Treatment of Danish adolescent diabetic patients with CSII – a matched study to MDI
Abstract
Objective: To compare two intensified insulin therapy regimens – continuous subcutaneous insulin infusion (CSII) against multiple daily insulin injection (MDI) – in Danish adolescents examined in a prospective, matched controlled study design.
Research design and methods: Thirty type 1 diabetic adolescents at CSII and 26 matched MDI controls were included in this open intention-to-treat study. Actrapid was used in both groups. Before study entry, all participants followed a brush-up course in order to minimize study effect. At each visit, the following parameters were recorded: hemoglobin A1c (HbA1c), insulin dose, weight, number of hypoglycemic and diabetic ketoacidosis (DKA) events, and the time resources used. At entry and exit of the study, diet registration and validated quality-of-life (QoL) questionnaires were filled by the participants.
Results: A non-significant decline in HbA1c was seen in both groups (p = 0.468); HbA1c decreased from 9.5 to 8.9% and from 9.7 to 9.5% in the CSII and MDI group, respectively. The insulin dose and the number of severe hypoglycemic events per patient were lower (non-significant) in the CSII group. Both groups showed increased body mass index – highest in the CSII group – and mild to moderate DKA episodes were only seen among CSII users. No differences could be demonstrated within the QoL or diet registrations.
Conclusions: CSII treatment is beneficial as an intensified insulin therapy for selected type 1 diabetic patients and both MDI and CSII can be offered by the professional diabetes team to better tailor therapy. In future, there is a strong need to identify the characteristics of responders to CSII treatment in order to increase the efficacy and safety of CSII treatment.
Metabolic control of type 1 diabetic adolescents is generally troublesome and difficult to achieve for the patient and a challenge for the health care providers. The reasons for this are probably multifactorial – involving, e.g., puberty-related increased insulin resistance; psychological factors associated with adolescence, e.g., the wish of being like peers without type 1 diabetes; the process of seeking independence from the parents normally responsible for diabetes management in childhood; and probably many other factors.
Increased hemoglobin A1c (HbA1c) is associated with increased risk of late diabetic complications (1). Thus, it is essential to aim at near-normal metabolic control even in childhood and puberty. However, it has been shown that even a limited period of years with good metabolic control may reduce the risk of late diabetic complications in the long run (2), despite the actual metabolic control.
During the recent years, continuous subcutaneous insulin infusion (CSII) has been increasingly popular as a method to aim for near-normal metabolic control in type 1 diabetic patients, and CSII has been reported to be promising [for meta-analyses, see (3, 4), and for reviews, see (5, 6)]. However, only two prospective, matched designed studies including a total number of participants exceeding 50 individuals in childhood and/or adolescents with type 1 diabetes have been published so far. First, Boland et al. in 1999 showed a decrease in HbA1c in both the CSII and the ‘multiple daily insulin injection’ (MDI)-treated group compared to baseline levels. The two groups, however, were not compared to each other (7). Second, Alemzadeh et al. recently demonstrated an improvement in HbA1c in the entire CSII-treated group, whereas MDI therapy improved HbA1c in only a subgroup of patients (8).
The aim of the present study was to compare two different intensified insulin therapy regimens – CSII against MDI in Danish adolescents examined in a prospective, matched controlled study design.
Research design and methods
Thirty type 1 diabetic adolescents at CSII and 26 matched MDI controls were included in this open intention-to-treat study. All patients followed at the Steno Diabetes Center, Gentofte, Copenhagen, or at the Pediatric Department at Hillerød Hospital, Hillerød, who were at the age of 13–19 yr at entry and had a diabetes duration of >1 yr, were invited to participate in the study. Only participants with written consent from their parents were included. Patients were excluded if they had psychiatric diagnoses, did not complete the ‘brush-up’ course during the run-in phase, missed three consecutive scheduled outpatient visits, or were not able to understand the education when given in Danish. No patients had late diabetic complications. The local ethic committees approved the study. This study has a power of 80 to show a difference of 1% point in HbA1c at a 5% significance level when 56–60 patients were included.
Among the participants, 30 chose CSII and the participants for the MDI group were randomly selected from the remaining outpatient population, as it was secured that they matched according to HbA1c, age, diabetes duration, and gender (Table 1). All participants were on MDI insulin regimen and had been introduced to carbohydrate (CHO) (CHO) counting) before entry. None had previous CSII experience. Furthermore, all participants followed a brush-up course in order to refresh their knowledge regarding blood-sugar-based insulin adjustments, diet counseling including CHO counting and diet-based insulin adjustments, precautions at physical exercise, and sick day management prior to entry. A leaflet summarizing these issues was introduced and handed out – hence, the only specific education given to the participants in the CSII group regarded the function and handling of the insulin pump – and education in basic diabetes management was given equally to all participants. In both groups, a minimum of four daily blood sugar measurements were advocated and insulin adjustments were made on the basis of this self-monitoring at the outpatient visits every 6–8 wk during the study period of 12 months. At each visit, the following parameters were recorded: HbA1c, insulin dose, weight, number of hypoglycemic events (defined as ‘Moderate’ by ‘Help from an assisting person, but conscious’, and as ‘Severe’ by ‘Unconsciousness or seizures’), number of diabetic ketoacidosis (DKA) events (defined as ‘Mild’ by HCO3−: > 15 mmol/L and as Moderate by HCO3−: 10–15 mmol/L), and finally, the time used at each contact was recorded.
| CSII | MDI | |
|---|---|---|
| HbA1c (%) | 9.5 ± 1.5 | 9.7 ± 1.6 |
| Age (yr) | 15.6 ± 1.9 | 16.2 ± 2.3 |
| Diabetes duration (yr) | 6.7 ± 3.9 | 7.8 ± 4.0 |
| Gender (M/F) | 16/14 | 15/11 |
- CSII, continuous subcutaneous insulin infusion; HbA1c, hemoglobin A1c; MDI, multiple daily insulin injection; M/F, male/female.
- * Patients in the CSII and MDI groups were matched according to HbA1c, age, diabetes duration (mean ± SD), and gender.
Short-acting insulin preparations (Actrapid) were used in all patients on MDI. In order to avoid any bias related to insulin preparation, Actrapid was also used in CSII participants in order to increase the focus to the effect of the different insulin administration principles. The MDI group had at least three preprandial Actrapid injections during the day and used neutral protamine Hagedorn insulin as basal insulin at bedtime (22:00 hours). At entry, the individual pump users’ total daily insulin dosage before entry (at MDI) was reduced by approximately 20% and the the remaining insulin was initially administered according to the following algorithm: 50% as basal and 50% as bolus insulin. The hourly basal insulin rate was calculated and 70% of the hourly basal insulin rate was given from 00:00 (midnight) to 03:30 hours, 130% from 03:30 hours to 19:00 hours, and 100% from 19:00 hours until the following midnight. The insulin dosage and administration algorithm was individually adjusted at the following outpatient visits. The daily blood glucose targets were a preprandial blood glucose in the range 5–7 mmol/L and a postprandial blood glucose ranging 10–12 mmol/L, aiming at reducing the HbA1c by 1% point in both groups. If this goal was not achieved, extra resources (e.g., more outpatient visits and telephone calls) were applied to the patient regardless of treatment arm.
A 24-h telephone hotline was established for all participants.
At entry and exit of the study (i) diet registration and (ii) validated quality-of-life (QoL) questionnaires were filled by the participants. The diet registration was self-reported registration of daily food intake. The amount and division of various basic food elements, the number of meals per day in addition to registration of the frequency of fast-food and sweets intake, and the use of CHO counting were reported. The QoL questions were categorized into four different groups: ‘Diabetes affecting daily life’, ‘Worries regarding diabetes’, ‘Satisfaction in daily life’, and ‘General aspects’ comprising 23 + 11 + 7 + 18 questions, respectively, in total 58 questions (9).
Continuous outcomes (HbA1c, insulin dose, and weight) were analyzed with mixed model (10), with random effects of patient and patient by time on study (Stochastic regression). Thus, we fitted separate linear trends over time for each patient. The average slope in the two patient groups was used as descriptors of treatment. It was checked whether there was homogeneity of variance and whether the residuals were normally distributed by means of graphical display.
The answers from each question from the QoL questionnaire were converted into a value ranging from 1 to 6, and the scores were summed within each of the four categories and analyzed using Student’s t-test. The diet registration was compared using chi-squared test. The number of hypoglycemic events within each group was tested using Fisher’s exact test, whereas the DKA events were too few to apply statistical comparison.
Results
Hemoglobin A1c
At the 411 visits conducted, the HbA1c was measured on 404 occasions. The model estimated the average HbA1c at trial onset to 9.56% in MDI and 9.18% in CSII (p = 0.33) groups. A decline in HbA1c was seen in both groups: 0.016% per month [confidence interval (CI) −0.049, 0.016] in the MDI group and 0.0004% per month (CI −0.029, 0.028) in the CSII group (p value for the difference 0.468). The measured mean HbA1c values at onset and at 3-month intervals are illustrated in Fig. 1, showing a drop in the measured HbA1c from 9.5% at entry to 8.9% at exit and from 9.7% at entry to 9.5% at exit in the CSII and MDI group, respectively (NS).
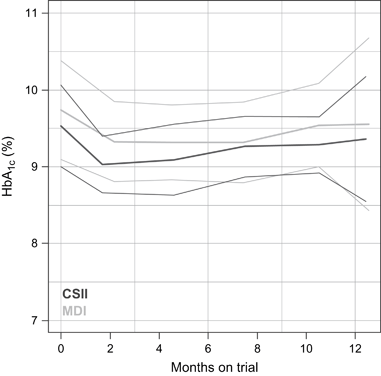
Hemoglobin A1c (HbA1c) over time for continuous subcutaneous insulin infusion and multiple daily insulin injection. Plots of the empirical means with 95% confidence interval. A decline in HbA1c is seen in both groups; however, the overall decline was not different between groups. The study effect was observed at 3 months where the groups differed significantly.
Insulin dose
At study entry, the insulin dose was reduced approximately 20%, equaling 0.31 IE/kg/d in the CSII group according to the protocol. This is statistically significant (p = 0.001). The average chance in insulin dose was −0.0021 IE/kg/24 h/month (95% CI 0.0122, 0.0081) in the MDI group and +0.0024 IE/kg/24 h/month (95% CI 0.0072, 0.0119) in the CSII group (p value for the difference 0.553); hence, no change in insulin dose was observed between or within the two groups during the study period.
Body mass index
The average change in body mass index (BMI) was 0.042 kg/m2/month (95% CI 0.004, 0.078) in the MDI group and 0.077 kg/m2/month (95% CI 0.044, 0.111) in the CSII group (p value for the difference 0.013). As the estimates are derived from a random effects model with random slope for each patient, inclusion of the initial BMI value will not change this result.
Resources used
The number of visits varied from 5 to 10 with an average of 6.4 in MDI vs. 7.9 in CSII (p < 0.001), with more visits for the pump group at entry. However, the mean time spent in the consultation per visit was 32.1 and 34.6 min for the MDI and the CSII group, respectively (NS).
Hypoglycemia and DKA
The number of hypoglycemic events is shown in Table 2. No difference was observed between the two groups. However, more severe hypoglycemic episodes per patient were observed in the MDI group compared to the CSII group (Table 2).
| Moderate and severe | Severe only | |||||
|---|---|---|---|---|---|---|
| Number of events | Number of persons | Number of events | Number of persons | |||
| >1 | 0 | >1 | 0 | |||
| MDI | 19 | 8 | 18 | 11 | 5 | 21 |
| CSII | 16 | 7 | 23 | 4 | 4 | 26 |
| Fisher’s exact test | p = 0.561 | p = 0.719 | ||||
- CSII, continuous subcutaneous insulin infusion; MDI, multiple daily insulin injection.
- * The number of hypoglycemic events and individuals with at least one event for ‘severe’ and ‘combined severe and moderate’ hypoglycemia is given. It is seen that there is a lower ratio of severe hypoglycemia per individual in the CSII group (4:4) vs. MDI group (11:5).
A total of 14 DKA episodes (in eight individuals, one individual had seven episodes and was transferred back to MDI) were observed in the CSII group: nine mild and five moderate events. No DKA episodes were seen in the MDI-treated patients.
Diet registration
No significant differences were observed between the MDI and the CSII group at entry or at exit, and no change was seen for either the MDI or the CSII group when compared at entry to exit of the study.
Quality of life
No significant difference in QoL scores was observed for any groups – neither in total nor between the four subgroups (i) between CSII and MDI at entry or at exit or (ii) over time for neither CSII nor MDI.
Discussion
The present study evaluated the metabolic effects of two different intensified insulin therapy regimens: CSII compared to MDI in type 1 diabetic patients during a period of 12 months. The results indicate no difference between the two treatment modalities over this time period. However, a trend of fewer severe hypoglycemic events per patient was observed in the CSII cohort.
Most previously published studies regarding the effect of insulin pump treatment in adolescents have been performed as either retrospective follow-up designs or prospective studies without a control arm and typically with the inclusion of less than a total of 50 participants. The four largest, non-controlled, follow-up studies demonstrate a reduced HbA1c after CSII treatment (11–14), as well as a reduction in the frequency of hypoglycemia. These findings have been confirmed in a prospective, matched study design comprising 40 CSII- and 40 MDI-treated adolescent type 1 diabetic patients (8). However, in line with our data, two recent smaller prospective, controlled studies could not confirm a beneficial effect on the metabolic control using CSII (15, 16).
The major concern regarding the non-controlled, follow-up studies is the study effect and the selection of patients offered pump treatment. All adolescents who fulfilled the inclusion criteria were offered pump treatment in our study, regardless of their current metabolic control; hence, no selection as to the composition of the study population were seen in our setting. However, possibly because of the voluntary selection of CSII treatment, our data demonstrated a study effect despite a run-in phase including a brush-up course, as the HbA1c dropped significantly after 3 months in the CSII arm of the study – an improvement that could not be sustained.
In contrast to most other studies, we have used short-acting insulin in both treatment arms, in order to focus on the methods for insulin delivery. It has previously been advocated that insulin analogs are superior to regular insulin because of more appropriate pharmacokinetics; however, no difference in HbA1c could be demonstrated when comparing short-acting insulin and insulin Aspart or insulin Lispro used in CSII (17). As all MDI subjects used short-acting insulin, this was also used in the CSII group in our group to avoid a potential bias because of different insulin preparations.
In line with most other studies, we observed fewer severe hypoglycemic events per individual in the CSII cohort; however, no overall reduction in hypoglycemia was seen. This is in itself an important finding as repeated episodes of hypoglycemia are predictive for more hypoglycemic episodes (18).
The fact that we in this study only had DKA episodes occurring in the CSII group could be because of insufficient handling of hyperglycemia within this group, as 10 of the 14 episodes happened within the first 3 months of the studyHowever, some of the mild episodes of hyperglycemia within the CSII group might have led to contact with the health care provider because of higher awareness especially regarding this aspect from the pump users (and their families) – episodes that might be managed at home without the health care providers’ knowledge in the MDI group.
To our knowledge, this is the first study to cover diet registration in both an MDI and a CSII group. CHO counting was used in both groups. Our study could not demonstrate a more flexible food planning in the either group. In contrast, two recent studies have demonstrated improved metabolic control after the introduction of (i) insulin matching CHO counting program (19) [as used in the DAFNE programme (20)] and (ii) an insulin dosage calculation device (21), but whether this is because of altered meal planning or changed dosages of insulin remains unknown.
It has been repeatedly demonstrated that intensifying metabolic control seeking near-normalized blood glucose levels has been at the risk of increasing BMI. Increase in BMI has been demonstrated in CSII users, decreasing their HbA1c (7). In our study, we confirmed this observation as our data showed an increase in BMI for both groups; however, significantly higher in the CSII group, despite no significant improvement of metabolic control. This is in contrast to the observation that CSII does not by itself lead to weight gain, as demonstrated by Raile et al. (22).
Studies have shown that good metabolic control associates to improved QoL (9, 23). However, improved metabolic control by any method might be associated to better QoL (24), and to our knowledge, no specific insulin therapy regimen so far has been demonstrated superior to others in improving QoL, given identical metabolic control. This is consistent with the findings in our study and in line with the findings of Weintrob et al. (15). However, as pointed out by Weintrob et al., it is important to distinguish between QoL and treatment satisfaction – as they tested for treatment satisfaction specifically as well as for QoL in general – this finding increased treatment satisfaction but unchanged QoL in their CSII/MDI crossover study (15). Hence, the questionnaire presently used comprising four subgroups – Diabetes affecting daily life, Worries regarding diabetes, Satisfaction in daily life, and General aspects – may not be able to measure ‘treatment satisfaction’ to a degree needed to identify a difference between the groups tested.
CSII is safe and beneficial for many adolescents. However, it is difficult to predict for whom it is safe and beneficial. Willi et al. have compared responders to non-responders of CSII treatment (defined as a fall in HbA1c of >1%, a fall in HbA1c to an absolute level of <7%, or both at 12 months post-CSII), demonstrating no differences with respect to gender, socioeconomic status, weight standard deviating score, BMI, initial HbA1c, frequency of hypoglycemia, or number of visits before CSII (14). However, Bode et al. have demonstrated that blood glucose self-monitoring and CHO counting were associated to better metabolic control among CSII users (12). Hence, motivation for near-normalized blood glucose control seems to be most essential in type 1 diabetes treatment. This is further illustrated by the fact that skipped boluses in CSII associate to higher HbA1c: two missed boluses per week led to an HbA1c increase of 0.5% and more than four missed boluses led to an HbA1c increase of 1.0% (25), – a phenomenon probably also existing in MDI patients; however, being much more difficult to detect in the MDI setting, as no automatic electronic recordings for MDI exists. Finally, no separate effect of CSII was seen when both CSII and MDI groups regularly used CHO-adjusted insulin doses (19). In our study, we may not have chosen a CHO counting system that satisfied the adolescents to the extend needed for optimal usage.
Hence, there seems no doubt that CSII treatment is beneficial as an intensified insulin therapy at least for selected type 1 diabetic patients. Both MDI and CSII should be offered by the diabetic team to better tailor therapy for the individual. Finally, there is a need to continuously educate health care providers and professionals in order to support and motivate the patients and their families in active type 1 diabetes management. In future, there is a need to identify the characteristics of responders to CSII treatment in order to increase the efficacy and safety of CSII treatment.
Acknowledgements
MiniMed, Denmark, is thanked for providing the insulin pumps (Model 508). Sponsors Diabetes Foreningen, Poul og Erna Sehested Hansens Fond, Glashoffs Fond, Nordsjællands Regional Fond, and The Fond for Clinical Development, Steno Diabetes Center are thanked for the ongoing financial support of pump supplies (catheters etc.)




