Diffusion limitations and metabolic factors associated with inhibition and recovery of photosynthesis from drought stress in a C3 perennial grass species
Abstract
Stomatal closure and metabolic impairment under drought stress limits photosynthesis. The objective of this study was to determine major stomatal and metabolic factors involved in photosynthetic responses to drought and recovery upon re-watering in a C3 perennial grass species, Kentucky bluegrass (Poa pratensis L.). Two genotypes differing in drought resistance, ‘Midnight’ (tolerant) and ‘Brilliant’ (sensitive), were subjected to drought stress for 15 days and then re-watered for 10 days in growth chambers. Single-leaf net photosynthetic rate (A), stomatal conductance (gs) and transpiration rate (Tr) decreased during drought, with a less rapid decline in ‘Midnight’ than in ‘Brilliant’. Photochemical efficiency, Rubisco activity and activation state declined during drought, but were significantly higher in ‘Midnight’ than in ‘Brilliant’. The relationship between A and internal leaf CO2 concentration (A/Ci curve) during drought and re-watering was analyzed to estimate the relative influence of stomatal and non-stomatal components on photosynthesis. Stomatal limitation (Ls %), non-stomatal limitation (Lns %), CO2 compensation point (CP) and dark respiration (Rd) increased with stress duration in both genotypes, but to a lesser extent in ‘Midnight’. Maximum CO2 assimilation rate (Amax), carboxylation efficiency (CE) and mesophyll conductance (gm) declined, but ‘Midnight’ had significantly higher levels of Amax, CE and gm than ‘Brilliant’. Maximum carboxylation rate of Rubisco (Vcmax) and ribulose-1,5-bisphospate (RuBP) regeneration capacity mediated by maximum electron transport rate (Jmax) decreased from moderate to severe drought stress in both genotypes, but to a greater extent in ‘Brilliant’ than in ‘Midnight’. After re-watering, RWC restored to about 90% of the control levels in both genotypes, whereas A, gs, Tr and Fv/Fm was only partially recovered, with a higher recovery level in ‘Midnight’ than in ‘Brilliant’. Rubisco activity and activation state restored to the control level after re-watering, with more rapid increase in ‘Midnight’ than in ‘Brilliant’. The values of Ls, Lns, CP and Rd declined, and Amax, CE, Vcmax, Jmax and gm increased after re-watering, with more rapid change in all parameters in ‘Midnight’ than in ‘Brilliant’. These results indicated that the maintenance of higher A and Amax under drought stress in drought-tolerant Kentucky bluegrass could be attributed to higher Rubico activation state, higher CE and less stomatal limitation. The ability to resume metabolic activity (Amax, CE, Fv/Fm and Rubisco) was observed in the drought-tolerant genotype and is the most likely cause for the increased recuperative ability of photosynthesis. Incomplete recovery of photosynthesis upon re-watering could be attributable to lasting stomatal limitations caused by severe drought damage in both genotypes. Promoting rapid stomatal recovery from drought stress may be critical for plants to resume full photosynthetic capacity in C3 perennial grass species.
Abbreviations-
-
- A
-
- net photosynthetic rate
-
- Amax
-
- maximum CO2 assimilation rate
-
- Ca
-
- ambient CO2 concentration
-
- Ci
-
- internal leaf CO2 concentration
-
- CE
-
- carboxylation efficiency
-
- CP
-
- CO2 compensation point
-
- DW
-
- dry weight
-
- FW
-
- fresh weight
-
- gm
-
- mesophyll conductance
-
- gs
-
- stomatal conductance
-
- Jmax
-
- the rate of ribulose 1,5-bisphosphate regeneration mediated by electron transport
-
- Lns
-
- non-stomatal limitation
-
- Ls
-
- stomatal limitation
-
- LSD
-
- least significant difference
-
- NADH
-
- nicotinamide-adenine dinucleotide
-
- Rd
-
- dark respiration
-
- RuBP
-
- ribulose 1,5-bisphosphate
-
- Rubisco
-
- ribulose 1,5-bisphosphate carboxylase/oxygenase
-
- SWC
-
- soil water content
-
- Tr
-
- transpiration rate
-
- TW
-
- turgid weight
-
- Vcmax
-
- maximum velocity of Rubisco for carboxylation
Introduction
Drought stress induces changes in various physiological and biochemical processes (Boyer 1982). Photosynthesis is one of the most sensitive processes to drought stress (Chaves 1991). The inhibitory effects of drought on photosynthesis can be associated with low CO2 availability caused by limitations of diffusion through the stomata and the mesophyll (Flexas et al. 2004) and/or the alterations of carbon assimilation metabolism (Lawlor 2002, Lawlor and Cornic 2002). Stomatal closure is an early response to drought and an efficient way to reduce water loss in water-limiting environment; however, it limits CO2 diffusion into the leaves for photosynthesis (Cornic 2000). In addition, mesophyll conductance (gm, the conductance for CO2 diffusion from the intercellular space to the chloroplast stroma) also plays an important role in limiting photosynthesis (Grassi and Magnani 2005). Loreto et al. (1992) demonstrated that gm is finite and, therefore, introduces another diffusive photosynthetic limitation in addition to stomatal conductance (gs). The sum of both stomatal and mesophyll limitations represents the diffusional limitations of a leaf. Inhibition of metabolic activities occurred with prolonged periods of drought stress (Parry et al. 2002). Biochemical or metabolic (non-stomatal) limitation of photosynthesis under drought stress has been associated with low carboxylation efficiency (CE), maximum velocity for carboxylation of Rubisco (Vcmax), ribulose-1,5-bisphospate (RuBP) regeneration capacity mediated by maximum electron transport rate (Jmax), activity of PSII and Rubisco activity (Jia and Gray 2004, Pena-Rojas et al. 2004). Effects of water stress on the activity of Rubisco varies with plant species and the level of drought stress, ranging from a dramatic reduction in Rubisco activity (Parry et al. 2002) to little or no inhibition of the enzyme (Pankovic et al. 1999, Pelloux et al. 2001). Others studies have demonstrated that Rubisco did not limit photosynthesis until severe drought stress or long-term water stress was encountered (Flexas 2006a). The perturbation of the biochemical processes involving the PSII reaction centers also impose limitations to photosynthesis under drought stress (Giardi et al. 1996, Krause 1988). Analysis of A/Ci curves—the response of net photosynthesis (A) to varying intercellular CO2 concentration (Ci)—has been used to distinguish the stomatal and non-stomatal limitations to photosynthesis under water stress (Flexas et al. 2004, Lawlor 2002). As maximal CO2 assimilation rate (Amax) reflexes the result of the mesophyllic impairments, its determination allows for the evaluation of non-stomatal limitations and hence the degrees of drought resistance of the photosynthetic machinery.
For perennial plant species, one of the most important drought survival strategies is the ability to recover rapidly from the stress when water again becomes available (Moreira et al. 1990). Selecting traits for high recuperative ability may be of more economic importance than selecting for improved growth during drought in turfgrasses (Norris and Thomas 1982). Recovery in perennial grasses from drought stress has been attributed to various morphological, physiological and biochemical factors, including maintenance of membrane stability (Jiang and Huang 2001), osmotic adjustment (Elmi and West 1995), phytohormone accumulation (Wang et al. 2003), delayed leaf rolling during stress (Huang and Wang 2005) and increased carbon partitioning and carbohydrate storage in leaf bases and stems (DaCosta and Huang 2006, Volaire et al. 1998). Maintenance of high photochemical efficiency and chlorophyll content during drought stress has also been associated with better recovery in shoot growth upon re-watering (Abraham et al. 2004, Huang et al. 1998). The ability to maintain photosynthesis under water stress is no doubt of major importance for plant survival of drought stress (Zlatev and Yordanov 2004), and may be critical for plant recovery following drought upon re-watering (Kaiser 1987). Although many studies have addressed different aspects of photosynthetic limitations during the period of drought stress, analyses of changes in stomatal and non-stomatal factors for photosynthetic recovery after water stress are limited (Flexas et al. 2006b). Little is known about physiological characteristics of recovery from drought stress and how different photosynthetic components (stomatal and metabolic) are involved in photosynthetic response to re-watering or what are the main limiting factors for recovery during re-watering in perennial grass species. Such information is critical for further understanding adaptation and survival mechanisms and developing drought-resistant grass germplasm for environments with limited water resources.
The objectives of this study were to examine the relative responses of stomatal and non-stomatal components of photosynthesis to drought stress and re-watering, and to determine major factors regulating photosynthesis during drought and recovery upon re-watering in a C3 perennial grass species. Specific aims of the study are to evaluate the extent to which photosynthesis can recover from drought damage, the factors controlling photosynthesis recovery upon re-watering, and whether there is genetic variation between cultivars of Kentucky bluegrass. Kentucky bluegrass is a widely used C3 turf and forage grass species that has a broad range of genetic variation in drought tolerance (Bonos and Murphy 1999, Ebdon and Petrovic 1998, Wang et al. 2003, Wang et al. 2004). In this study, two genotypes contrasting in drought tolerance were evaluated, which were drought-tolerant ‘Midnight’ and drought-sensitive ‘Brilliant’ (Wang et al. 2003, Wang et al. 2004). Single-leaf A and the stomatal factors (gs and transpiration) and non-stomatal components (photochemical efficiency, Rubisco activity, Rubisco activation state) were examined during drought and re-watering. The relative limitation of stomatal and non-stomatal components during drought and recovery was also determined, and Vcmax, Jmax, gm and Rd were estimated with non-linear regression technique based on the biochemical model described by Farquhar et al. (1980) through A/Ci curve response analysis.
Materials and methods
Plant material and growth conditions
Plants of ‘Midnight’ and ‘Brilliant’ were collected from 3-year-old field plots at the turfgrass farm, Rutgers University, Adelphia, NJ. Grass sods with roots being trimmed off (10 cm in diameter) were planted in plastic pots (10 cm in diameter and 40-cm deep) filled with a mixture of sand and topsoil (fine, montmorillonitic, mesic, aquic arqui-dolls) (1:3, v/v). Plants were maintained in a greenhouse at Rutgers University for 50 days to allow establishment of canopy and roots. Controlled-release fertilizer (16N-4P-8K) was top-dressed to provide a total amount of 20 g N m−2 during the study period. After 50-day establishment in the greenhouse, plants were transferred to a growth chamber with a temperature regime of 23/18°C (day/night), a 12-h photoperiod, 65% relative humidity and a photosynthetically active radiation of 600 µmol m−2 s−1 at the canopy level. Plants were grown in well-watered conditions (watered three times per week until soil water reached field capacity or drainage occurred from the bottom of the pot). Plants were acclimated to growth chamber conditions for 2 weeks before treatments were imposed.
Treatments and experimental design
The experiment consisted of well-watered control, drought stress and re-watering treatments. For well-watered control, plants were watered every other day to completely saturate the soil in the pot (until water drained from the bottom of the pot). Drought stress was imposed by withholding irrigation for 15 days. At the 15th day of drought, drought-stressed plants were re-watered to soil reaching field capacity for the examination of recovery. Each treatment for each genotype had four pots, which were randomly placed in four growth chambers (four replicates). Treatments and grass genotypes were arranged as a completely randomized block design.
Evaluation of soil and leaf water status
Soil volumetric water content (SWC) and leaf relative water content (RWC) of fully expanded leaves were measured to evaluate soil water availability and plant water status, respectively. The level of SWC in a 0–20 cm soil layer of each pot was monitored by time domain reflectometry (Soil Moisture Equipment Corp., Santa Barbara, CA). Leaf RWC was determined using 10–15 whole, fully expanded leaves from each pot according to Barrs and Weatherley's (1962) method. Leaf samples were detached from plants and immediately weighed to determine fresh weight (FW). Samples were placed into covered petri dishes filled with water for leaves to reach full hydration. After approximately 24 h at 4°C, leaf samples were blotted dry with paper towels and then weighed immediately to determine turgid weight (TW). Leaf tissue was then dried in an oven at 80°C for 48 h to determine dry weight (DW). Leaf RWC was calculated as (FW—DW)/(TW—DW) × 100.
Analysis of photosynthesis and stomatal and non-stomatal components
A, gs and transpiration rate (Tr) were measured with 10 whole, fully expanded leaves (second from the top) from each pot. The A/Ci response curves for changes in A with leaf internal CO2 (Ci) were generated using a portable infrared gas analyzer (Li-6400, LICOR, Inc, Lincoln, NB) following the method described in Manter and Kerrigan (2004). The analyzer was set at 500 µmol s−1 flow rate (leaf temperature of 23 ± 0.4°C, 60 ± 5% relative humidity) and a light emitting diode external light source providing a photosynthetic photon flux density of 800 µmol m−2 s−1. The A/Ci curves were constructed (6–8 min for each data point) as the A responses to varying ambient CO2 concentration in the cuvette (Ca). Measurements started at Ca of 400 µmol mol−1, and then CO2 in the cuvette was decreased to 50 µmol mol−1. Subsequently, Ca was progressively increased up to 1000 µmol mol−1 using a CO2 mass flow controller. Each datum of A was recorded at a given Ca after equilibration to a steady state (coefficient of variation <1%). Generally, 10 data points were used for the construction of an A/Ci curve.
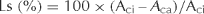 (1)
(1)A/Ci curves were analyzed using Photosyn Assistant (Dundee Scientific, Dundee, Scotland, UK), which uses the biochemical model described by Farquhar et al. (1980), and subsequently modified by von Caemmerer and Farquhar (1981), Sharkey (1985), Harley and Sharkey (1991) and Harley et al. (1992). The model estimated the potential biochemical limitations to photosynthesis: maximum Rubisco carboxylation rate (Vcmax), maximum rate of ribulose 1,5-bisphosphate regeneration mediated by electron transport (Jmax), leaf dark respiration in light (Rd) and gm. A non-linear regression technique was used to estimate Vcmax, Jmax, Rd and gm, and values were standardized to the leaf temperature of 25°C as suggested by Sharkey et al. (2007).
Leaf photochemical efficiency (maximum quantum efficiency of photosystem II) was estimated by measuring the ratio of variable to maximum fluorescence of chlorophyll (Fv/Fm) with a chlorophyll fluorometer (OS1-FL, OPTI-SCIENCES, Hudson, NH). Measurements were made on intact leaves with the fluorometer after plants were adapted in darkness for 30 min.
Rubisco extraction and activity measurements were performed using a method described by Cheng and Fuchigami (2000) with modifications. Briefly, 0.2 g of leaves were taken from each pot at 11:00–12:00 am and immediately frozen in liquid nitrogen and kept at −80°C before use. For extraction, ground samples were placed in an ice-cold test tube with 3 ml extraction buffer [50 mM Hepes–KOH (pH 7.5 at 25°C), 10 mM MgCl2, 2 mM EDTA, 10 mM DTT, 10% glycerol (v/v), 1% BSA (w/v), 1% Triton X-100 (v/v) and 1.5% insoluble polyvinyl polypyrrolidone (w/v)]. Samples were homogenized for 30 s and then centrifuged at 14 000 g for 10 min at 4°C. The supernatant was used immediately for Rubisco activity assay.
Rubisco activity was measured at 25°C by enzymatically coupling ribulose 1,5-bisphosphate (RuBP) carboxylation to nicotinamide-adenine dinucleotide (NADH) oxidation (Lilley and Walker 1974). NADH oxidation was monitored at 340 nm using a spectrophotometer (Helios Alpha, Thermospectronic, Rochester, NY). For initial Rubisco activity, a 50 µl sample extract was added to a semi-microcuvette containing 900 µl of assay solution, immediately followed by adding 50 µl 10 mM RuBP, and then mixed well. The absorbance at 340 nm was recorded every 6 s for 1 min. For total Rubisco activity, the sample extract was added to the assay solution and incubated at 25°C for 15 min to allow activation of all Rubisco, and then 50 µl 10 mM RuBP was added and mixed well. The assay solution for both initial and total Rubisco activity measurements contained 100 mM bicine (pH 8.0 at 25°C), 25 mM KHCO3, 20 mM MgCl2, 3.5 mM ATP, 5 mM phosphocreatine, 5 units glyceraldehyde-3-phosphate dehydrogenase, 5 units 3-phosphoglyceric phosphokinase, 17.5 units creatine phosphokinase and 0.25 mM NADH. Rubisco activity was calculated based on the following formula (Cheng and Fuchigami 2000):
Activity (µmol s−1 g−1 FW) = (A340/min) × (extraction volume/sample volume) × (0.00134 µmol s−1) × (1/FW). Rubisco activation state was calculated as the ratio of initial activity to total activity.
Statistical analysis
All data were subjected to analysis of variance (SAS 9.0, SAS Institute Inc., Cary, NC) to determine treatment effects and genotypic variations in SWC, RWC, Fv/Fm ratio, gs, A, Tr, Rubisco activity and activation state, Ls, Lns, Amax, CP, CE, Vcmax, Jmax, Rd and gm. Treatment means and genotype differences were separated using the least significant difference (LSD) test at P = 0.05.
Results
Soil and plant water status
At the initiation of drought treatment, SWC was at the field capacity (about 30%) (Fig. 1). SWC declined to about 10, 5 and 4% at 5, 10 and 15 days of drought, respectively. Upon re-watering, SWC returned to the field capacity level. No significant differences in SWC were detected between pots in two genotypes under well-watered, drought or re-watering conditions.
Effects of drought stress on SWC in ‘Midnight’ and ‘Brilliant’. Vertical bars on the top indicate LSD values (P = 0.05) for the comparison of two genotypes at a given day of treatment.
Well-watered plants maintained about 95% of leaf RWC during 25 days of the experimental period in both genotypes (Fig. 2). Drought-stressed plants of both genotypes showed a progressive decrease in RWC during the 15-day treatment period (Fig. 2). No significant difference in RWC was observed at 5 days of treatment between well-watered plants and drought-stressed ones. At 10 and 15 days, RWC in ‘Midnight’ decreased to 87 and 53%, and RWC in ‘Brilliant’ decreased to 74 and 39%, respectively. ‘Midnight’ had significantly (13%) higher RWC than ‘Brilliant’ at 10 and 15 days of drought stress. Leaf RWC of drought-stressed plants in both genotypes restored to the prestress level (0 day of drought) or the well-watered control level at 2 and 10 days of re-watering.

Effects of drought stress on leaf RWC in ‘Midnight’ and ‘Brilliant’. Vertical bars on the top indicate LSD values (P = 0.05) for the comparison of two genotypes at a given day of treatment.
Changes in leaf gas exchange characteristics and photochemical efficiency (Fv/Fm) under drought stress and re-watering
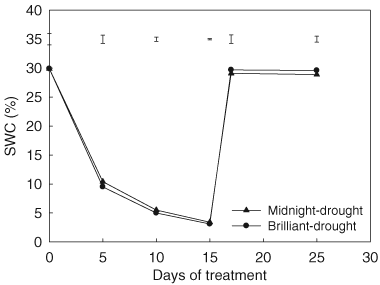
Drought stress led to a significant decline in A, gs and Tr for both genotypes, beginning at 5 days of treatment, compared with their respective well-watered controls (Fig. 3). Leaf A of ‘Midnight’ were 75, 46 and 22% of the control level at 5, 10 and 15 days of drought stress, respectively; whereas in ‘Brilliant’, the corresponding figures were 72, 29 and 6%, respectively (Fig. 3A). Leaf gs of ‘Midnight’ decreased to 48, 25 and 13% of the control level at 5, 10 and 15 days of drought stress, respectively. For ‘Brilliant’, gs was 46, 13 and 0.8% of the control level at 5, 10 and 15 days, respectively (Fig. 3B). Leaf Tr of ‘Midnight’ decreased to 51, 31 and 22% of the control level at 5, 10 and 15 days of drought stress, respectively, whereas in ‘Brilliant’, the corresponding figures were 49, 16 and 4%, respectively (Fig. 3C). ‘Midnight’ maintained significantly higher A, gs and Tr than ‘Brilliant’ at 10 and 15 days of drought stress.
Effects of drought stress on gas exchange in ‘Midnight’ and ‘Brilliant’ (A, A; B, gs; C, Tr). Vertical bars on the top indicate LSD values (P = 0.05) for the comparison of two genotypes at a given day of treatment.
After re-watering, leaf gas exchange parameters recovered gradually, but the extent of recovery varied between the two genotypes. Leaf A was restored to 49 and 81% of the control level at 2 and 10 days of re-watering in ‘Midnight’; whereas for ‘Brilliant’, A was restored to 27 and 64% of the control value at 2 and 10 days of re-watering, respectively (Fig. 3A). Leaf gs restored to 30 and 77% of the control level for ‘Midnight’ at 2 and 10 days of re-watering and the corresponding figures were 17 and 50% for ‘Brilliant’ (Fig. 3B). The Tr restored to 38 and 83% of the control level at 2 and 10 days of re-watering in ‘Midnight’, but only restored to 16 and 53% of the control level in ‘Brilliant’(Fig. 3C). Midnight had significantly higher A, gs and Tr than Brilliant at both 2 and 10 days of re-watering.
Leaf Fv/Fm in well-watered plants maintained above 0.79 throughout the experiment in both genotypes (Fig. 4). No reduction was observed at 5 days of drought stress in both genotypes. The decline in Fv/Fm was observed in ‘Brilliant’ at 10 days of drought, and not until 15 days in ‘Midnight’. Fv/Fm was 98 and 72% of the control level at 10 and 15 days of drought in ‘Midnight’, respectively; the corresponding figures were 84 and 48% of the control value in ‘Brilliant’. Fv/Fm recovered to 73 and 92% of the control plants at 2 and 10 days in ‘Midnight’, respectively, and 53 and 66% of the control level in ‘Brilliant’. Fv/Fm values of re-watered plants in ‘Midnight’ were significantly higher than those in ‘Brilliant’ at both 2 and 10 days of re-watering.

Effects of drought stress on photochemical efficiency in ‘Midnight’ and ‘Brilliant’. Vertical bars on the top indicate LSD values (P = 0.05) for the comparison of two genotypes at a given day of treatment.
Changes in Rubisco enzyme under drought stress and re-watering
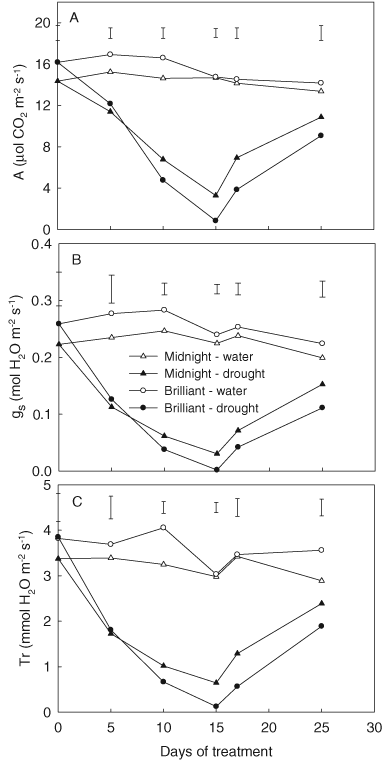
During the entire experimental period, no significant differences were observed for both Rubisco activity and activation state in well-watered plants between ‘Midnight’ and ‘Brilliant’ (Fig. 5A, B). During 15 days of drought stress, Rubisco activity and activation state of Rubisco in ‘Midnight’ did not exhibit significant decline while that for ‘Brilliant’ decreased to a significant lower level than the control at 10 and 15 days of drought (Fig. 5A, B). ‘Midnight’ had 15 and 28% higher Rubisco activity, and 10 and 22% higher activation state than that of ‘Brilliant’ at 10 and 15 days of drought, respectively.
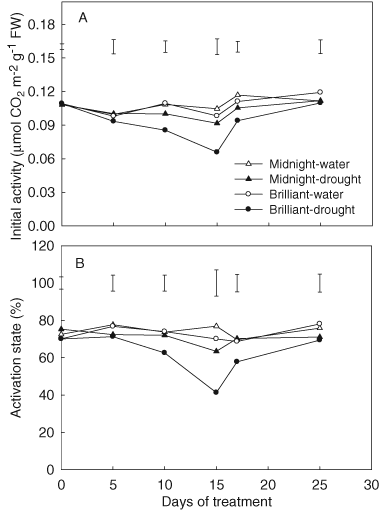
Effects of drought stress on Rubisco activity (A) and activation state (B) in ‘Midnight’ and ‘Brilliant’. Vertical bars on the top indicate LSD values (P = 0.05) for the comparison of two genotypes at a given day of treatment.
Rubisco activity and activation rate of Rubisco increased to the control level after 2 and 10 days of re-watering for ‘Midnight’ (Fig. 5A, B). For ‘Brilliant’, there was still 15% lower Rubisco activity and 12% lower activation state than the control at 2 days of re-watering, which increased to the control level at 10 days of re-watering.
Analysis of response of CO2 assimilation to intercellular CO2 concentration under drought stress and recovery
Changes in A as a function of the intercellular CO2 concentration (Ci) were used to determine stomatal limitations (Ls) and non-stomatal limitation (Lns) under drought stress and during re-watering (Fig. 6). CP, which was the Ci value at which A equaled zero, was determined from the A/Ci curve. The initial slope of the A/Ci curves was calculated as an estimate of CE. The Amax was the highest level of A from the A/Ci curve at saturated light and CO2. The data in all these parameters during the 15-day drought stress and 2 and 10 days of re-watering are presented in Table 1.
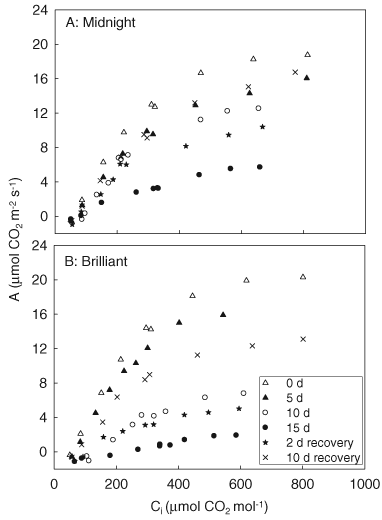
Responses of A vs intercellular leaf CO2 concentration (Ci) (A/Ci curves) under drought stress and recovery in ‘Midnight’ (A) and ‘Brilliant’ (B). Each data point represents the average of four replications. Measurements were made at a photosynthetic photon flux density of 800 µmol m−2 s−1, a leaf temperature of 23 ± 0.4°C, and 60 ± 5% relative humidity.
| Parameters | Treatment | Drought | Re-watering | ||||
|---|---|---|---|---|---|---|---|
| Days | 0 | 5 | 10 | 15 | 2 | 10 | |
| Amax | Midnight | 18.8 a | 16.1 b | 13.5 c | 5.7 d | 10.4 c | 16.8 ab |
| (µmol m−2 s−1) | Brilliant | 20.3 a | 15.9 b | 6.8 c * | 1.9 d * | 5.0 c * | 13.1 b * |
| Ls (%) | Midnight | 13.7 e | 16.7 de | 43.4 b | 59.9 a | 30.1 c | 22.3 d |
| Brilliant | 16.2 e | 29.5 c * | 58.7 b * | 86.1 a * | 52.3 b * | 27.4 d | |
| Lns (%) | Midnight | 0 | 14.4 d | 27.9 c | 69.6 a | 44.6 b | 10.7 d |
| Brilliant | 0 | 21.7 e | 66.6 c * | 90.6 a * | 75.3 b * | 35.4 d * | |
| CP (µmol mol−1) | Midnight | 58.4 d | 69 c | 83 b | 90 a | 85.2 ab | 64.3 cd |
| Brilliant | 56.6 d | 67 d | 134.8 b * | 180.3 a * | 98.1 c * | 70.3 d | |
| CE (µmol m−2 s−1) | Midnight | 0.02441 a | 0.02087 b | 0.02100 b | 0.01005 d | 0.01510 c | 0.02312 ab |
| Brilliant | 0.02670 a | 0.02457 a | 0.01481 c * | 0.00611 e * | 0.01128 d * | 0.01648 b * | |
Net photosynthetic rate increased with increasing concentrations of CO2 in both genotypes under well-watered conditions (0 day) or under drought stress (Fig. 6). The values of Amax decreased with drought stress at 10 and 15 d in both genotypes (Fig. 6 and Table 1). Drought-induced reduction in Amax was more dramatic in ‘Brilliant’ than in ‘Midnight’ (Table 1). The Amax was 86, 72 and 30% of the prestress level at 5, 10 and 15 days of drought in ‘Midnight’, respectively; whereas the corresponding figures were only 78, 33 and 9% in ‘Brilliant’. ‘Midnight’ maintained a significant higher Amax than ‘Brilliant’ at 10 and 15 days of treatment. The Amax value increased to 55% of the prestress level at 2 days of re-watering in ‘Midnight’, but only increased to 25% of the prestress level in ‘Brilliant’ (Table 1). ‘Midnight’ maintained significantly higher Amax than ‘Brilliant’ at 2 days of re-watering. The Amax value following 10 days of re-watering increased significantly compared with the level at 15 days of drought stress, but was still significantly lower than the prestress level for both genotypes (89% of the prestress level in ‘Midnight’ and 65% of the prestress level in ‘Brilliant’).
No significant difference was observed in Ls between ‘Midnight’ (13.7%) and ‘Brilliant’ (16.2%) under well-watered conditions (0 day) (Table 1). Leaf Ls increased with the progression of drought stress in both genotypes, but more dramatic increases occurred in ‘Brilliant’ than in ‘Midnight’. Leaf Ls in ‘Brilliant’ was 13, 15 and 26% higher than ‘Midnight’ at 5, 10 and 15 days of drought, respectively. Leaf Lns also increased with drought duration in both genotypes, and was significantly higher in ‘Brilliant’ than in ‘Midnight’ during 15 days of drought (Table 1).
The CP value under well-watered conditions was 57 µmol CO2 mol−1 for both genotypes (Table 1). Drought stress led to a significant increase in CP in both genotypes, but to a greater extent for ‘Brilliant’. CP increased by 18, 42 and 54% at 5, 10 and 15 days of drought in ‘Midnight’, compared with the prestress level (0 day); whereas in ‘Brilliant’, the corresponding figures were 18, 138 and 219%, respectively. The CE did not show variation between the two genotypes under well-watered conditions (0 day) (Table 1). Significant differences were observed on 10 and 15 days of drought treatment in both genotypes. ‘Midnight’ had 29 and 39% higher CE than ‘Brilliant’ at 10 and 15 days of drought, respectively.
After 2 days of re-watering, Ls, Lns and CP in both ‘Midnight’ and ‘Brilliant’ declined to a significantly lower level than the value of drought-stressed plants at 15 days, but was still significantly higher than the value measured during prestress conditions (Table 1). The CE value for ‘Midnight’ increased to 62% of the prestress level, whereas for ‘Brilliant’, CE was only 42% of the prestress level at 2 days of re-watering. ‘Midnight’ maintained significantly higher CE, and lower Ls, Lns and CP than ‘Brilliant’ at 2 days of re-watering.
Following 10 days of re-watering, Ls and Lns in both genotypes decreased to significantly lower levels than that at 15 days of drought, but still higher than their respective prestress levels (Table 1). The CP levels at 10 days of re-watering significantly decreased compared with the 15-day drought level, reaching to the respective prestress levels in both genotypes. The CE in 10-day re-watered plants increased to significantly higher levels than the 15-day drought-stressed plants in both genotypes, and to the prestress level in ‘Midnight’ and 62% of the prestress level in ‘Brilliant’ (Table 1). The CE for ‘Midnight’ (0.02312 µmol m−2 s−1) was significantly higher than that of ‘Brilliant’ (0.01648 µmol m−2 s−1) at 10 days of re-watering.
The values of Vcmax (maximum carboxylation rate of Rubisco), Jmax (maximum electron transport rate), leaf Rd and gm were estimated by fitting the Farquhar's model using non-linear regression techniques to the Rubisco limited portion of the A/Ci curves at low values of Ci (< 200 µmol mol−1) and to the RuBP regeneration limited one at higher level of Ci(> 300 µmol mol−1), respectively. No significant decline or even an increase was observed in both genotypes for Vcmax and Jmax at 5 days of drought (Table 2). Vcmax and Jmax declined beginning at 10 days of drought in both genotypes, with more rapid decrease in ‘Midnight’ than in ‘Brilliant’. Vcmax decreased to 80 and 34% of the prestress level in ‘Midnight’ at 10 and 15 days of drought, whereas the corresponding figures were 39 and 18% in ‘Brilliant’. For Jmax, the values decreased to 87 and 25% of the prestress level in ‘Midnight’ at 10 and 15 days of drought; for ‘Brilliant’, the value decreased to 59 and 11% of the prestress level at 10 and 15 days of drought. Leaf Rd increased with drought progression. Leaf Rd increased by 37, 75 and 123% at 5, 10 and 15 days of drought in ‘Midnight’, compared with the prestress level (0 day); whereas in ‘Brilliant’, the corresponding figures were 59, 115 and 169%, respectively. Drought also induced a significant decrease in gm in both genotypes, with more rapid decline in ‘Brilliant’ than in ‘Midnight’. The gm decreased to 79, 75 and 16% of the prestress level at 5, 10 and 15 of drought in ‘Midnight’. For ‘Brilliant’, the gm decreased to 65, 31 and 5% of the prestress level at 5, 10 and 15 days of drought, respectively. ‘Midnight’ maintained a significantly higher Vcmax, Jmax and gm than ‘Brilliant’ at moderate to severe drought stress (10 and 15 days).
| Parameters | Treatment | Drought | Re-watering | ||||
|---|---|---|---|---|---|---|---|
| Days | 0 | 5 | 10 | 15 | 2 | 10 | |
| Vcmax | Midnight | 68.6 ab | 81.8 a | 54.8 b | 23.1 c | 59.7 b | 86.9 a |
| (µmol m−2 s−1) | Brilliant | 69.9 b | 86.3 a | 27.0 d * | 12.8 e * | 23.8 d * | 48.2 c * |
| Jmax | Midnight | 100.3 a | 90.6 ab | 87.8 b | 25.2 d | 74.1 c | 107.5 a |
| (µmol m−2 s−1) | Brilliant | 110.1 a | 110.1 a | 64.8 b * | 12.1 d * | 35.3 c * | 78.7 b * |
| Rd | Midnight | 2.69 d | 3.67 c | 4.71 b | 5.99 a | 3.19 c | 2.24 d |
| (µmol m−2 s−1) | Brilliant | 2.77 d | 4.39 c | 5.96 b * | 7.44 a * | 4.89 c * | 2.90 d |
| gm | Midnight | 2.63 a | 2.09 ab | 1.98 b | 0.41 d | 1.20 c | 1.94 b |
| (µmol m−2 s−1 Pa−1) | Brilliant | 2.97 a | 1.93 b | 0.91 d * | 0.14 e * | 0.90 d * | 1.44 *c |
Upon re-watering, Vcmax, Jmax and gm increased to a significant higher level and Rd decreased to a significant lower level compared with 15 d of drought in both genotypes (Table 2). The Vcmax and Jmax value increased to 87 and 74% of the prestress level in ‘Midnight’ at 2 days of re-watering, and restored completely to the prestress level at 15 days of re-watering. For ‘Brilliant’, the Vcmax and Jmax value was only restored to 34 and 32% of the prestress level at 2 days of re-watering, and 69 and 71% of the prestress level at 10 days of re-watering. The gm value increased after re-watering in both genotypes, compared with 15 days of drought, but still significantly lower than the prestress level. The Rd value decreased after re-watering in both genotypes compared with 15 days of drought, and completely restored to the prestress level at 10 days of re-watering. ‘Midnight’ remained a significantly higher Vcmax, Jmax and gm during 10 days of re-watering.
Discussion
Photosynthetic responses to drought stress: stomatal and metabolic limitations
The analysis of A/Ci curves demonstrated that the decline in A in both genotypes during drought stress was associated with both stomatal limitations (Ls) and non-stomatal limitations (Lns). However, the relative contribution of Ls and Lns to A varied with drought stress duration and genotypes. For both genotypes, both Ls and Lns increased with stress duration, but Ls was higher than Lns at 5 days of drought and Lns became higher than Ls after 15 days of drought (Table 1). These results suggested that Ls was more important for A during early phase of drought, whereas Lns became more limiting for A following prolonged periods of stress in Kentucky bluegrass. This pattern of response is consistent with that described by other authors under controlled conditions (Bota et al. 2004). Previous studies also reported increases in Ls and Lns with the severity of water stress, and Ls was found to be higher (Escalona et al. 1999, Gulias et al. 2002) or lower (Lawlor 2002, Tezara et al. 2003) than Lns, depending on stress severity and plant species. A comparison between the two genotypes shows that ‘Midnight’ had significantly lower Ls from 5 to 15 days of drought and lower Lns from 10 to 15 days, suggesting that the drought-tolerant ‘Midnight’ was able to maintain higher net photosynthesis and photosynthetic capacity by lowering both stomatal and non-stomatal limitations.
Our measurements of various photosynthetic components supported the results derived from the A/Ci curves. Under short-term (5 days) drought stress when leaf RWC was 74–87% (moderate stress), the decline in A was accompanied by dramatic decreases in gs and Tr (Fig. 3) while Fv/Fm remained unaffected in both genotypes (Fig. 4), and other photosynthetic components (Rubisco activity and activation state, Amax, CE) and photosynthetic capacity (Vcmax and Jmax) exhibited no change in ‘Midnight’ or some reduction in ‘Brilliant’. The limitations to CO2 assimilation imposed by stomatal closure may promote an imbalance between photochemical activity at PSII and electron requirement for photosynthesis, leading to an overexcitation and subsequent damage of PSII reaction centers caused by photoinhibition (Krause 1988). In this way, damage to the photosynthetic machinery may eventually occur, imposing an additional non-stomatal limitation to the process. Investigations based on assessments of chlorophyll fluorescence have shown that PSII is resistant to water deficit, being either unaffected (Shangguan et al. 2000) or affected only under severe drought conditions (Saccardy et al. 1998). Fv/Fm reflects the maximal efficiency of excitation energy capture by ‘open’ PSII reaction centers. A decrease in this parameter indicated down-regulation of photosynthesis or photoinhibition during drought stress (Souza et al. 2004). The results of our study, based on chlorophyll fluorescence measurements, showed that photochemical activity did not decline until more severe water stress (RWC of 40–50%) or a prolonged period of drought (15 days), and ‘Midnight’ had significantly higher Fv/Fm than ‘Brilliant’. These results indicated that photochemical efficiency was relatively less responsive to drought than stomatal components in Kentucky bluegrass. Thus, the early inhibition of photosynthesis by drought stress in Kentucky bluegrass could be induced by stomatal closure, but not be associated with the damage of PSII at this stage. If stomatal limitation is exclusively involved in the inhibition of A, exposure to high CO2 concentrations should be effective in restoring A in drought-stressed plants to values comparable to the control, as increased ambient CO2 concentrations can increase the leaf internal CO2 concentrations (He et al. 1992). In our experiment, as RWC declined from 95–87% in ‘Midnight’ and from 96% to 92% in ‘Brilliant’, it appeared that stomatal limitation was mainly responsible for the inhibition of A, as considerable increases in A were observed with increasing CO2 concentrations under drought stress. The decline in photosynthetic capacity (Vcmax, Jmax) in response to drought has been observed in many other plant species (Flexas et al. 2004, Wilson et al. 2000, Xu and Baldocchi 2003). Decreases in Amax and CE at moderate to severe drought stress (Table 1), which were accompanied by reductions in Vcmax and Jmax (Table 2), indicate a down-regulation of CO2 assimilation to adjust mesophyll capacity to the decreased CO2 supply (Chaves et al. 2002, Lawlor and Cornic 2002) caused by stomatal closure. Decreases in Vcmax may result from reductions in the amount of active Rubisco (Pena-Rojas et al. 2004). The reduction in Jmax is associated with limited RuBP regeneration, which may be due to inadequate ATP or NADPH supply, or low enzymatic activity of the PCR cycle such as sedoheptulose- 1,7-bisphosphatase and fructose-1,6-bisphosphatase (Flexas et al. 2004, Lawlor 2002, Parry et al. 2002).
With prolonged periods of drought or more severe drought when RWC dropped to below 87% in ‘Midnight’ and 92% in ‘Brilliant’, metabolic limitation could become more important, as A exhibited slow increases with increasing CO2 concentrations. Prolonged water limitation (10 and 15 days) causing severe drought (RWC of 40–50%) caused a reduction in Amax and CE, and an increase in CP in both genotypes (Table 1), suggesting that there are increased effects of non-stomatal limitations on photosynthesis during severe drought. The CP increased in both genotypes, and the CP values were significantly higher in ‘Brilliant’ than in ‘Midnight’. This increase in the CP induced by water stress was mainly due to the decrease in assimilation capacity, and the contribution of non-stomata1 factors on the decrease in assimilation capacity was larger than that of stomatal limitation (Ishida et al. 2000). High CP has been associated with high respiration, which results in loss of carbon fixed through photosynthesis. Our results indicated that drought-stressed plants, particularly drought-sensitive plants had a higher Rd, which may require more CO2 for photosynthesis to maintain positive carbon gain. The inhibition in photosynthesis under drought stress has also been associated with reduced activities of enzymes involved in carboxylation such as Rubisco (Parry et al. 2002). Some studies demonstrated that Rubisco limitation in photosynthesis did not occur until severe drought stress or long-term water stress occurred (Flexas et al. 2006a, Hu et al. 2009). In vitro Rubisco activity and activation state declined with drought stress only in drought-sensitive ‘Brilliant’, while Rubisco activity in ‘Midnight’ was significantly higher than in ‘Brilliant’. These results indicated that photosynthesis limitations under prolonged or severe drought stress were associated with the lower Rubisco activity and activation state in drought-sensitive Kentucky bluegrass genotype. Similar results have been reported in bermudagrass cultivars contrasting in drought tolerance (Hu et al. 2009).
Various mechanisms have been associated with Kentucky bluegrass adaption to drought stress. Some studies suggested that drought resistance in Kentucky bluegrass genotypes was associated with lower canopy temperature, deeper root system and higher mass production, and lower stomatal resistance (Bonos and Murphy 1999). Others have demonstrated hormonal responses to drought resistance in Kentucky bluegrass and found a negative association of drought tolerance to ABA accumulation (Wang et al. 2003, Wang et al. 2004, Wang and Huang 2004). Our results showed that the superior drought resistance in ‘Midnight’ Kentucky bluegrass could be related to the maintenance of higher photosynthetic capacity and superior photosynthetic activity, as manifested by the higher photochemical efficiency of PSII, in vitro Rubisco activity and activation state, in vivo CE and RuBP regeneration capacity. The varying degrees of decline in these photosynthetic components in the two genotypes may contribute to the variations in photosynthesis under drought stress and during recovery in Kentucky bluegrass. In addition, ‘Midnight’ maintained higher RWC and higher Tr without depleting the soil water more than ‘Brilliant’ during drought treatment, which could be due to a smaller leaf area such that they compensated for the higher Tr per unit leaf area and/or a greater ability to transfer water from the soil to the shoot, e.g. by increased root hydraulic conductivity. However, how these factors play roles in drought-resistant in Kentucky bluegrass are not well understood, which deserve further investigation.
Recovery in stomatal and non-stomatal components during re-watering
Following 2 and 10 days of re-watering, SWC was brought back to field capacity and RWC recovered to the prestress or the well-watered control level in both genotypes, indicating that plants were fully rehydrated upon re-watering. Net photosynthetic rate of drought-tolerant ‘Midnight’ returned near the well-watered control level earlier than that for drought-sensitive ‘Brilliant’ after re-watering, which could reflect the less severe damage in photosynthesis systems during drought stress or less recovery required to return to non-stressed values. van Rensburg and Kruger (1993) used four different tobacco (Nicotiana tabacum L.) cultivars to show that the time required for full recovery of photosynthesis was strongly dependent upon the different water stress-tolerance capacities. At 2 and 10 days of re-watering, Ls and Lns values decreased significantly below the levels at 15 days of drought, which may be the result of stomatal opening and resumption of metabolic activities from rehydration. ‘Midnight’ had significantly lower Ls than ‘Brilliant’ at 2 days of re-watering and both Ls and Lns at 10 days of re-watering, suggesting a lowering stomatal restriction as an early response (2 days) to re-watering and better recovery in both stomatal and non-stomatal factors during later phase of re-watering (10 days). The drought-induced decrease in photosynthetic capacity (Vcmax, Jmax) was rapidly reversed following re-watering in drought-tolerant ‘Midnight’, indicating rapid recovery in Rubisco carboxylation and light saturated electron transport rates or less damages in both carbon fixation and light reaction of photosynthesis under drought stress in this genotype. Photochemical efficiency (Fv/Fm) only decreased after 10 days of drought and restored fast to the control level also supporting the notion that the photosynthetic machinery in leaves of drought-stressed ‘Midnight’ was maintained in a relatively highly photoprotected state under drought stress and during recovery.
The degree of recovery in different stomatal components (Ls, gs and Tr), non-stomatal components (Lns, Fv/Fm, Amax, CE and Rubisco) and photosynthetic capacity (Vcmax, Jmax) also varied between the two genotypes. None of the stomatal or non-stomatal parameters recovered fully in ‘Brilliant’ after re-watering, which suggests that 15 days of drought leading to severe water deficit (RWC of 40–50%) resulted in irreversible damage of the stomata and at the chloroplast level in PSII systems and carboxylation processes, and the incomplete recovery in both stomatal and non-stomatal factors limited recovery in photosynthesis upon re-watering in drought-sensitive ‘Brilliant’. Kirschbaum (1988) suggested that photosynthesis during re-watering was limited by both incomplete stomatal opening and metabolic components in Eucalyptus pauciflora. Ennahli and Earl (2005) reported that limited recovery of metabolic activities was the most important limitation for photosynthetic recovery in cotton (Gossypium hirsutum L.) plants subjected to severe water stress. Contrary to ‘Brilliant’, for ‘Midnight’ non-stomatal components and photosynthetic capacity (Vcmax, Jmax) recovered completely to the well-watered control level after 10 days of re-watering, but A, gs and Tr did not resume completely to the prestress or the well-watered control level. Thimmanaik et al. (2002) showed an almost complete recovery of several enzymes in the Calvin cycle only 2 days after re-watering. The incomplete recovery in photosynthesis in ‘Midnight’ appeared to be mainly related to remaining diffusion limitations (stomatal and mesophyll) after re-watering, as gm, Ls were still not recovered completely to the prestress or control level, even though Fv/Fm, Rubisco activity, Rubisco activation state and CE resumed to the prestress level. Some studies suggest that drought-induced stomatal occlusion of waxes may contribute to the remaining stomatal limitation after re-watering by blocking stomatal aperture (Gallé and Feller, 2007). Others found that incomplete return to full stomatal opening may be associated with the stomatal limitation of full recovery of photosynthesis after re-watering (Boyer 1971). Our results suggested that the stomatal limitations seemed to be irreversible upon re-watering after plants were exposed to drought stress for 15 days when RWC dropped to below 40–50% in Kentucky bluegrass. Nevertheless, the lack of full recovery in A, gs, gm and Tr after re-watering in both genotypes indicated drought-induced permanent damages in stomata in Kentucky bluegrass, restricting gas diffusion for photosynthesis, which could be due to stomatal closure and/or occlusion, and/or CO2 diffusion from the intercellular space to the site of carboxylation.
In summary, our results indicated that the maintenance of higher A, Amax under drought stress in drought-tolerant relative to drought-sensitive genotype in Kentucky bluegrass attributed to higher Rubisco activation state, higher CE and photosynthetic capacity, and lower stomatal limitations. The ability of resuming metabolic activities (Amax, CE, Fv/Fm and Rubisco) and photosynthetic capacity (Vcmax, Jmax) was associated with the recuperative ability in photosynthesis in Kentucky bluegrass following re-watering, particularly for the drought-tolerant genotype. Incomplete recovery of photosynthesis upon re-watering could be attributable to lasting diffusion limitations (stomatal and mesophyll) resulted from severe drought damages in both genotypes of Kentucky bluegrass. Promoting rapid stomatal and gm recovery from drought stress may be critical for plants to resume full photosynthetic capacity in C3 perennial grass species.
Acknowledgements
The authors thank Dr Chenping Xu, David Jespersen, Emily Merewitz and Yan Xu for comments and review of this manuscript. Thanks also go to Rutgers Center of Turfgrass Science for funding support of this study.




