Improved treatment satisfaction but no difference in metabolic control when using continuous subcutaneous insulin infusion vs. multiple daily injections in children at onset of type 1 diabetes mellitus
Abstract
Objective: The aim of this study was to compare safety, metabolic control, and treatment satisfaction in children/adolescents at onset of type 1 diabetes mellitus who were treated with either continuous subcutaneous insulin infusion (CSII) or multiple daily injections (MDI).
Research design and methods: Seventy-two children/adolescents (7–17 yr of age) were enrolled in this open, randomized, parallel, multicenter study. Approximately half of the patients were treated with MDI (natural protamine hagedorn [NPH] insulin twice daily and rapid-acting insulin three to –four times daily, n = 38) by pen, and the other half received CSII (n = 34). The patients were followed for 24 months with clinical visits at the entry of the study and after 1, 6, 12, and 24 months. During these visits, hemoglobin A1c, insulin doses, weight, and height were registered. Severe episodes of hypoglycemia and ketoacidosis as well as technical problems were recorded. In addition, the patients/parents answered the Diabetes Treatment Satisfaction Questionnaire.
Results: There was no significant difference in metabolic control between the treatment groups. Treatment satisfaction was significantly higher in the group treated with CSII compared with the MDI group (p ≤ 0.01 at all screening visits). There were no episodes of ketoacidosis and there was no significant difference regarding severe hypoglycemia between the treatment groups.
Conclusions: CSII treatment proved to be a safe therapy in children/adolescents followed for 24 months after onset of their diabetes. Treatment satisfaction was higher in the CSII group, although there was no difference in metabolic control compared with the MDI group.
Continuous subcutaneous insulin infusion (CSII) therapy was introduced more than 25 yr ago as an approach to more closely simulate normal plasma insulin fluctuations than could be achieved by conventional injection therapy (1, 2). Many studies including the Diabetes Control and Complication Trial (DCCT) (3–6) have demonstrated that CSII can provide better metabolic control with a lower risk of severe hypoglycemia and a smaller weight gain compared with multiple daily insulin injections (MDI). Pickup et al. recently performed a meta-analysis of randomized controlled trials in order to investigate the effect of CSII compared with MDI treatment. The analysis showed that the CSII treatment resulted in a small improvement in glycemic control and reduced the risk of microvascular complication (7).
Today, CSII is a well-established treatment of type 1 diabetes in adults as well as in young people. Improvement in pump technology in conjunction with the results of DCCT and other studies has radically increased the use of CSII in children during the past decade. In Sweden, for example, the usage of CSII in the pediatric population with diabetes is now more than 20% (8). Young people who received CSII found coping with their diabetes less difficult compared with those who obtained MDI treatment. The flexibility and effectiveness of CSII is well suited to the lifestyle of young people. CSII appears to be a safe alternative therapy in children and adolescents with diabetes (9–11).
Insulin pump therapy in newly diagnosed children and adolescents with type 1 diabetes has not been extensively investigated, and only a few studies have been conducted so far. de Beaufort et al. reported in 1989 that children treated with CSII from the onset of their diabetes had better metabolic control from 2 months compared with patients treated with conventional injection therapy (one to two injections per day) (12). In a follow-up study, the CSII-treated children expressed significantly less physical complaints and physical restrictions compared with the children treated with conventional injection (13). In contrast, Pozzilli et al. found no difference in metabolic control between CSII and MDI treatments from the onset of diabetes in a pilot trial in 2003 in patients aged 12–35 yr (14). Furthermore, Ramchandani et al. found, in a study of 28 children with newly diagnosed type 1 diabetes, an improved metabolic control after 18 months with CSII from onset. However, in that study, no control group was recruited (15).
Data from studies of children with 1- (5) or 2-yr experience (16) show that patients are more satisfied with CSII treatment than with MDI. It has also been found that patients with poor glycemic control are more satisfied with pump treatment (5, 17). However, there have been no recent studies concerning the treatment satisfaction of insulin pump therapy on newly diagnosed children or adolescents with diabetes. As treatment with an insulin pump becomes a more frequent choice of therapy and is offered occasionally to newly diagnosed patients, it is important to assess the effect and satisfaction with this treatment in this population. We therefore designed this study to compare CSII with MDI in newly diagnosed diabetic children and adolescents. Our outcome measurements were metabolic control, safety, and treatment satisfaction.
Methods
This investigation was a multicenter study with nine participating pediatric departments in Sweden. The study was a randomized, open study with two parallel groups, one group received CSII and the other group received MDI treatment. The patients were stratified by gender and puberty (prepuberty and puberty). Puberty was defined as B2 Tanner in girls and testicular volume of ≥4 mL in boys. The patients were between 7 and 17 yr of age when they were enrolled in the study, and they were followed for 24 months. The recruitment period was between December 2001 and April 2004. None of the patients had any other relevant diseases. This study was approved by Ethics committee, Uppsala University, Sweden. Signed consent was obtained from all patients/parents.
All pediatric departments in Sweden treat patients with newly diagnosed diabetes mellitus according to the National guidelines for children with diabetes in Sweden (18), including hospitalization for 7–10 d. During this time, the patients and/or their parents were informed about the study and asked about participation. If they volunteered, the patients were randomized into CSII or MDI groups within 3 wk of the diagnosis. During hospitalization, all patients and their parents were educated and trained by the pediatric diabetes team according to the National guidelines (18). The only difference in education between the two groups was that the patients in the CSII group received an additional half a day of education in operating the insulin pump.
Insulin treatment
The MDI group received morning and bedtime intermediate-acting insulin NPH (Insulatard; Novo Nordisk, Bagsvaerd, Denmark) and rapid-acting insulin, aspart (NovoRapid; Novo Nordisk, Bagsvaerd, Denmark) three to four times a day as mealtime insulin. The NPH injections were given as separate injections, that is, not mixed with the rapid-acting insulin. The CSII group used insulin aspart (NovoRapid; Novo Nordisk). The insulin pumps that were used in this study were H-Tron (Roche, Burgdorf, Switzerland).
Clinical visits
The patients were followed regularly by the pediatric diabetes team according to the National guidelines (18). The visits were more frequent in the beginning and thereafter every third month. However, telephone contacts could occur on a more frequent basis. Study visits were at the start of the study (0 months = within 3 wk after the diabetes diagnosis) and after 1, 6, 12, and 24 months. At each study visit, the following parameters were registered: (i) anthropometric data (height and weight), (ii) pubertal status, (iii) Hemoglobin A1c (HbA1c), (iv) insulin dosage, (v) number of contacts with the hospital for ketoacidosis (defined as pH < 7.30), (vi) severe hypoglycemic episodes and/or technical problems, and (vii) the Diabetes Treatment Satisfaction Questionnaire (DTSQ) (19).
Anthropometric data, height, and weight were converted to standard deviation (SD) of body mass index (BMI) according to Swedish population reference standards (20). HbA1c was analyzed at the central laboratory, University Hospital, Uppsala, Sweden, with a high-performance liquid chromatography method (Bio-Rad Laboratories, Hercules, CA, USA), calibrated according to the Swedish national reference that gives values that are approximately 1% below DCCT numbers (reference interval 3.6–5.0) (21). HbA1c measured at the onset of diabetes and at 18 months were analyzed at the local laboratory using the same national reference. Insulin dosages were registered as total daily dose (TDD) and also mealtime/bolus insulin as percentage of TDD (% bolus of TDD). For pump users, these numbers were taken from the pump download, and for the pen users, their stated doses were registered. In addition, the patients listed in a diary of their own, severe hypoglycemic episodes (defined as those requiring assistance for recovery from another person) and technical problems (if this involved contacting the hospital or the company that supplied the insulin pump or insulin pen).
DTSQ
All patients in the study completed the DTSQ at 1, 6, 12, and 24 months (19). The younger patients completed the questionnaire together with their parents, and the teenagers completed the questionnaire by themselves. The DTSQ is designed to assess satisfaction with diabetes treatment regardless of the type of treatment. It has been shown to be reliable, valid, and sensitive to change (22–24). The questionnaire consists of eight questions that are answered on a 7-point scale (range 0–6). Six items contribute to the treatment satisfaction score, and two items assess perceived frequency of hyperglycemia and hypoglycemia. Higher scores on the satisfaction scale indicate greater satisfaction. Higher scores on the hyperglycemia and hypoglycemia items indicate greater problems.
Statistical analyses
Data are given as arithmetical mean values and 95% confidence interval. The statistical data analysis was performed using Statistical Package for the Social Sciences (spss, version 14). Descriptive statistics were used to describe the study participants. Follow-up data were compared with the baseline characteristics. The differences between outcomes of CSII and MDI treatments were tested by Student’s t-test (two-tailed). In addition, for estimation of the differences in outcomes, repeated measures analysis of variance (anova) was also used. However, there were no differences in significance between the two tests; therefore, the p value from the Student’s t-test is presented throughout the article. The level of statistical significance was set uniformly at α = 0.05. Power calculations showed that a sample size of 40 individuals was needed to be able to detect a 0.7% significant difference in HbA1c.
Results
Seventy-two patients were enrolled in this study (Table 1), 42 boys and 30 girls. Forty five were pubertal and 27 prepubertal. Thirty-four patients were randomized to insulin pump treatment (CSII group), and 38 were randomized to insulin pen treatment (MDI group). Age at the start of the study was 11.8 ± 4.9 yr in the CSII group and 12.3 ± 4.5 yr in the MDI group (p = 0.47). Pump therapy was well accepted by all patients in the CSII group.
| Study start | 1 month | 6 months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CSII (n = 34) | MDI (n = 38) | p Value | CSII (n = 34) | MDI (n = 38) | p Value | CSII (n = 34) | MDI (n = 38) | p Value | |
| Hemoglobin A1c (%) | 8.2 ± 0.4 | 8.4 ± 0.5 | 0.57 | 6.4 ± 0.3 | 6.7 ± 0.4 | 0.21 | 5.5 ± 0.2 | 5.7 ± 0.3 | 0.33 |
| Standard deviation of body mass index (BMI Δ SD) | 0.40 ± 0.15 | 0.27 ± 0.13 | 0.18 | 0.31 ± 0.22 | 0.04 ± 0.18 | 0.07 | |||
| Insulin (U/kg) | 0.74 ± 0.10 | 0.85 ± 0.15 | 0.25 | 0.47 ± 0.06 | 0.54 ± 0.09 | 0.26 | 0.53 ± 0.06 | 0.65 ± 0.10 | 0.07 |
| Percentage mealtime/bolus insulin of total daily dose | 0.50 ± 0.02 | 0.52 ± 0.03 | 0.43 | 0.46 ± 0.03 | 0.51 ± 0.05 | 0.12 | 0.53 ± 0.03 | 0.50 ± 0.03 | 0.17 |
| Treatment satisfaction | 31.5 ± 1.4 | 28.4 ± 1.8 | 0.01 | 33.4 ± 1.0 | 28.5 ± 1.7 | <0.001 | |||
| Perceived hyperglycemia | 0.9 ± 0.3 | 1.0 ± 0.4 | 0.75 | 1.7 ± 0.5 | 2.1 ± 0.6 | 0.25 | |||
| Perceived hypoglycemia | 1.6 ± 0.4 | 2.7 ± 0.5 | 0.001 | 1.5 ± 0.4 | 1.8 ± 0.5 | 0.38 | |||
| 12 months | 24 months | |||||
|---|---|---|---|---|---|---|
| CSII (n = 34) | MDI (n = 37) | p Value | CSII (n = 34) | MDI (n = 33) | p Value | |
| Hemoglobin A1c (%) | 6.0 ± 0.3 | 6.0 ± 0.4 | 0.99 | 6.5 ± 0.4 | 6.7 ± 0.5 | 0.66 |
| Standard deviation of body mass index (BMI Δ SD) | 0.27 ± 0.27 | 0.14 ± 0.23 | 0.45 | 0.13 ± 0.31 | 0.30 ± 0.21 | 0.37 |
| Insulin (U/kg) | 0.62 ± 0.08 | 0.89 ± 0.12 | <0.001 | 0.74 ± 0.09 | 1.07 ± 0.16 | 0.001 |
| Percentage mealtime/bolus insulin of total daily dose | 0.46 ± 0.04 | 0.51 ± 0.04 | 0.05 | 0.47 ± 0.03 | 0.53 ± 0.04 | 0.02 |
| Treatment satisfaction | 32.7 ± 0.9 | 28.2 ± 2.2 | 0.001 | 33.1 ± 0.9 | 27.5 ± 2.0 | <0.001 |
| Perceived hyperglycemia | 1.9 ± 0.5 | 2.7 ± 0.6 | 0.06 | 2.5 ± 0.5 | 3.5 ± 0.5 | 0.01 |
| Perceived hypoglycemia | 1.4 ± 0.5 | 1.8 ± 0.4 | 0.20 | 1.7 ± 0.4 | 1.7 ± 0.4 | 0.89 |
- CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; n, number of subjects.
- Values given as mean ± 95% confidence interval.
Of the 72 patients, 67 completed the whole study. Two patients in the MDI group changed to insulin pump between 18 and 21 months at their own/parents’ request. Two patients, also in the MDI group, were switched to the new long-acting insulin analogue, glargine, after 18 months (initiated by the diabetes team). Furthermore, one additional patient in the MDI group dropped out around 12 months. These patients did not differ from the other patients in the MDI group regarding metabolic control or treatment satisfaction when they dropped out of the study.
The time between the day of diabetes diagnosis and the day of entering the study was 12.2 ± 2.0 d for the CSII group and 10.4 ± 1.7 d for the MDI group (p = 0.19).
After stratification by gender and puberty, no differences were found between the groups with the exception of % bolus of TDD (see below).
Metabolic control
There was no significant difference at any study point between the CSII and the MDI groups regarding metabolic control. HbA1c dropped during the first 6 months of the study and had a modest increase during the later part of the study in both groups (Fig. 1). HbA1c at the start of the study was 8.2 ± 0.4% in the CSII group and 8.4 ± 0.5% in the MDI group (p = 0.57) and after 24 months was 6.5 ± 0.4% and 6.7 ± 0.5%, respectively (p = 0.66) (Table 1).
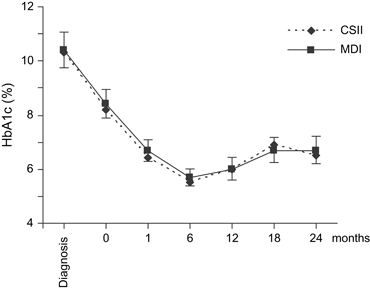
HbA1c levels during the study period. Values are presented as mean ± 95% confidence interval. The values for the CSII group are presented as black triangles connected by an interrupted line. The values for the MDI group are presented as black squares with a filled line in between. All the HbA1c measurements were analyzed at a central laboratory (University Hospital, Uppsala, Sweden) except for the diagnosis and 18-month time points that were analyzed at the local laboratories using the same national reference. CSII, continuous subcutaneous insulin infusion; HbA1c, hemoglobin A1c; MDI, multiple daily injections.
BMI
BMI, calculated as BMI Δ SD, compared with the time when the patients entered the study increased in both groups throughout the study. The CSII group had a larger increase in the beginning of the study, although not significant compared with the MDI group. At 6 months, BMI Δ SD was 0.31 ± 0.22 in the CSII group and 0.04 ± 0.18 in the MDI group (p = 0.07) and after 24 months 0.13 ± 0.31 vs. 0.30 ± 0.21, respectively (p = 0.37) (Table 1). Of note, there was no significant difference in BMI between the groups when entering the study.
Insulin doses
Insulin doses, measured as U/kg, increased during the study period in both groups (except between 0 and 1 month) (Table 1). The increase was more pronounced in the MDI group with a significantly greater increase at 12 and 24 months (0.89 ± 0.12 vs. 0.62 ± 0.08, p < 0.001 and 1.07 ± 0.16 vs. 0.74 ± 0.09, p = 0.001). The % bolus of TDD was also significantly different between the groups at the end of the study where the CSII group had a lower percentage (0.46 ± 0.04 vs. 0.51 ± 0.04, p = 0.049 at 12 months and 0.47 ± 0.03 vs. 0.53 ± 0.04, p = 0.021 at 24 months). When the cohort was further stratified according to gender and puberty, only minor differences were found. The prepuberty patients in the MDI group had a significant increase in % bolus of TDD after 24 months compared with those of the CSII group (0.57 ± 0.06 vs. 0.46 ± 0.14, p = 0.008). Female patients in the MDI group had a significantly higher percentage after 24 months compared with female patients in the CSII group (0.53 ± 0.19 vs. 0.43 ± 0.17, p = 0.021).
Satisfaction with the treatment
Treatment satisfaction was found to be significantly higher in the CSII group compared with the MDI group after 1 month of treatment and continued to be higher throughout the study (Fig. 2). This difference was more pronounced after every subsequent study visit (except between 6 and 12 months) (Table 1). After 1 month of treatment, the satisfaction score for the CSII group was 31.5 ± 1.4 and 28.4 ± 1.8 for the MDI group, p = 0.01. At 24 months, the scores were 33.1 ± 0.9 and 27.5 ± 2.0, respectively, p < 0.001.
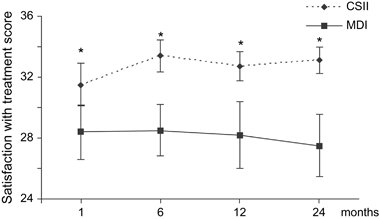
Treatment satisfaction during the study period. The satisfaction with treatment scores is presented as mean ± 95% confidence interval. The scores for the CSII group are presented as black triangles connected by an interrupted line. The scores for the MDI group are presented as black squares with a filled line in between. Stars indicate a significant p value between the CSII and the MDI groups. At 1 month: p = 0.01, 6 months: p < 0.001, 12 months: p = 0.001, and 24 months: p < 0.001. CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections.
Perceived frequencies of hypoglycemia and hyperglycemia
At the 1-month follow-up, the MDI group scored that they had had significantly more episodes of unacceptably low blood sugar, as reported on the DTSQ, compared with the CSII group (2.7 ± 0.5 vs. 1.6 ± 0.4, p = 0.001). At 24 months follow-up, the MDI group reported significantly more perceived unacceptably high blood sugar episodes compared with the CSII group (3.5 ± 0.5 vs. 2.5 ± 0.5, p = 0.01).
Adverse events
During the study period, there were no registered episodes of ketoacidosis, which required contact with the hospital in either group. Cases with technical problems were low, only five episodes in the CSII group and one in the MDI group. Severe hypoglycemic episodes were almost equal between the two groups, 13 episodes in the CSII group and 12 in the MDI group during the study period (19 and 17 episodes per 100 patient-year, respectively).
Discussion
In this open, randomized, parallel group study of children and adolescents with newly diagnosed type 1 diabetes, we found that CSII is a safe and well-accepted therapy. The CSII treatment group showed significantly greater treatment satisfaction but without any difference in metabolic control when compared with MDI treatment during the study period of 24 months. The strength of our study lies in the randomized trial design, the relative large cohort, the duration of the study, and completeness of follow-up.
Our finding of no difference in metabolic control is in contrast to the study of de Beaufort et al. from the late 1980s where they detected a considerable improvement in metabolic control in the CSII group compared with the injection therapy group in newly diagnosed diabetic children (12). However, their finding could probably be explained by the fact that the injection group only received one to two injections per day, which is in sharp contrast to this study where MDI treatment with five to six daily injections was used. In line with our findings, Pozzilli et al. in 2003 showed no difference in metabolic control between the CSII and the MDI treatments in a pilot study of patients with newly diagnosed type 1 diabetes (14). The most reasonable explanation for the lack of difference in metabolic control might be that there is a similar effectiveness on glycemic control between the two treatment regimes. However, the lack of a significant difference in improved metabolic control might be that the study period was too short to detect a difference in outcome because many patients could have had a remission phase that lasted for some time into the study (25). Another explanation could be that, we did not set any specific target for blood glucose levels or HbA1c apart from ‘as good metabolic control as possible’. A tighter target for HbA1c may have been easier to accomplish with CSII, thereby differentiating in effectiveness between the two treatment methods. One more important result in our study was that the CSII group required significantly less insulin at 12 and 24 months compared with the MDI group, which is a common finding in many studies comparing the treatments (5, 9, 26).
A difference in treatment satisfaction was found between the randomized groups even after the first month of the study, as measured by DTSQ (22, 23). The score for the MDI group was approximately that which would be expected as a baseline finding for patients on long-term MDI treatment with no experience of CSII (19), whereas the CSII group had a significantly higher score. The difference was maintained during the course of the study. The patients using CSII were more satisfied with their treatment option and therefore were likely to find diabetes less difficult to manage. It can be expected that these patients will have fewer worries about their disease and that there will be a reduced negative impact of diabetes in daily life. However, we must be careful not to over interpret this result, indicating a positive effect on quality of life (QoL). Diabetes itself has a negative impact on QoL (27), and this has been observed despite high levels of treatment satisfaction (as measured by the DTSQ) (28).
Studies investigating metabolic control and the frequency of adverse events in patients using CSII compared with MDI treatment show contradictory results (6, 9, 16, 29). In our study, there was no difference in severe hypoglycemic episodes between the treatment groups. Of note, the registration of hypoglycemic episodes was only recorded in each patient’s diary, and although the criteria for hypoglycemia were clearly described in the study protocol, this parameter could have been interpreted differently between the patients. No episodes of ketoacidosis requiring hospital admission were found in this study. This could be a result of a thorough education program for the patients/families at the onset of their diabetes and the fact that many patients still hade some residual beta-cell function throughout the study (30). Another commonly reported adverse effect of insulin pump therapy is significant weight gain (6). In this study, this trend was also found during the first study year, although not significantly different between the treatment groups.
In our study, we used NPH insulin as intermediate-acting insulin for the MDI group because the new long-acting insulin analogue, glargine, was not registered for treatment of children in Sweden at the start of our study. However, we do not believe that the use of the glargine would have altered our result because most other studies comparing those two insulin types have not shown any or only minor differences in metabolic control (31–33), although there are pharmacokinetics and pharmacodynamics differences between glargine and NPH insulin (34).
This study presents important information regarding CSII treatment in children at onset of type 1 diabetes and could be of use in clinical practice. Even if treatment satisfaction was improved in the CSII group compared with the MDI group, there was no difference in metabolic control and therefore one must also take health economical aspects [the additional cost for CSII compared with MDI treatment is about $2700 per patient and year (35–37)] into account before changing therapy regime for young people with newly diagnosed type 1 diabetes. We can only speculate if the improved treatment satisfaction will compensate for the increased expenses. Nevertheless, this study will bring important information to professionals, patients, and heath authorities concerning the choice of treatment in children at onset of diabetes.
Acknowledgements
We thank the participating pediatric diabetes teams for their assistance with this study. This research was partly supported by ‘Research and Development Center, county of Gävleborg’, Sweden, ‘The Swedish Children’s Diabetes Foundation’, Novo Nordisk Scandinavia, and Roche Diagnostics, Sweden. We thank Hans Högberg (Research and Development Center, county of Gävleborg, Sweden) for statistical assistance and Karin Swenning for valuable administration assistance. Finally, this study would not have been possible without the support of all patients and their families. This study has been registered at International Standard Randomized Controlled Trial Number (ISRCTN), trial no: CCT-NAPN-16461.




