Assessing the relative contributions of phytochelatins and the cell wall to cadmium resistance in white lupin
Abstract
In response to Cd stress, higher plants utilise a number of defence systems, such as retention in cell walls, binding by organic molecules in the cytosol and sequestration in the vacuole. White lupin is a Cd-resistant legume that is of interest for phytoremediation of acidified and Cd-contaminated soils. The aim of this research was to evaluate the contributions of various mechanisms of Cd detoxification used by this species, focusing on cell-wall retention and binding by thiol-rich compounds. Retention of Cd by the cell wall of white lupin was well described by a Langmuir isotherm model. The percentage of total Cd adsorbed by the cell wall ranged from 29 to 47% in leaves, from 38 to 51% in stems and from 26 to 42% in roots depending on the Cd supply. Cadmium induced the synthesis of high levels of phytochelatins (PCs) in lupin plants, mainly in roots, with PC3 being the major PC. The amount of Cd complexed by thiols accounted for approximately 20% of the total Cd in leaves, 40% in stems and 20% in roots. Therefore, cell-wall retention could account for more than twice the amount of Cd complexed by PCs in leaves and roots. In stems, both mechanisms contributed equally to Cd detoxification. These studies indicate that white lupin plants use cell-wall binding and, secondarily, the production of PCs, as effective mechanisms of Cd detoxification.
Abbreviations –
-
- CEC
-
- cation exchange capacity
-
- HPLC
-
- high-performance liquid chromatography
-
- ICP-MS
-
- inductively coupled argon plasma mass spectrometry
-
- GSH
-
- glutathione
-
- PCs
-
- phytochelatins
Introduction
Cadmium is a widespread heavy metal that is of importance because of its relatively high mobility in soils, and its acute and chronic toxicity to plants and animals. Cadmium that is available in soil is taken up by the plant by the same uptake mechanisms used for nutrients such as Zn. Therefore, the Cd concentration in the cytosol of plant cells must be tightly controlled to maintain the level of Cd below critical limits (Sanità di Toppi and Gabrielli 1999).
A first barrier against Cd stress, operating mainly at the root level, is retention of Cd in the cell wall (Nishizono et al. 1989). Several studies have described the key role of plant cell walls in reducing Cd accumulation in the protoplast (Al-asheh and Duvnjak 1999, Khan et al. 1984, Lozano-Rodríguez et al. 1997). Once Cd enters the cytosol, other detoxification mechanisms are induced, primarily the formation of complexes between Cd and phytochelatins (PCs) and their subsequent compartmentalization (Cobbett and Goldsbrough 2002).
PC synthase, the enzyme responsible for synthesis of PCs, requires a metal–glutathione (GSH) complex as one of its substrate (Vatamaniuk et al. 1999). PCs are therefore synthesized when the cytoplasmic concentration of heavy metal ions is able to provide sufficient levels of this substrate. In some cases, PCs can be detected in plant tissues prior to an increase in metal concentration and PC synthesis increases with exposure to higher levels of cadmium (Cobbett and Goldsbrough 2002). High correlations between shoot PC concentrations and the degree of Cd-induced growth inhibition in maize and wheat have been reported by Keltjens and van Beusichem (1998). Therefore, analysis of PCs in plants could be a useful biochemical indicator of Cd stress.
A large number of studies have demonstrated the critical role of PCs in Cd detoxification and tolerance of plants to Cd (Howden et al. 1995, Inouhe et al. 2000). In contrast, studies with plant species that exhibit unusual hypertolerance to Cd, such as Silene vulgaris and Thlaspi caerulescens indicate that PCs are not responsible for the observed metal tolerance phenotypes (De Knecht et al. 1992, Ebbs et al. 2002).
White lupin (Lupinus albus L.) possesses a strong root system and great capacity for mobilization and uptake of elements from soils. Moreover, white lupin has a high level of tolerance to many abiotic stress conditions including acidified (pH 4–7) soils (Huyghe 1997, Römer et al. 2000). Previous studies have shown that white lupin has good resistance to Cd toxicity (Carpena et al. 2003a, Zornoza et al. 2002). In addition, field experiments have demonstrated the potential use of this species for phytoremediation of heavy metals in low pH soils (Carpena et al. 2003b, Vázquez 2004).
The primary aim of this research was to study the retention of Cd by lupin cell walls. A secondary goal was to identify and quantify the accumulation of Cd-induced PCs in white lupin. These experiments focused on the relationship between PCs and Cd levels in roots, stems and leaves; and the potential use of PCs as a biochemical indicator for Cd stress. Finally, the relative contributions of cell walls and PCs to the overall detoxification of Cd by this species were also investigated.
Materials and methods
Plant material and experimental design
Two experiments were carried out to study Cd retention by cell walls and the synthesis of PCs under increasing Cd concentrations.
White lupin seeds (Lupinus albus L. cv. Marta) were surface sterilized and germinated on moist filter paper at 28°C in the dark for 3 days. Seedlings were transferred to a hydroponic culture system, where in the first experiment 14 plants were grown in each container with 3000 ml of nutrient solution; in the second experiment, five plants were grown in each container with 750 ml of nutrient solution. The composition of nutrient solution was as follows: 1.5 mM Ca(NO3)2, 1.5 mM KH2PO4, 1 mM MgSO4, 2 mM KNO3, 35.9 μM Fe-ethylendiamine-N,N′ bis(o-hydroxyphenyl) acetic acid, 32.8 μM MnSO4, 1.6 μM CuSO4, 1.6 μM ZnSO4, 46.2 μM HBO3, 1 μM (NH4)6Mo7O24, 1 μM NiCl2 and 1 μM CoCl2. Deionised water was used to prepare all solutions, which were continuously aerated with an aquarium air pump and changed weekly.
For the first experiment, lupin plants were grown in hydroponic culture in a growth chamber. Temperature and relative humidity range from 18 to 21°C and from 40 to 60%, respectively. Photon flux density was 520 μmol m−2 s−1 during the 11-h photoperiod. For the second experiment, lupin plants were grown in a greenhouse under the following conditions: temperature of 18–21°C, relative humidity between 80 and 100% and photosynthetic photon flux density of approximately 1300 μmol m−2 s−1 with a similar 11-h photoperiod.
After 7 days, Cd was added as 3CdSO4.8H2O. In the first experiment, the Cd concentrations used were 0, 0.2, 0.6, 2, 6, 20, 40 and 60 μM and plants were sampled after 15 days. In the second experiment, lupin plants were treated with 0, 2, 6, 18 and 54 μM Cd doses for 15 days. The Cd concentrations were selected based on previous experiments (Zornoza et al. 2002). Three replicates (containers) of each treatment were performed in a randomised block design. After sampling, plants were divided into leaves, stems and roots and weighed.
Stress indicators
Total leaf chlorophyll and total thiols were measured as indicators of Cd stress in the first experiment.
Total thiols were assayed using 100 mg fresh weight of frozen pulverised plant material with 0.4 ml of NaOH (1 M) containing NaBH4 (1 mg ml−1) and 0.2 ml of deionised water. After centrifugation (11 000 g, 10 min), 0.5 ml of supernatant was added to 0.5 ml of 5,5′-dithiobis (2-nitrobenzoic acid) (300 μM) dissolved in neutralizing buffer (0.5 M potassium phosphate, pH 7.2). Absorbance was measured at 412 nm (Jocelyn 1987).
Leaf chlorophyll was extracted with 80% (v/v) acetone. Absorbance of the acetone extracts at 645 and 663 nm was used to determine the concentration of chlorophyll a and b (Lichtenthaler and Wellburn 1983).
Cell-wall extraction
Five grams fresh weight of leaves, stems and roots were homogenised in a chilled mortar and pestle in 10 ml of 0.1 M Tris–HCl buffer (pH 7). The homogenate was centrifuged for 20 min at 10 000 g (Chakravarty and Srivastava 1997). The pellet was incubated in ethanol (80%) using a water bath with constant stirring (40°C, 60 min). Afterwards, the residue was successively washed with ethanol (80%), chloroform/methanol (2/1, v/v) and acetone (Zornoza et al. 2002). The supernatant was removed by centrifugation at 3500 g for 5 min and the pellet of cell wall retained for further analysis.
Mineral analysis
The remaining leaves, stems and roots were lyophilized, and dry weight was determined. Lyophilized plant material was ground and then digested in an acid mixture (HNO3:H2O2:H2O; 3:2:10, v/v/v) for 30 min in closed containers under pressure (Lozano-Rodríguez et al. 1995). Cadmium in plant tissue was analysed by inductively coupled argon plasma mass spectrometry (ICP-MS) (Perkin–Elmer Elan 6000).
Quantitation of GSH and PCs by high-performance liquid chromatography
For analysis of GSH and PCs, approximately 0.5 g of plant material was homogenised in 0.1 N HCl (1 ml g−1 tissue) with a mortar and pestle. Samples were vortexed and incubated on ice for 10 min before insoluble material was removed by centrifugation. 220 μl of extract was mixed with 60 μl of 2 mM N-acetyl-Cys (included as a standard) and filtered through a 0.2 μm membrane. Two hundred fifty microlitres of this mixture was analysed by high-performance liquid chromatography (HPLC) using postcolumn derivatization to detect GSH and PCs (Gupta and Goldsbrough 1991). The detection limit of the HPLC assay for PC2 was 0.2 nmol.
Identification of PCs by electrospray ionization-mass spectrometry (ESI-MS)
Samples of lupin roots treated with 18 μM Cd were used to verify the identity of PCs. Extracts were separated by HPLC without using postcolumn derivatization. The eluant was collected at the retention times corresponding to the three major Cd-induced thiol compounds, predicted to be PC2, PC3 and PC4. These samples were concentrated by lyophilization and analysed by ESI-MS.
Statistical analyses
Values in the tables and figures indicate mean values ± standard error. Differences among Cd treatments were analysed by one-way analysis of variance (Bender et al. 1989), followed by a post hoc multiple comparisons of means using Duncan test (P < 0.05). The statistical program spss 11.5 was used.
Results
Plant growth and chlorophyll content
Exposure to Cd inhibited plant growth impacting both shoot and root fresh weights (Fig. 1). Cadmium concentrations up to 6 μM had a small effect, primarily on shoots; however, higher Cd concentrations resulted in dramatic decreases in fresh weight (from 44 to 34% at 20 μM Cd and from 70 to 57% at 60 μM Cd, in shoots and roots, respectively). Likewise, root elongation was also affected by Cd supply in both experiments (data not shown). In the second experiment, high negative correlations were found between root length and PC accumulation in leaves (R2 = −0.91), stems (R2 = −0.92) and roots R2 = −0.92) for lupin plants under increasing Cd doses.
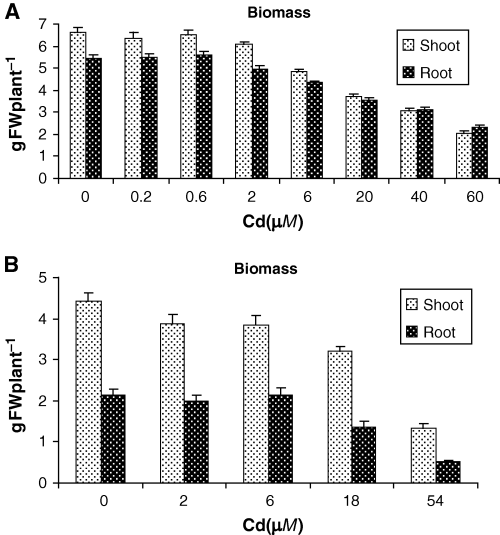
Fresh weight of shoot and root of lupin plants exposed to increasing Cd doses for 15 days corresponding to first (A) and second (B) experiment. Error bars indicate standard error of three replicates.
To assess the general stress imposed on white lupin by these Cd treatments, chlorophyll content was measured. In the first experiment, chlorophyll was not affected until 6 μM Cd treatment. Higher Cd concentrations resulted in significantly lower chlorophyll levels (Table 1). Taken together these measurements indicate that Cd treatments of 6 μM and above impose a significant level of stress on white lupin.
| Cd (μM) | Chlorophylls (μg g−1 FW) | Total thiols (nmol SH g−1 FW) | ||
|---|---|---|---|---|
| Leaves | Stems | Roots | ||
| 0 | 1691.8 ± 66.8 | 471.7 ± 3.0 | 170.2 ± 4.8 | 104.7 ± 3.1 |
| 0.2 | 1635.1 ± 93.4 | 475.1 ± 2.0 | 182.2 ± 5.7 | 107.8 ± 2.9 |
| 0.6 | 1603.7 ± 91.9 | 500.4 ± 7.8 | 204.0 ± 10.3 | 110.6 ± 3.5 |
| 2 | 1591.8 ± 151.2 | 542.2 ± 4.9 | 200.3 ± 4.9 | 174.9 ± 9.6 |
| 6 | 1500.7 ± 110.1 | 550.8 ± 4.9 | 236.0 ± 9.8 | 266.0 ± 15.0 |
| 20 | 1409.7 ± 125.1 | 547.4 ± 6.4 | 470.8 ± 25.7 | 791.6 ± 53.1 |
| 40 | 1457.1 ± 28.9 | 679.6 ± 9.7 | 888.1 ± 11.9 | 1072.1 ± 26.0 |
| 60 | 1090.1 ± 98.7 | 751.8 ± 26.8 | 985.2 ± 3.5 | 1224.7 ± 46.2 |
Distribution of cadmium and thiols in plants
The concentration of Cd in leaves, stems and roots generally increased with increasing Cd doses in both experiments (2, 3). However, in the second experiment Cd accumulation in roots was four-fold higher than in the first experiment; moreover, the leaves and stems of plants exposed to the highest Cd concentration in the second experiment (54 μM) showed a significant decrease in Cd accumulation compared with the 18 μM Cd treatment (Fig. 3). Differences in growth conditions in the two experiments may explain these results.
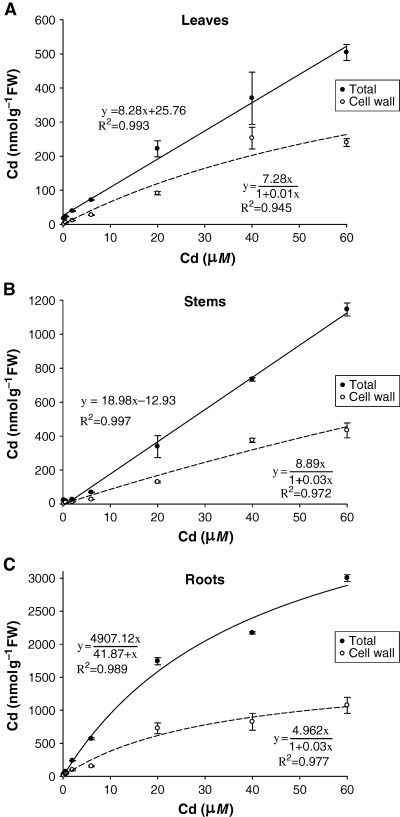
Total and cell-wall Cd concentrations in leaves (A), stems (B) and roots (C) of lupin plants exposed to increasing Cd doses for 15 days. Error bars indicate standard error of three replicates.
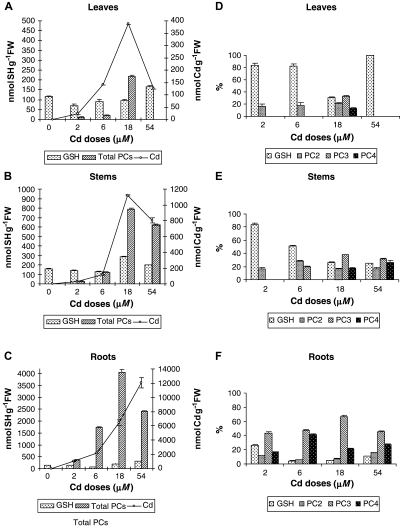
Glutathione, total phytochelatins and Cd concentrations in leaves (A), stems (B) and roots (C) and relative abundance of thiol compounds in leaves (D), stems (E) and roots (F) of lupin plants exposed to increasing Cd doses for 15 days. Error bars indicate standard error of three replicates.
The highest Cd accumulation was found in roots followed by stems (0.4–0.1 of root Cd) and leaves (0.2–0.03 of root Cd) for both experiments (2, 3). The concentration of thiols in each organ followed the same trends as Cd accumulation. Thus, 72–96% of total thiols were located in roots, 3–20% in stems and 1–8% in leaves (Table 1 and Fig. 3). Consequently, high positive correlations were observed between the concentrations of Cd and of total thiols in leaves (R2 = 0.75; 0.91), stems (R2 = 0.96; 0.99) and roots (R2 = 0.97; 0.88) of Cd-treated plants, in the first and second experiments, respectively.
Retention of Cd by the cell wall
Total Cd concentrations showed good linear correlations with the Cd treatment concentrations, except for roots where this linearity was lost at 40 μM Cd and higher (Fig. 2). In contrast, the Cd retained by cell walls exhibited a non-linear relationship with external Cd treatment deviating from the linear model at 40 and 60 μM Cd (Fig. 2). Thus, Cd concentration in the cell wall was better described by the Langmuir isotherm model (1) (Tan 1993) showing good determination coefficients in leaves (R2 = −0.945), stems (R2 = −0.972) and roots R2 = −0.977).
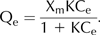
Qe is the adsorption density at the equilibrium solute concentration Ce (mg of adsorbate per g of absorbent); Ce is the concentration of adsorbate in solution (mg l−1); Xm is the maximum adsorption capacity corresponding to complete monolayer coverage (mg of solute adsorbed per g of absorbent) and K is the Langmuir constant (litre of adsorbent per mg adsorbate).
The maximum calculated adsorption capacities (Xm) were significantly higher in roots (4.61) than in stems (3.47) and leaves (2.05), respectively.
Fig. 4 shows the amount of Cd retained by the cell wall as a fraction of the total Cd in each plant organ. The percentage of Cd retained by cell wall ranges between 29 and 47% in leaves, between 38 and 51% in stems and between 26 and 42% in roots. Therefore, in all organs of white lupin plants a substantial fraction of the total Cd load is bound to cell-wall components.
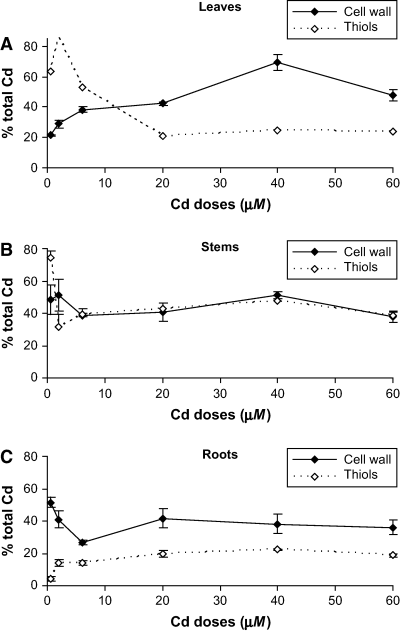
Percentage of total Cd retained by cell wall and estimated percentage of total Cd complexed by Cd-induced thiols in leaves (A), stems (B) and roots (C) of lupin plants exposed to increasing Cd doses for 15 days. Error bars indicate standard error of three replicates.
Identification of PCs induced by Cd exposure
In the second experiment, individual PCs in white lupin were characterised for the first time. Mass spectra obtained for the three different PCs (m/z: PC2 540.2, PC3 772.4 and PC4 1004.5) were used for the identification of each peptide. PC3 was the most abundant thiol in roots at all concentrations of Cd, in stems of plants exposed to 18 and 54 μM Cd doses and in leaves after treatment with 18 μM Cd (Fig. 3D, E). Both the size and the concentration of PCs increased with increasing Cd supply. High positive correlations were found between the concentrations of Cd and of PCs in leaves (R2 = 0.92), stems (R2 = 0.99) and roots (R2 = 0.98) of Cd-treated plants. However, at the highest Cd concentration (54 μM) PC accumulation in all plant organs decreased compared with the 18 μM Cd treatment (Fig. 3A–C). Although there were some variations in GSH in plants exposed to Cd, they were in general similar to those in control plants (Fig. 3A–C).
Discussion
The experiments described in this report examine the response of white lupin to Cd. Cadmium treatments reduced the growth of lupin plants as has been reported for other legume species (Hernández et al. 1998, Leita et al. 1993). White lupin exhibited a significant decrease in fresh weight only with treatments higher than 6 μM Cd (Fig. 1). When lupin plants grown on perlite, Zornoza et al. (2002) found a significant growth inhibition only with concentrations of 4 μM Cd and higher, reaffirming the capacity of this species to resist relatively high Cd concentrations. Another indicator of stress, chlorophyll content exhibited a similar response to increasing Cd concentrations (Table 1). This suggests that white lupin is able to avoid Cd toxicity up to a threshold of 6 μM, much higher than the Cd concentration typically found in a polluted soil (Sanità di Toppi and Gabbrielli 1999).
Most of the Cd (80–90%) taken up by lupin plants was retained in roots with only a small fraction translocated to stems and leaves (2, 3). Similar trends in root–shoot distribution of Cd have been previously reported for white lupin (Römer et al. 2000, Zornoza et al. 2002) and other crops (Wu-zhong et al. 2002, Zhang et al. 2002). The capacity of white lupin to limit Cd uptake into shoots could be explained by immobilization of Cd in root cell walls thereby reducing translocation of Cd to shoots. The percentages of Cd retained by the root cell wall ranged between 26 and 42% depending on the Cd treatment (Fig. 4), slightly lower than in lupin plants exposed to Cd for longer periods (Zornoza et al. 2002). The proportion of Cd bound to root cell walls of white lupin was similar to that found in bean, rice and curly kale, but higher than in maize (Guo and Marschner 1995) or white lupin grown at lower Cd levels (Costa and Morel 1993). In general, cell walls of leguminous plants possess higher cation exchange capacity (CEC) than cereals; and among legumes, cell walls of white lupin roots have a higher CEC (Meychik and Yermakov 1999).
A Langmuir isotherm model that took into account Cd binding by the cell wall provided a good fit to measurements of Cd concentrations in leaves, stems and roots (Fig. 2). This means that the cell wall could be regarded as a low-specific exchanger with relatively high binding capacity for Cd ions in the surrounding apoplast solution. This behaviour of cell wall acting like a ‘filter’ for Cd has been described by Khan et al. (1984) and Nishizono et al. (1989). The maximum calculated Cd adsorption capacities (Xm) in white lupin tissues were significantly higher for roots (4.61 mg g−1) than for stems (3.47 mg g−1) or leaves (2.05 mg g−1). These data suggest that root cell walls have a higher adsorption capacity compared with those of stem and leaves. García-Gomez et al. (2002) used raw paper pulp (as a substitute for cell wall) and showed that over 90% of the Cd in solution was sorbed by the pulp at low Cd concentrations, but only about 20% was sorbed at the highest Cd concentration. The lower percentage of Cd retained by cell wall at higher Cd concentrations was also found in our studies, suggesting the progressive saturation of Cd binding sites in the cell wall (Fig. 2).
In addition to extracellular binding of Cd, white lupin also synthesize PCs, and the distribution of these thiol peptides reflected the concentration of Cd in the plant (Fig. 3). Both and the total thiol concentration and the chain length of PCs increased in plants exposed to higher concentrations of Cd as also seen in tobacco (Vögeli-Lange and Wagner 1996) and wheat (Stolt et al. 2003). The most abundant Cd-induced PC in white lupin was PC3 followed by PC4 (Fig. 3). PC3 has also been reported as the main Cd-induced PC in others species (Rainieri et al. 1995, Stolt et al. 2003). The chain length of PCs depends not only on the plant species but also on the inducer of PC synthesis. In white lupin plants treated with arsenate, at concentrations similar to those used here for Cd, PC2 was the most abundant PC isoform (Vázquez et al. 2005).
The highest concentrations of PCs in white lupin were similar to those measured in Nicotiana rustica and Brassica napus (Carrier et al. 2003, Vögeli-Lange and Wagner 1996) but higher than those found in Thlaspi arvense and Triticum arvense (Ebbs et al. 2002, Stolt et al. 2003). White lupin therefore appears to be a plant species with a higher capacity to produce PCs, especially in roots.
A direct relationship between PC concentrations and the level of stress caused by Cd in white lupin, i.e. the degree of growth inhibition was observed. In plant cell cultures PC synthesis can be detected within minutes of exposure to Cd (Cobbett and Goldsbrough 2002). Therefore, the rapid and sensitive response of PCs may allow PCs to be used as a biomarker of Cd stress in white lupin. One problem with this proposal is the reduced synthesis of PCs at high concentrations of Cd, probably because of Cd toxicity. Reduced Cd levels were also observed in leaves and stems at the highest Cd exposure level, so a reduced Cd translocation from root to shoot could account for the lower PC accumulation in these organs.
Depletion of GSH in response to Cd has been reported in several plant species (Steffens 1990). However, there was little variation in GSH levels in plants exposed to different Cd concentrations (Fig. 3). The ability of white lupin to maintain the levels of GSH even while producing large amounts of PCs suggests that this species has a substantial capacity to synthesize GSH. Similar results were obtained with tobacco seedlings under increasing Cd stress (Vögeli-Lange and Wagner 1996). Maintenance of the GSH pool within a range may be critical, because GSH can provide protection against reactive oxygen species in a number of ways (Dietz et al. 1999).
The formation of Cd–GSH complexes is one mechanism proposed for detoxification of Cd in plants, especially as a first line of defence to avoid Cd damage within the cytosol (Howden et al. 1995). GSH was able to complex all Cd present in Cd-treated leaves of tobacco plants (Vögeli-Lange and Wagner 1996). Two sulphydryl (SH) groups from GSH and/or PCs should complex one Cd2+ ion. GSH–Cd and PC–Cd complexes with a 2:1 stoichiometry for SH:Cd have been reported (Díaz-Cruz et al. 1997, Steffens 1990). The estimated percentage of total Cd that is complexed by thiols (at a 2:1 stoichiometry) is shown in Fig. 4. The contribution of the cell wall to Cd detoxification was greater than that of thiols, especially in leaves and roots, with average percentages over 20% in leaves, 40% in stems and 20% in roots. Similar percentages in roots were reported by Wojcik and Tukendorf (1999) for wheat and by Stolt et al. (2003) for maize. Cell walls would account for twice the amount of Cd complexed with thiol compounds in leaves and roots, whereas both mechanisms contributed equally in stems (Fig. 4). These results support the hypothesis that the cell wall plays a major role in Cd detoxification followed by PCs, which operate in the cytosol when Cd levels exceeded the binding of the cell wall.
In spite of Cd binding by the cell wall and thiols compounds, a considerable fraction of Cd in leaves and roots remained as free Cd or complexed by other ligands, probably organic acids (Boominathan and Doran 2003). This suggests that other mechanisms could contribute to Cd detoxification in white lupin. In this context, Cd can be transported into the vacuole as a PC complex where high molecular weight Cd–PC complexes that contain CdS are formed. This may allow recycling of some PCs as well as the accumulation of Cd in tissues without a concomitant increase in PC concentrations (Maier et al. 2003).
In summary, these studies demonstrate that the cell wall of white lupin reduces the entrance of Cd into cells because of its high ion-exchange capacity. However, at Cd concentrations higher than 6 μM, a considerable amount of Cd entered root cells, leading to translocation of Cd to the shoot. Cd-induced stress is accompanied by the synthesis of PCs, which by complexation and vacuolar compartmentation contributed to Cd detoxification. The Cd toxicity in plant tissues is evident by growth inhibition, decrease of chlorophylls levels. The synthesis of PCs in response to Cd may allow PCs to be used as an effective early biomarker of Cd stress in lupin plants. Further studies, with this interesting species, that focus on developing a better understanding of the mechanisms involved in metal detoxification and their responses under field conditions are in progress.
Edited by J. K. Schjørring
Acknowledgments
Acknowledgements – The authors acknowledge financial support from the Spanish Ministry of Education and Science (project CTM2004-06715-C02-01), the Ramon Areces Foundation and the United States Department of Agriculture–National Research Institute (USDA-NRI) Plant Response to the Environment programme. Seeds were kindly supplied by Andres Gil Aragón (Servicio de Información y Documentación Tecnológica (SIDT), Junta de Extremadura). Cadmium was analysed in the ICP-MS laboratory from the Servicio Interdepartamental de Investigación–Universidad Autónoma de Madrid (SIDI-UAM) with the assistance of Inmaculada Rivas. The authors wish to thank Dr E Esteban for the revision of the manuscript.




