Impact of high ozone on isoprene emission, photosynthesis and histology of developing Populus alba leaves directly or indirectly exposed to the pollutant
Abstract
The direct and indirect impact of ozone on Populus alba was studied by exposing leaves enclosed in specially designed cuvettes for 30 days to high ozone (150 ppb, 11 h per day), while leaves developing above the cuvettes were exposed to ambient ozone. Gas exchanges and histo-anatomical parameters were measured to specifically understand whether ozone indirectly affects the anatomy and physiology of leaves. Three leaf classes were investigated: (1) those expanding above the cuvettes (A leaves); (2) those already developed inside the cuvettes (B leaves) and (3) those developing inside the cuvettes, since the beginning of the ozone treatment (C leaves). The anatomy and morphology of the first leaf developing outside the cuvette (A1) were strongly affected by ozone, whereas photosynthesis was not perturbed. However, in leaves of ozone-treated plants developing after A1, a large reduction of starch accumulation was observed, which suggests a delayed biosynthesis, or a very rapid export of starch toward other sinks. Isoprene emission was higher and isoprene synthase messenger RNA was more expressed in ozone-treated A1 leaves than in control leaves with similar ontogeny. This indicates that isoprene synthesis is stimulated by ozone, and reveals that isoprene emission is controlled at a transcriptional level. Leaves already developed inside the cuvette (B leaves) rapidly sensed ozone stress, which inhibited photosynthesis, stomatal conductance and isoprene emission. The observation that new leaves were developing inside the cuvettes during the treatment (C leaves) suggests that resistance to ozone may be acquired by plants. Leaves C showed a more packed and thinner mesophyll than controls of similar development, which may help reduce ozone penetration inside cells. They also showed a lower photosynthesis in comparison to controls and to other leaf classes, probably because of ribulose 1,5-bisphosphate carboxylase/oxygenase activity limitation, as inferred from photosynthesis response to intercellular CO2. However, isoprene emission was slightly stimulated also in C leaves, confirming that a large fraction of carbon is invested into isoprene formation under ozone stress.
Abbreviations –
-
- BVOCs
-
- biogenic volatile organic compounds
-
- Ci
-
- intercellular CO2 concentration
-
- Fm
-
- maximal fluorescence
-
- Fs
-
- steady-state fluorescence
-
- Fv
-
- variable fluorescence
-
- mRNA
-
- messenger RNA
-
- Rubisco
-
- ribulose 1,5-bisphosphate carboxylase/oxygenase
-
- RuBP
-
- ribulose 1,5-bisphosphate
-
- UV
-
- ultraviolet
Introduction
Recent studies have shown multiple interactions between isoprenoids, a group of biogenic volatile organic compounds (BVOCs) largely emitted by vegetation, and atmospheric ozone. In particular (1) it has been shown that isoprene released by plants may contribute to the formation of tropospheric ozone in urban and periurban areas in which high loads of anthropogenic NOx are also present (Chameides et al. 1988); (2) ozone episodes have been found to stimulate isoprenoid emission by leaves (Loreto et al. 2004); (3) isoprenoids have been reported to reduce ozone damage in leaves, by yet not fully understood mechanisms (Loreto et al. 2001, 2004) but probably (4) isoprene reduces intercellular NO and H2O2 formation under ozone episodes, interfering with molecules signalling cellular damage and programmed cell death (Velikova et al. 2005).
It is important to improve our understanding of the interaction between isoprene and ozone, because present and predicted climate change is expected to cause a concurrent increase of the formation of these gases. Today the ozone concentration in the low troposphere may episodically reach levels that can cause acute damage to vegetation (Pell et al. 1997). Cumulative exposure to ozone chronically at high level may also cause damage and reduce plant growth and productivity (Fuhrer et al. 1997). Modellers predict an increase of 50% of the atmospheric concentration of ozone by the year 2100 because of increasing anthropogenic sources of NOx and greenhouse gases, and to the consequent climate warming, in turn changing the chemical weather of Earth (Fowler et al. 1999). This is expected to exacerbate ozone toxic or negative effects on vegetation. Climate warming is also expected to increase isoprenoid emissions by plants because of the strong temperature dependence of isoprenoid biosynthesis (Loreto and Sharkey 1990). This may have a dual effect, further enhancing ozone production at the atmospheric level, while contributing to reduction in ozone damage to leaves.
Ozone impact on vegetation is often studied with enclosure systems, in which plants or parts of them are fumigated with atmospheric or enriched ozone levels (Loreto and Velikova 2001). These systems allow to detect plant response to acute exposures but may not be practical to determine the impact of chronic exposures. Moreover, ozone rapidly reacts with other chemical species and it is not clear whether its negative effects can be exerted at distances from the site of acute pollution. In this work, an enclosure system was created to allow long-term fumigations with high levels of ozone in fully expanded leaves while letting to expand new leaves at atmospheric ozone levels. The objective of this study was to know whether ozone only affects the physiology and anatomy of leaves directly exposed to high levels of the pollutant, or if it also affects new leaves not directly exposed to the pollutant. In particular, we wanted to investigate the impact of ozone on the emission of isoprene in newly developed leaves, determining whether ozone can stimulate isoprene formation also remotely from the site of ozone uptake by vegetation.
Material and methods
Plant material
Three-year-old plants of Populus alba L. were used for this study. Plants were potted in 50-l pots filled with commercial soil and grown and maintained in a greenhouse under controlled conditions. Experiments were run in late spring when the air temperature was 24–30°C and the air humidity was around 50%, the light intensity at the canopy level 850 μmol photons m−2 s−1. Plants were irrigated daily and fertilized weekly to avoid drought and nutritional stress during the experiments.
The ozone fumigation system
Branch cuvettes (3 l of internal volume) especially developed for this experiment were carefully installed at the bottom of the stem including the basal (three to four) leaves (Fig. 1). The cuvettes were made of soft Teflon film fastened to a rigid ring of PVC to avoid the direct contact with leaves. The top part of the cuvettes was then gently wrapped on the stem and firmly tied to it to avoid leaks. The stems exiting the top part of the cuvettes were cut to allow the sprouting of one bud only per stem during the ozone fumigation. New buds appeared after 1 week and new shoots grew within 3 weeks after cutting. The lateral part of the cuvettes housed two holes and connectors to allow the flux of air in and out of the cuvette. All connecting pipelines were made by Teflon to avoid high ozone uptake by other plastic materials and early wearing out of the materials. Air was pumped in the cuvettes with a compressor at a flux of 6 l min−1. This high flux allowed to avoid condensation of transpired water vapour inside the cuvette and also inflated and self-sustained the cuvette walls distant from the rigid polyvinyl frame. Sensors of light and temperature were also placed in the cuvettes through the air-inlet hole. The enclosures caused an attenuation of 15% of the light intensity and a maximum increase of air temperature of 2°C with respect to the temperature measured in the greenhouse during the hottest hours of the day.

View of the experimental system used for ozone fumigation. In this figure, the 3-l branch Teflon cuvettes developed for this experiment are visible, as well as the Teflon lines to circulate ozonated or non-ozonated air inside the cuvette at controlled concentrations and flow rates. Details about the system are reported in the text.
The air entering the cuvette was enriched with ozone in half of the cuvettes (five plants referred to as treatment). Ozone was generated by flowing part of the air, pushed by a diaphragm pump, through a winding quartz glass illuminated with an ultraviolet (UV) light source (Helios Italquartz, Milan, Italy) placed at short distance from the cuvette inlets. This method offered the possibility to change the amount of ozone generated by changing the air flow through the winding quartz glass or by changing the intensity of the UV light source. The lamp was set to generate ozone at a concentration of 150 ppb, and fumigation was carried out for 11 h per day (07.00–18.00 h) and for 1 month. Five other plants thereafter referred to as controls, were grown in cuvettes as for the ozone-treated plants but the air in was not enriched with ozone. In control cuvettes, the ozone concentration followed a daily trend. Ozone concentration was 0 ppb at night but built up photochemically to a maximum of 40 ppb during the central hours of the day. The buds developing on the stem out of the cuvettes grew at ambient ozone concentration both in treated and control plants. The ozone concentration in the cuvettes of treated and control plants was monitored with a photometric O3 analyser (1008 Dasibi Environmental Corp., Glendale, CA) connected with the cuvette inlet and outlet and periodically switching between cuvettes with Teflon valves (Velikova et al. 2005). The ozone-enriched air outflow from the cuvettes was conveyed out of the greenhouse. In the greenhouse, the ozone concentration was similar to that recorded in the control cuvettes.
Measurements were carried out on the following leaf material: (1) the first three leaves expanding out of the cuvettes. These leaves will be named A1 (first leaf), A2 (second leaf) and A3 (third leaf); (2) the leaves growing inside the cuvette since the beginning of the experiment. These leaves will be named B; and (3) the leaves expanding from buds inside the cuvettes during the experiment. These leaves will be named C.
Gas-exchange and fluorescence measurements
The exchange of CO2 and H2O between leaves and air was measured with a Li-6400-40 portable gas-exchange system (LI-COR, Lincoln, NE), and photosynthesis, transpiration, stomatal conductance to CO2 and H2O and intercellular CO2 concentration (Ci) were calculated with the instrument software. Chlorophyll fluorescence was also measured in vivo and simultaneously to gas exchanges with the same apparatus. In particular, we measured the quantum yield of photosystem 2 (PS2) in darkened leaves (the ratio between variable and maximal fluorescence, Fv/Fm) and in illuminated leaves (the ratio between maximal − steady-state fluorescence and maximal fluorescence (Fm′ − Fs)/Fm′ = ΔF/Fm′). Details about nomenclature and connotation of fluorescence parameter, and about the protocol of fluorescence measurements are given in van Kooten and Snel (1990). Measurements were carried out on the central part of the leaf (2 cm2), which was clamped in the Li-6400 gas-exchange cuvette and exposed to a flux (0.45 l min−1) of synthetic air (20% O2, 80% N2, 380 ppm CO2) free of ozone, BVOCs and other pollutants. During all gas-exchange measurements, the leaf temperature was maintained at 30°C and the leaf was illuminated with a light intensity of 800 μmol photons m−2 s−1. The CO2 concentration in the air was modulated to generate a response of photosynthesis to CO2 concentrations between 40 and 1000 ppm. Isoprene emission from leaves was detected simultaneously to CO2 and H2O gas-exchange measurements, connecting the outflow of the Li-6400 cuvette to a proton-transfer-reaction mass-spectrometer (Ionicon, Innsbruck, Austria). Measurements were done at the same time of the day (10.00–11.00 h) to minimize physiological changes driven by environmental factors or by accumulation of photosynthates during the day. Measurements of B and C leaves were carried out by temporarily removing the plants from the fumigation cuvettes. Measurements were carried out on B leaves at days 0, 6, 14 and 21 of the experiment. Measurements of leaves A and C were carried out at days 30–35 of the experiment.
Histological observations of leaf anatomy
Histological observations were carried out in leaves at day 30 of the experiment. Leaf tissue pieces (1–2 mm2) were excised from four portions of each leaf (along the margin and far from the margin of upper and lower halves) on five replicates per treatment. Samples were collected at the same hour of the day (12.00 h) to avoid diurnal variability of sugar accumulation in leaves. Leaf tissues were immediately fixed in 5% (w/v) glutaraldehyde in 0.075 M sodium cacodylate buffer, pH 7.2, for 12 h. Samples were then washed three times for 15 min each in 0.075 M sodium cacodylate buffer, pH 7.2, and were postfixed in 1% (w/v) OsO4. Samples were then dehydrated in increasing concentrations of ethanol and included in resin (Epon 812 resin, 2-dodecenylsuccinic anhydride, and methylnadic anhydride mixture, Sigma Aldrich, St. Louis, MO). A preinclusion at room temperature in increasing concentrations of resin dissolved in propylene oxide was followed by the final inclusion in freshly prepared resin followed by the polymerization at 35°C for 12 h, 45°C for 12 h and 60°C for 12 h. Semithin (1–2 μm) and ultrathin (<1 μm) sections were cut with an ultramicrotome (Om U2, Reichter, Heidelberg, Germany) equipped with a glass blade. The semithin sections were stained with toluidine blue and were observed under a light microscope (DMR HC, Leica, Wetzlar, Germany) equipped with a system to take micrographs. Histo-anatomical parameters were measured with an image analysis software (IM1000, Leica, Cambridge, UK). Ultrathin sections were mounted on uncoated copper grids (200 mesh) and were contrasted by adding uranyl acetate and an aqueous solution of lead citrate before observation with a transmission electron microscope (TEM 400 T, Philips, Monza, Italy).
Isoprene synthase (PaISPs) RNA isolation and analysis
Total RNA was extracted from frozen, homogenized leaf tissue (0.1–0.15 g FW) of control and ozone-treated poplar plants with the NucleoSpin® RNA Plant kit (Macherey-Nagel, Düren, Germany) according to the manufacturer’s instruction. A given amount of total RNA (1–2 μg) was reverse transcribed for 1 h at 42°C using 200 units of SuperscriptII RT (Invitrogen, Carlsbad, CA), with 1× corresponding buffer, 10 mM dithiothreitol (DTT), 0.4 mM of each deoxynucleotide triphosphates (dNTPs) and 0.5 μg μl−1 of oligo dT (5′-T(25)–VX-3′) primer (Invitrogen, Carlsbad, CA). The complementary DNA was used for PCR with 1 unit Taq polymerase (Amersham Bioscience, Uppsala, Sweden), 1× corresponding buffer, 0.2 mM of each dNTP and 10 μM of the actin and PaIspS primers (Invitrogen, Carlsbad, CA). For the analysis of the genes we used the following primers: actin forward 5′-TGCTGAGCGATTCCGTTGCCCAGAG-3′ and reverse 5′-TGGTGGTGCAACCACCTTGA-3′ designed on the published sequence with National Center for Biotechnology Information (NCBI) accession number AB025795 and PaIspS forward 5′-AGCTTCCATTGCATCGCAGGACG-3′ and reverse 5′-TGCTGAAGCCAGGTCGTTGC-3′ designed on the published sequence with NCBI accession number AB198180. The authenticity of the PCR products was checked by two directional sequencing using ABI Prism 310 Genetic Analyzer (Perkin Elmer Life and Analytical Sciences, Boston, MA).
Statistics
The data presented are means from at least four replications for each data point. Mean values ± standard errors are shown for gas exchange, fluorescence measurements and anatomical features. Anatomical microphotograph show only one sample but they were repeated on at least 20 samples yielding similar results. Differences of isoprene emission and PaISPs messenger RNA (mRNA) expression between controls and ozone-treated leaves of the same classes were tested with a t-test, and asterisks (*** or *) indicate differences significant at P < 0.01 or 0.10, respectively. A multiple range test was used to compare the means of anatomical features and fluorescence data in an analysis of variance scheme, and means significantly different were separated by different letters (P < 0.01 for anatomical features and P < 0.05 for fluorescence data).
Results
The high ozone level was rapidly sensed by the leaves B inside the cuvette. Visual signs of necrosis caused from ozone exposure were observed after 3 days of treatment (Fig. 2A). All physiological parameters, including photosynthesis, stomatal conductance and isoprene emission, decreased rapidly, within 3 weeks of treatment (Fig. 2B) and were undetectable in further measurements. Many of the leaves were shed by day 30 of the experiment.
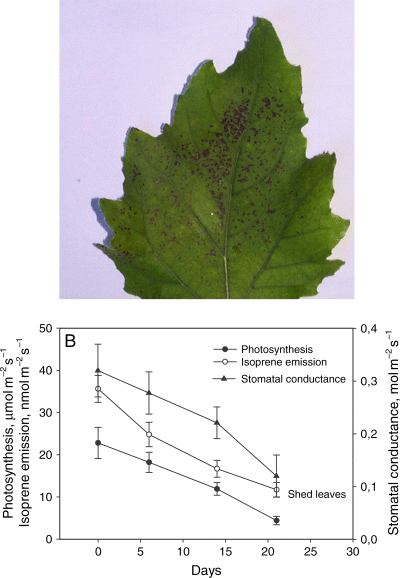
Effects of ozone fumigation on fully expanded leaves (class B) directly exposed to the pollutant for 3 days (A). (B) Photosynthesis, isoprene emission and stomatal conductance of these leaves were followed until leaf abscission, which occurred between 21 and 30 days after beginning the ozone treatment. Means ± standard error (n = 4) are shown.
The first leaves that developed outside the cuvette within 1 week from the beginning of the ozone treatment (A1) showed peculiar morphology and anatomy. The leaf laminas were small (<5 cm) and lost the typical shape of poplar leaves being oval and with regular edges (data not shown). The lamina was much thicker than in control leaves and in all other leaves of classes A and C, and of the corresponding controls growing in plants which were not fumigated with ozone (Table 1). All components of the lamina (parenchyma and the two layers of palisade tissues) contributed to make A1 leaves thicker and their mesophyll more packed than those of controls (Table 1). These features were rapidly lost in the A leaves developing after A1, which were very similar to control leaves. New leaves developed inside the cuvettes during the experiment (C leaves) and were clearly resistant to ozone. C leaves developed with a time frame similar to A3 leaves (they started to expand 15 days after beginning the ozone treatment) and were thinner in ozone-exposed plants than in control plants, especially because of a reduced height of the palisade parenchyma and a reduced size of intercellular spaces in the spongy parenchyma (Table 1 and Fig. 3). The mesophyll of ozone-exposed C leaves was densely packed and palisade cells were smaller in C leaves growing in plants not exposed to ozone.
| Leaf class | Thickness of leaf lamina (μm) | Height of palisade parenchyma (μm) | Height of palisade tissue (first layer) (μm) | Height of palisade tissue (second layer) (μm) | Diameter of palisade cells (μm) |
|---|---|---|---|---|---|
| Controls | |||||
| C | 126 ± 2b | 51 ± 1b | 28 ± 1ab | 25 ± 1b | 17 ± 1b |
| A1 | 128 ± 2b | 66 ± 1cd | 29 ± 1b | 32 ± 2c | 14 ± 2a |
| A2 | 130 ± 1b | 64 ± 1c | 31 ± 2c | 34 ± 1c | 14 ± 1a |
| A3 | 103 ± 1a | 51 ± 1b | 27 ± 1a | 24 ± 1ab | 14 ± 1a |
| Ozone treated | |||||
| C | 106 ± 2a | 46 ± 1a | 25 ± 1a | 22 ± 1a | 14 ± 1a |
| A1 | 147 ± 3c | 68 ± 2d | 35 ± 2d | 36 ± 2cd | 17 ± 1b |
| A2 | 128 ± 2b | 62 ± 1c | 31 ± 1c | 31 ± 1c | 15 ± 1a |
| A3 | 114 ± 2ab | 54 ± 1b | 29 ± 1b | 26 ± 1b | 15 ± 1a |
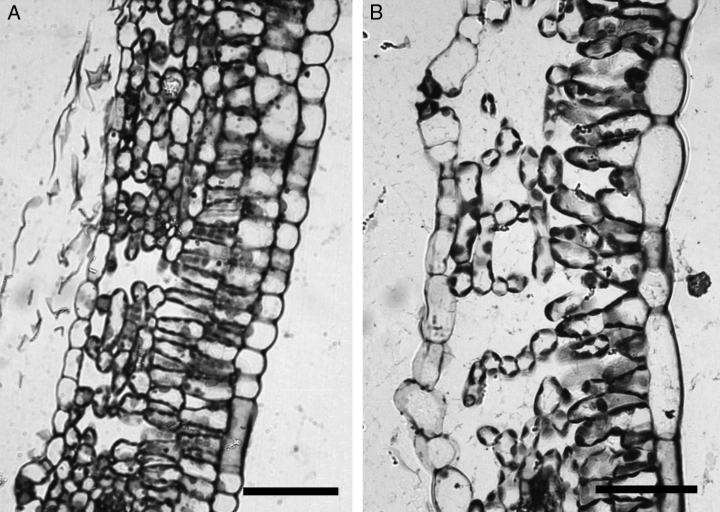
Light microscopy images of sections of leaves developing in the cuvettes during the experiment (referred to as C leaves in the text). In leaves growing in cuvettes under high ozone (A) the total thickness is lower and the mesophyll is more packed than in leaves growing in control conditions, i.e. in cuvettes without ozone enrichment (B). Bars represent 50 μm in both images.
Measurements of the relationship between photosynthesis and Ci are often used to reveal photosynthesis limitations. This approach clearly indicated a difference between C and A leaves in ozonated samples, the latter behaving as the corresponding control, whereas photosynthesis response to increasing Ci was less steep, indicating a lower ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activity, in C leaves (Fig. 4). There were also differences between A1 and A2 leaves of ozone-treated plants in the response of photosynthesis to Ci. However, in this case the difference was observed at Ci higher than ambient, where photosynthesis response to Ci was not linear and should be limited by the regeneration rate of ribulose 1,5-bisphosphate (RuBP). Moreover, control leaves of comparable age behaved as A1 and A2 leaves, respectively, which indicated a strong developmental control on the response of photosynthesis to Ci, rather than a direct effect of the ozone treatment. For instance C leaves of control plants and A3 leaves of ozone-treated and control plants, ontogenetically similar, showed the same response of photosynthesis to Ci (Fig. 4). No differences were found in the quantum yield of dark-adapted leaves measured by the fluorescence parameter Fv/Fm (Table 2) but in illuminated leaves the quantum yield of C leaves was clearly affected by growth under high ozone. Moreover, A leaves again showed a very clear developmental control. The ΔF/Fm′ parameter was higher in control and ozone-treated A1 leaves than in control and ozone-treated A2 and A3 leaves (Table 2). This confirmed the indications supplied by gas-exchange data (Fig. 4) that photosynthesis of developing leaves was limited by the electron transport–driven regeneration of RuBP, irrespective of the ozone treatment.
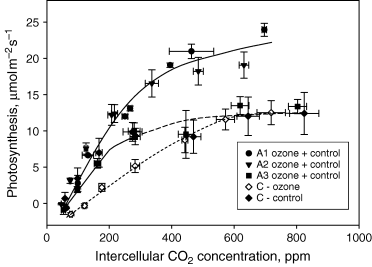
Photosynthesis response to intercellular CO2 concentration in leaves expanding above the cuvettes (A), and in leaves developing inside the cuvettes since the beginning of the ozone treatment (C). The number following class A legend denotes the sequence of leaf expansion, being A1, A2, A3, the first, second and third leaf, respectively, to expand above the cuvettes. In class A leaves, no difference was observed in the response of ozone fumigated and control plants and the two treatments are pooled (n = 8). The ozone-treated plants of class C leaves (n = 4) are shown separately from controls (n = 4). The responses have been interpolated with second-order best-fit lines generated by the Sigmaplot 2002 software (Systat, Erkrath, Germany). The solid line connects data points of A1 and A2 leaves of control and ozone-treated plants. A second solid line interpolates those data points of C control and A3 control and ozone-treated plants that are not significantly different from those of A1 and A2 leaves. Lines with dashed patterns connect those data points of C control, C ozone treated and A3 control and ozone-treated plants, which are statistically different (confidence limits = 95%) from those of A1 and A2 leaves.
| Leaf class | Fv/Fm | ΔF/Fm′ |
|---|---|---|
| Controls | ||
| C | 0.79 ± 0.02a | 0.23 ± 0.5b |
| A1 | 0.81 ± 0.04a | 0.22 ± 0.4b |
| A2 | 0.77 ± 0.02a | 0.16 ± 0.3a |
| A3 | 0.77 ± 0.04a | 0.14 ± 0.3a |
| Ozone treated | ||
| C | 0.80 ± 0.02a | 0.11 ± 0.5a |
| A1 | 0.77 ± 0.05a | 0.21 ± 0.5ab |
| A2 | 0.79 ± 0.03a | 0.14 ± 0.3a |
| A3 | 0.78 ± 0.01a | 0.13 ± 0.4a |
Electron micrographs showed the accumulation of many starch granules in A leaves of control plants. In A3 leaves of ozone-treated plants, however, no starch accumulation was observed (Fig. 5). Starch accumulation in A2 leaves of ozone-treated plants was about 50% of that observed in A2 controls. The accumulation of starch in A1 leaves of controls and ozone-treated plants was not significantly different (data not shown).
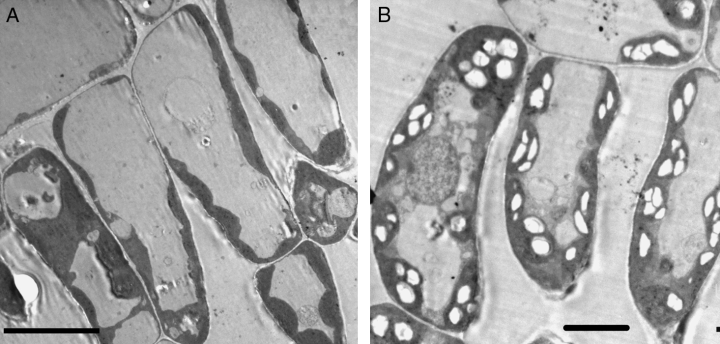
Electron microphotograph of cells of the third leaves (A3) expanding above the cuvettes in which ozone was (A) or was not fumigated (B). Chloroplasts of control A3 leaves present numerous white starch grains, which are absent in chloroplasts of A3 leaves developing from ozone fumigated cuvettes. Bars represent 10 μm in panel A and 5 μm in panel B.
The ozone treatment caused a stimulation of the emission of isoprene by poplar leaves. This increase was statistically significant in C leaves and, particularly, in A1 leaves, when compared with control leaves of similar age (Fig. 6). In A1 leaves, the stimulation of isoprene emission was associated to a larger expression of P. alba PaISPs with respect to the control of correspondent age (Fig. 7). PaISPs expression also matched the age-dependent reduction of isoprene emission in A leaves (Fig. 6). In C leaves, a very low expression of PaISPs was detected without significant differences between ozone-treated and control leaves.
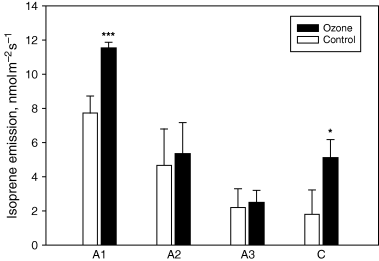
Emission of isoprene in leaves expanding above the cuvettes (A), and in leaves developing inside the cuvettes since the beginning of the ozone treatment (C). The number following class A legend denotes the sequence of expansion, being A1, A2, A3, the first, second and third leaf, respectively, to expand above the cuvettes. Means ± standard error (n = 4) are shown. Differences between controls and ozone-treated leaves of the same classes were tested with a t-test, and asterisks (*** or *) indicate differences significant at P < 0.01 or 0.10, respectively.
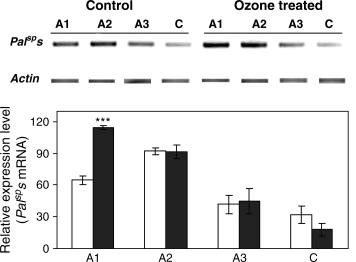
Expression analysis of isoprene synthase mRNA (PaISPs) in Populus alba leaves expanding above the cuvettes (A), and in leaves developing inside the cuvettes since the beginning of the ozone treatment (C). The number following class A legend denotes the sequence of expansion, being A1, A2, A3, the first, second and third leaf, respectively, to expand above the cuvettes. One to 2 μg of total RNA was reverse transcribed and amplified by semiquantitative reverse transcription–PCR. Messenger RNA levels loaded in each lane were determined by coamplification and normalization with an internal standard (actin). Close to the blot, a graph with the relative intensities of the signals (means ± standard error, n = 4) is shown. Differences between controls (empty bars) and ozone-treated leaves (filled bars) of the same classes were tested with a t-test, and asterisks (***) indicate differences significant at P < 0.01 level.
Discussion
Ozone damage occurred fast and was severe in P. alba leaves directly exposed to ozone, confirming that this plant species is sensitive to ozone (Bortier et al. 2000). By using an innovative cuvette system, the objective of this study was to detect whether ozone could affect the morphology and physiology of leaves of the same plant but which were developing in condition of direct or indirect exposure to the stressor. In fact, the anatomical changes of the first leaves developing at ambient ozone level (A1) were dramatic and indicated that ozone can strongly impact on their development. However, the impact of ozone was rapidly lost in leaves developing after A1, suggesting that ozone only affects relevantly the closest leaf to the ozone source. The signalling system, which brings about these interesting developmental changes, is to our knowledge unknown and deserves further investigation. It may be possible, alternatively, that ozone sensitivity be mediated by the different developmental stage of the leaves, being stronger in leaves already developed (e.g. A1) than in those still developing (e.g. A3). This also needs further investigation. Interestingly, despite ozone impact on leaf anatomy, A1 leaves revealed a very well-preserved physiology, the photochemistry and biochemistry of photosynthesis being very similar to those observed in control leaves of corresponding developmental stage. However, a very low accumulation of starch was noted in leaves developing after A1 in ozone-treated plants. This indicates that ozone may affect starch biosynthesis on expanding leaves. These results suggest that the biosynthesis of starch is delayed, as this would explain why no differences were found in fully expanded leaves (e.g. A1), whereas newly developing (A3) leaves of ozone-treated plants were unable to accumulate starch. However, it cannot be excluded that starch was in fact produced, but exported very rapidly from A3 leaves to stronger sinks of ozone-treated plants. The perturbed starch biosynthesis may ultimately affect plant growth and productivity in plants experiencing ozone exposure. More experiments are needed to investigate whether this strong starch depletion also occurs in response to less dramatic ozone exposure, which may indicate its very common occurrence in nature. Starch depletion has been observed by other authors in leaves directly exposed to ozone and associated to photosynthesis inhibition (Oksanen 2003). In this experiment, however, starch is depleted in leaves with unperturbed photosynthesis.
Ozone also had a very strong impact on isoprene emission in the first leaf developing at ambient ozone. The observed isoprene stimulation in A1 leaves developing above those fumigated with ozone expands the observation that leaves recovering from ozone stress emit more isoprene (Velikova et al. 2005) or monoterpenes (Loreto et al. 2004). As in the case of other physiological and anatomical features, also isoprene emission was not affected in leaves developing at more distance from the ozone fumigated leaves, although a residual but not significant stimulation was also noted in A2 leaves. It should be considered that isoprene emission is under a strong developmental regulation, the low emission of young leaves being regulated at a transcription level because of the low amount of isoprene synthase mRNA and protein (Wiberley et al. 2005). Our finding confirms that PaISPs mRNA is very low in leaves that start to develop and suggests that indirect exposure to ozone does not affect early stages of isoprene induction, at least on leaves that develop far from the ozone source. It remains to be tested, however, whether ozone may induce earlier emission of isoprene in those leaves developing immediately above the ozone source (A1 leaves).
The biochemical steps that are involved in isoprene stimulation under oxidative stress are unknown. Isoprene emission is often associated to the availability of photosynthetic intermediates and this is also true for B leaves growing in the cuvettes at high ozone. However, this is not the case in A leaves, which showed no photosynthesis stimulation with respect to A leaves of control plants. Isoprene stimulation in A1 leaves was associated with a greater expression of isoprene synthase gene, indicating that there might have been an increase of the protein amount, and that isoprene emission of leaves developing above those which are exposed to ozone can be regulated at the transcriptional level, as also observed in leaves exposed to light and heat stress (Sasaki et al. 2005).
The observation that new leaves (C leaves) developed inside the cuvettes under high levels of ozone, while already developed leaves (B leaves) showed larger damage and were eventually shed, reveals adjustments leading to the acquisition of ozone resistance. This is in our view an important observation whose biochemical basis should be further investigated. The mesophyll of C leaves exposed to ozone was more packed than that of C leaves of controls and other leaf classes, suggesting that ozone diffusion might be reduced and that this may be related to the observed resistance. Ozone exposure often results in a reduction of mesophyll thickness and to more packed cell structure (Oksanen et al. 2005). Probably, C leaves also have a more developed antioxidant system protecting them from reactive oxygen species formed by ozone (Diara et al. 2005, Pell et al. 1997). Isoprene was described to have an antioxidant action protecting leaves from ozone (Loreto and Velikova 2001). We have detected a stimulation of isoprene emission in C leaves directly exposed to ozone in comparison to the emission of corresponding control leaves. However, the emission of ozone-treated C leaves was lower than that in A1 leaves and in B leaves at the beginning of the stress treatment. Two aspects should be considered when analysing this data set. First, C leaves were analysed at a very young stage, comparable to those of A3 leaves. This is reflected in a yet low developmental capacity to produce isoprene as also indicated by the very low level of PaISPs mRNA detected in C leaves. Second, despite being resistant to ozone, the photosynthetic metabolism of C leaves was perturbed by direct ozone exposure and this may have further limited isoprene production. A very large limitation of photosynthesis was revealed in ozone-exposed C leaves by the response of photosynthesis to increasing Ci. The limitation was particularly evident at low CO2 levels at which photosynthesis responds linearly to CO2 and is limited by Rubisco activity (von Caemmerer and Farquhar 1981). Rubisco is known to be negatively affected by ozone, being a very common cause of photosynthesis limitation in ozone-stressed leaves (Pell et al. 1997). It is suggested that Rubisco remains a parameter sensitive to ozone, even in leaves that acquire resistance to the pollutant.
In summary, our novel fumigation apparatus allowed to study at the same time the direct and indirect impact of ozone on developing P. alba leaves. Large changes in the anatomy of the leaves developing immediately after those exposed to ozone were detected. These leaves also showed a stimulation of isoprene emission not associated with higher photosynthesis levels, indicating that a larger fraction of the carbon budget was allocated to form isoprene as an indirect consequence of ozone stress. Ozone also indirectly affected starch accumulation in developing leaves, probably delaying starch biosynthesis or causing a rapid translocation of starch to other plant parts. This study also revealed that direct exposure to ozone may lead to the development of a thin class of leaves, with packed mesophyll and resistant to the pollutant. These leaves also show isoprene stimulation, again revealing a larger carbon investment into the isoprenoid pathway, whereas photosynthetic rates are severely limited by Rubisco activity.
Edited by K.-J. Dietz
Acknowledgments
Acknowledgements – This study was supported by The European Commission (contract MC-RTN-CT-2003-504720, “ISONET”), by the European Science Foundation programme Volatile Organic Compounds in the Biosphere-Atmosphere System (VOCBAS) and by the Italian Ministry for Research projects FIRB – RBAU018FWP: “Attivita’ antiossidante degli isoprenoidi volatili e loro ruolo nella protezione delle piante dagli stress abiotici” and PRIN –“Valutazione di rischio di livello II da esposizione ad ozono per la vegetazione mediterranea. Analisi sperimentali in condizioni controllate di laboratorio e in campo, misure e modellistica dei flussi di ozono”.




