but not CD34 in adult mice (Morisada et al., 2005), our studies indicate that the expression level of CD31 of LYVE-1-positive LECs is so low that it does not affect the reliability of using CD31 as a BEC marker in the gut and GALTs. However, this type of weak expression of CD31 on other leucocytes should be considered when sections are co-stained with other cell markers. As CD34 is not expressed on LECs, it may be a better marker for BECs. In spleen and liver, sinusoid blood vessels also express LYVE-1, and so it is not restricted to lymphatic vessels (Carreira et al., 2001). However, this problem is not applicable in the studies of lymphatic vessels in gut.
Podoplanin is also known as OTS-8, T1-Alpha, gp36 and E11 antigen. It is predominantly expressed in lymphatic endothelium and is regarded as a novel player that regulates various key aspects of lymphatic formation. It may be involved in maintaining the shape of lymphatic vessels, even their valve structure. Our immunohistochemical studies have revealed no convincing colocalization of LYVE1 and podoplanin, and the podoplanin-positive structures were obviously not lymphatic vessels. Hence, we consider that, for the study of the blood and lymphatic vasculature of mouse gut, the characterization of LECs in the gut with this anti-podoplanin antibody is unreliable. As, until now, there are two kinds of podoplanin antibodies are available, another anti-mouse podoplanin should be tested in further studies.
Blood and lymphatic vasculature of gut and GALT
In the present study, the blood and lymphatic vasculature of the lower digestive segment of mouse gut was studied by immunohistochemistry. In the small intestine, we have mainly studied the blood and lymphatic vasculature of the villus. The vasculature of the jejunum and ileum are similar. However, they are much different from that of the caecum and colon.
Anatomists have analysed the gut for decades, and yet, the number of lymphoid structures being discovered is still growing. The Peyer's patch is a typical GALT in the small intestine. In contrast to the Peyer's patch, other constituents of the GALT in intestine are less well characterized. The existence of mature and immature isolated lymphoid follicles, cryptopatches, lymphocyte-filled villi and submucosal lymphoid aggregates has been reported (Kanamori et al., 1996; Moghaddami et al., 1998; Hamada et al., 2002; Lorenz et al., 2003). Most of these structures have only recently been described, and the extent, to which the various species contain these structures, remains unclear. In this study, we have studied the blood and lymphatic vasculature of several constituent of GALTs in the lower part of gut by using immunohistochemistry. In the further study, the investigation of blood and vasculature will be combined with the innnervation and other immune cells on single section so that the spatial distribution of these structures with respect to the immune cells can be revealed. With the newly developed protocol in our lab, we have accomplished the six-colour staining of blood vessels, lymphatic vessels, nerves and other three cell populations in the villus of small intestine (data are not shown).
As the GALT and lymph nodes are all secondary lymphoid organs or tissues, they have some similarities in their blood and lymphatic vasculature. Because Peyer's patch lacks afferent vessels the lacteal and other draining lymphatic vessels may function as an afferent lymphatic vessel. Stimulated cells flow out via submucosa efferent vessels. Lymphocyte enters the GALT through postcapillary high endothelial venules, which are similar to the high endothelial venules in the lymph node. In the lymph node, the capillary network that lies mainly in the nodule area can also be found in Peyer's patch (Fig. 2b). The direction of lymph flow, which contains antigens and antigen-presenting cells, is similar in the two organs. In the case of spleen, there is no lymphatic vessels system, although spleen sinusoid endothelial cells have been reported as being LYVE1-positive (Morisada et al., 2005). In this case, the antigens and other macromolecules may arrive with the blood flow from the marginal sinus to the follicle. The red pulp, which contains several large populations of macrophages and some plasma cells, may have the similar functions to the medullar region of the lymph node. Moreover, white pulp is a follicle-like structure and is similar in the lymph node and some constituents of GALT. The comparison of the similarities of these secondary lymphoid organs may lead to a better understanding of the mucosal immunity activities of GALT although the hypothesis remains to be confirmed in further studies.
The small size of the GALTs and their distribution in large organs impose obvious problems regarding their anatomical study. Random sampling of the relevant tissue is thus inadequate for such investigations, and systemic scans of the whole structure should ideally be made. 3D reconstructions can conveniently be used to visualize a statistically significant numbers of individual structures, while retaining their localization with respect to each other in the organ. For example, in our study of gut, using thin sections (3–5 μm) whole overview of lacteal in the villus of the small intestine cannot be obtained. The 3D reconstruction of thick sections by using confocal microscopy therefore represents an excellent solution for this problem. Certainly, other approaches for the investigation and 3D reconstruction of blood and lymphatic vasculature and innervation of mouse gut are available, e.g. (1) the reconstruction of even thicker vibrotome sections or tissue chopper sections, whereby sections thicker than 100 μm are used for optical sectioning by confocal microscopy; (2) the reconstruction of thin serial gut sections producing a real 3D view of the gut and GALTs; (3) the imaging of explanted organs or in vivo imaging by using intravital microscopy, as in studies of lymphocyte circulation and homing, the immune response in inflammation, and infection in murine gut and GALT (Bargatze et al., 1995; Warnock et al., 2000; Okada et al., 2002).
Innervation and nerve/immune cell interaction in small intestine
The nervous system in the gut also plays a crucial role in gut mucosal immunity. One kind of most important targets for the enteric nerve system is the immunomodulatory and inflammatory cells of the gut that are involved in mucosal immunological, allergic and inflammatory response. In the past decades, the innervation of different lymphoid organs in different species has been studied. For example, the noradrenergic and peptidergic innervation of various lymphoid tissues have been investigated by several approaches (Felten et al., 1985). In our study, PGP 9.5 and GFAP have been employed as specific markers for the characterization of nerves in the gut. Certainly other marker such as neurofilaments (NF L, M, H, different subunits) (Defaweux et al., 2005), Beta III Tubulin (Draberova et al., 1998) can also be used for the characterization of nerve in the mouse gut tissues.
In the present study, we are interested in the innervation and nerve/immune cell interaction in the villus of small intestine and GALT. We first studied the distribution of nerves in the villus of intestine and Peyer's patch with respect to the B cells, T cells and dendritic cells. Some of our findings are in agreement with the data presented in the literature. For example, there are few nerves in the follicle region and more nerves in the inter-follicluar region, which is similar with the results of Defaweux et al. (2005). However, in the study of innervation of Peyer's patch using surface marker GFAP, one of very interesting findings was that many fine fibres associated with immune cells were observed in the different parts including the follicular region. However, these fibres were more difficult to detect by surface marker PGP9.5.
For a better understanding of the innvervation of different compartments of secondary lymphoid tissues, such as B- and T-cell region, the mouse spleen was also studied. With respect to the innervation studies of spleen, Felten et al. (1985) have shown that the innervation of spleen is generally directed into zones of T lymphocytes and plasma cells rather than into nodular regions or B-cell regions. However, our results indicate that fine fibres can also enter B-cell regions and have direct contacts with B cells.
For the nerve-immune cell interaction, it was observed that neuron cell bodies and nerve fibres have close association with B cells, T cells and B220CD3 double-positive cells. Close associations between nerve fibres and dendritic cells were also found. Indeed, some dendritic cells formed membrane–membrane contacts with the neuron axon and cell bodies. In the study of Defaweux et al. (2005), close connections between the dendritic cell and nerves in the Peyer's patch were revealed with double-colour immunohistochemistry although the detail anatomical analysis of these kinds of contacts was lacking. In our study, the morphology of these kinds of contacts was characterized not only in Peyer's patch, but also in the villus, which is also an important place for sampling the antigen from the gut lumen. The dendritic cells are capable of binding and carry prions (Huang et al., 2002) and exosomal transfer is an attractive general mechanism for the transfer of pathogenic prion between cells, because GPI-anchored proteins (which include the prion proteins) are preferentially incorporated into the ‘lipid raft’ of exosomes during their formation by vesicle budding in late endosomes (Denzer et al., 2000; Clayton et al., 2001). Thus, these kind of direct contact will be helpful to understand the transport mechanisms of prions to the nerve fibres (Defaweux et al., 2005).
In our study, it was observed that this kind of contact had a synapse-like morphology on the light microscopy level. According to the results, there are three kinds of contact between the dendritic cells and the nerves. The first is the dendritic cell-nerve terminal contact, which was found in the villus and Peyer's patch. The second kind of contact that is found also in the villus and Peyer's patch is the dendritic cell-neuron cell body contact. The third is the contact between the immune cells and fine nerve fibres, which is similar to the first kind of contact. These kinds of fibres, which were indirectly identified by the anti-GFAP antibodies, are abundant in the Peyer's patch, even in the lymphoid follicle region of it. A similar type of contact between nerve cells and other immune cells has previously been reported. For example, synaptic-like contacts between tyrosine-hydroxylase-positive nerve fibres and lymphocytes in the white pulp of rat have been described in studies of noradrenergic sympathetic innervation (Felten and Olschowka, 1987). Nerve fibres can also form similar contacts with mast cells (Blennerhassett et al., 1991; Suzuki et al., 2004; Furuno et al., 2005). In the study of thymus, based on the ultrastructural observations, structure of ‘classical’ synapse was observed (Williams et al., 1981). The contacts observed in our study also have some characters of the synapse although these structures are not a prerequisite for the transmitter release.
As the nerve in the intestine and Peyer's patch is static and the immune cells are mobile, these kind of contact must be dynamic, just like the immune synapse between dendritic cells and T cells (Friedl et al., 2005). Compared with finding in the literature, we have demonstrated more anatomical details of these nerves–immune cell contact, which will be very helpful for the study of the neuroinvasion and nerve/immunoregulation.
Further work is necessary to define this contact between the dendritic cells and nerves in the gut and GALT. (1) This type of contact has to be confirmed in tissue sections or in the co-culture of nerve cells and dendritic cells by using electron and immunoelectron microscopy. If the typical characteristics of a synapse can be found at the contact sites of the two membranes, then it can indeed be considered a synapse-like structure. (2) The neurotransmitters and neuropeptides, e.g. substance P and serotonin, must be identified in the nerves that have similar contacts with dendritic cells. (3) The transmitter receptors on the dendritic cells in the gut and GALT should be characterized. (4) The 3D reconstruction of this kind of contact structure must be carried out to improve our knowledge of this association. (5) The dynamics study of the formation of this contact should be performed in vitro by co-culturing nerve cells and dendritic cells.
To clarify this kind of close contact between immune cells and nerve fibres, two possibilities are available (Felten et al., 1985). The first is the detection of direct ligand–receptor interactions; the finding of ligand–receptor pairs on neurons and dendritic cells should confirm their close contacts. These interactions could affect cyclic nucleotide generation in dendritic cells, which in turn could alter the maturation, synthetic capabilities for specific peptide hormones through a biochemical cascade system, chemokine secretion, surface antigen expression and other internally linked functions. Similar interactions might also affect the phagocytic capabilities of dendritic cells and their homing abilities. The second is the detection of indirect contacts through transmitters. This could be achieved through cateolamine ligand–receptor interactions on secondary secretory cells, such as mast cells or other cells. As ACh is an important neurotransmitter in the enteric nerve system, the expression of its receptor in the mouse Peyer's patch was investigated in our study. ACh receptors consist of two major subtypes, the muscarinic-activated metabotropic receptors (second messenger coupled) and the fast-ionotropic cationic nicotine-activated channel receptors, both of which are activated by the endogenous neurotransmitter, ACh (Lindstrom, 1996; Hogg et al., 2003). For muscarinic receptors, five subtypes of have been determined, named M1-M5. In the past years growing evidence indicated that cells other than neurons throughout the body expressed nicotinic AChRs including lymphocytes, macrophages, dendritic cells, adipocytes, keratinocytes, endothelial cells and epithelial cells of the intestine and lung (Conti-Fine et al., 2000; Sharma and Vijayaraghavan, 2002; Sato et al., 1999). According the studies of Aicher et al. (2003), α7-nACh receptor was expressed by human DCs and upregulated by nicotine so that the nicotine strongly activated dendritic cell-mediated adaptive immunity. For muscarinic ACh receptor, expression of different subtype (M1-M5) mRNA were found on human leucocytes and various leukaemic cell lines including T cells, B cells and monocytes (Sato et al., 1999). In our study, the expression of one subtype of muscarinic ACh receptor (M2) was demonstrated on some immune cells populations, which would be helpful for understanding the nerve-immune interaction through neuronal transmitters. There are several possible models for this kind of interaction: (1) as T cells can produce ACh (Kawashima et al., 1998), the modulation of ACh can be through an autocrine or paracrine pathway. The ACh from the nerves and T cells can affect the activity of these T cells or other immune cells. The close associations of nerves and immune cells shown in our study will be helpful to reveal the anatomic basis of these kind of interactions; (2) the adaptive immunity of dendritic cells can be affected after ACh binds with its receptor on these cells, which is similar to pathway that nicotine activates the dendritic cell-mediated adaptive immunity (Aicher et al., 2003); (3) the ACh produced by the T cells may have effect on the nerve fibres associated with it, more likely through a metabolic pathway; (4) the ACh from nerves, T cells or other sources can bind with its receptor on blood or lymphatic vessels, intestinal epithelium cells or other cells, which play an important role in the immunological response and inflammation.
These kinds of interactions and associations between the immune and nervous systems described above might provide a route by which the autonomic nerve system can directly influence specific immune system effectors cells. Our study might thus aid our understanding of the anatomical basis of the neuroninvasion of the pathogens and neuro/immune regulation of secondary lymphoid tissues and organs. Modulating the nervous system, either centrally by psychopharmacological drugs or locally, at the synaptic level, by transmitter antagonists, agonists and uptake-blockers might be a promising approach for immune therapy (Straub et al., 1998). Thus, the local interaction of the autonomic nervous system and the immune system in gut and GALT may provide the rationale for treating selected inflammatory diseases by neuropharmacological or psychological intervention.




