Isolation of cDNA clones corresponding to genes differentially expressed in pericarp of mume (Prunus mume) in response to ripening, ethylene and wounding signals
Abstract
Ripening of climacteric fruit is a complex developmental process that includes many changes in gene expression. Some ripening-regulated genes are responsive to ethylene and/or wounding signals. Wounding increased Pm-ACS1 expression in Prunus mume (Japanese apricot), but was negatively regulated by ethylene. However, exposure of freshly harvested mature green mume fruit to ethylene induced PmACS1. Fifteen complementary DNA clones corresponding to messenger RNAs differentially expressed in the pericarp of P. mume fruit in response to ripening, ethylene and wounding signals were isolated by differential display. Quantitative real-time PCR analysis distinctly showed that these genes are differentially regulated. Genes that were upregulated during fruit ripening include Pm15 (cinnamyl-alcohol dehydrogenase), Pm21 (2-oxoacid-dependent dioxygenase), Pm22 (1-acyl-sn-glycerol-3-phosphate acyltransferase), Pm27 (unknown function), Pm38 (alcohol dehydrogenase), Pm41 (no homology), Pm52 (no homology), Pm65 (pectate lyase), Pm68 (expansin), Pm69 (serine carboxypeptidase) and Pm94 (alcohol acyltransferase). Expression of most of these genes was also inducible by ethylene and some of them were inducible by wounding. Pm3 (water channel protein, MIP) and Pm8 (unknown function) were downregulated during ripening. Expression of Pm71 (no homology) and Pm74 (NAC family protein) did not increase during ripening or in response to ethylene, but was upregulated in response to wounding. The possible physiological roles of these genes during ripening and in response to ethylene and wounding are discussed.
Abbreviations –
-
- ACC
-
- 1-aminocyclopropane-1-carboxylic acid
-
- AMV
-
- avian myeloblastosis virus
-
- cDNA
-
- complementary DNA
-
- DIG
-
- digoxigenin
-
- F
-
- forward primer
-
- mRNA
-
- messenger RNA
-
- NBD
-
- 2,5-norbornadiene
-
- PA
-
- phosphatidic acid
-
- RACE
-
- rapid amplification of cDNA ends
-
- R
-
- reverse primer
-
- RT-PCR
-
- reverse transcription–PCR
Introduction
Fruits are broadly classified as either climacteric or non-climacteric based on measurable physiological changes that occur during ripening. Although non-climacteric fruits, such as citrus, may respond to ethylene [an example being ethylene-induced messenger RNA (mRNA) and pigment accumulation in the flavedo of orange; Alonso et al. 1995], ethylene is not required for fruit ripening. Climacteric fruits such as tomato and banana are characterized by autocatalytic ethylene production and a distinct respiratory burst during the ripening process (Brady 1987). Exposure to exogenous ethylene, inhibition of ethylene synthesis, or the action of various chemical agents greatly affect the rate of ripening. Ripening of climacteric fruit is a complex developmental process that involves changes in gene expression and enzyme activity (Brady 1987, Fischer and Bennett 1991). Identification of genes whose expression is regulated during ripening is an important approach for understanding the molecular mechanisms responsible for ethylene-related phenomena during ripening.
Previous studies have resulted in the isolation of a number of genes whose expression is regulated during ripening of climacteric fruit through differential screening or differential display techniques (Aggelis et al. 1997, Clendennen and May 1997, Domínguez-Puigjaner et al. 1997, Hadfield et al. 2000, Ledger and Gardner 1994, Lincoln et al. 1987, Medina-Suárez et al. 1997, Pear et al. 1989, Santino et al. 1997, Zegzouti et al. 1999). Expression of most of these genes is ethylene inducible (Aggelis et al. 1997, Domínguez-Puigjaner et al. 1997, Lincoln et al. 1987, Medina-Suárez et al. 1997, Zegzouti et al. 1999). Non-climacteric fruit have also been analyzed for genes that are regulated during ripening (Manning 1998, Medina-Escobar et al. 1997a, 1997b, Nam et al. 1999, Proust et al. 1996, Wilkinson et al. 1995). In addition to the expression of genes associated with the synthesis or perception of ethylene, cell-wall metabolism and the accumulation of sugars and pigments during the ripening of climacteric fruit, expression of genes associated with senescence (Clendennen and May 1997), pathogenesis (Clendennen and May 1997, Fils-Lycaon et al. 1996, Itai et al. 2000, Medina-Suárez et al. 1997, Tattersall et al. 1997) and stress response (Clendennen and May 1997, Itai et al. 2000, Ledger and Gardner 1994, Medina-Suárez et al. 1997, Nam et al. 1999, Zegzouti et al. 1999) are also ripening regulated. Some ripening-enhanced genes are also inducible in response to wounding (Diallinas and Kanellis 1994, Lincoln et al. 1987, Parsons and Mattoo 1991).
Mume (Prunus mume Sieb. et Zucc.) is a climacteric fruit that produces large amounts of ethylene during ripening (Mita et al. 1999, Sawamura and Miyazaki 1989). The evolution of ethylene during ripening is associated with a marked increase in the expression of the ethylene-biosynthetic enzymes 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase (Mita et al. 1999). In other climacteric fruit, such as tomato, passion fruit and kiwifruit, prominent expression of ACC oxidase precedes the expression of ACC synthase and the burst of ethylene evolution during fruit ripening (Mita et al. 1998, Nakatsuka et al. 1997, Xu et al. 1998). Expression of the gene encoding ACC synthase has been proposed as the rate-limiting step that controls the burst of ethylene during fruit ripening in these plant species. By contrast, expression of ACC synthase is increased earlier than that of ACC oxidase during mume fruit ripening (Mita et al. 1999). It is likely that the ACC synthase and ACC oxidase genes of mume are activated sequentially for maximum production of ethylene during ripening (Mita et al. 1999). The early spike in expression of ACC synthase after harvesting mume fruit at the mature green stage should likely result in the quick ripening.
We used fluorescent differential display with total RNA isolated from pericarps of mume fruit to study the molecular aspects of ripening and responses to ethylene and wounding. We isolated 15 complementary DNA (cDNA) clones corresponding to mRNAs that are differentially expressed during fruit ripening and in response to ethylene and wounding. The possible functions of these genes in relation to fruit metabolism and to quality traits in mume are discussed.
Materials and methods
Plant material
Immature green and mature green mume fruit (Japanese apricot, P. mume Sieb. et Zucc. cv. Nanko) were obtained from a local grocer and from the Experimental Farm of Shizuoka University, from 1999 to 2003. Fruit at the immature green stage was harvested in the middle of May (70–85 days after full bloom) and at the mature green stage in the middle of June (100–115 days after full bloom). The mature green fruit were held at 20°C and allowed to ripen. Partially ripened fruit (2 days after harvest; ethylene evolution between 5 and 20 nl g−1 h−1) and fully ripened fruit (5 days after harvest; ethylene evolution above 200 nl g−1 h−1) were used. For treatment of fruit with ethylene, mature green fruit were placed in a 10-l desiccator into which ethylene was introduced at the required concentration (Hyodo and Fujinami 1989). For wounding experiments, pericarp tissue was sliced into blocks (0.8 × 0.8 × 0.5 cm3) and incubated on filter paper that had been moistened with 10 mM phosphate buffer (pH 7) and 50 μg l−1 chloramphenicol for 7 h at 20°C in a 10-l desiccator. Sliced fruit were also placed in a desiccator, into which ethylene and 2,5-norbornadiene (NBD) were introduced separately or in combination at the required concentration, as described by Hyodo and Fujinami (1989). NBD is a competitive inhibitor of the action of ethylene (Sisler and Yang 1984). Pericarp tissue for extraction of RNA was kept frozen at −80°C until use.
Extraction of RNA
Total RNA was extracted from the pericarp of at least 10 fruits with phenol and sodium dodecyl sulfate, and precipitated in lithium chloride as previously described (Nakamura et al. 1991). Poly(A)+ RNA was isolated with Oligotex dT-30 (Takara Shuzo, Kyoto, Japan).
Fluorescent differential display
Fluorescent differential display was performed with a Fluorescence Differential Display Kit (Takara Shuzo, Kyoto, Japan), according to the manufacturer’s instructions. Total RNA was extracted from mature green, ripe, ethylene-treated and wounded fruits. cDNAs were synthesized from 300 ng of total RNA in 20 μl of a reaction mixture, which contained 10 mM Tris–HCl (pH 8.3), 50 mM KCl, 5 mM MgCl2, 1 mM of each dNTP, 20 units of RNase inhibitor, 5 units avian myeloblastosis virus (AMV) reverse transcriptase and 2.5 μM of a fluorescein-labeled downstream primer (5′ fluorescein-labeled TnVV; n = 13–16, V = A or C or G). The reaction was incubated at 55°C for 30 min. The mixture was then heated to 99°C for 5 min to inactivate the reverse transcriptase. One-tenth of the resultant cDNA was amplified by PCR using combinations of arbitrary 10-mer upstream primers and fluorescein-labeled downstream primers that were the same as those used for the synthesis of cDNA, according to the manufacturer’s instructions. The parameters for PCR were as follows: denaturation at 94°C for 2 min, annealing at 38°C for 5 min and extension at 72°C for 5 min, for one cycle; denaturation at 94°C for 30 s, annealing at 38°C for 2 min and extension at 72°C for 1 min, for a total of 34 cycles, followed by 5 min extension at 72°C. Aliquots of each PCR reaction were removed for Southern hybridization (see below). After addition of an equal amount of loading buffer (95% formamide, 20 mM EDTA, 0.05% bromophenol, 0.05% xylene cyanol FF) to the remaining solution, aliquots of each sample were heated for 3 min at 94°C and separated by electrophoresis on a 4% Long Ranger gel system (Takara Shuzo, Kyoto, Japan; BMA, Rockland, ME) containing 7 M urea and 1× TBE (89 mM Tris–HCl, pH 8.0, 89 mM boric acid, 2 mM EDTA). The fingerprinting pattern of DNA fragments was scanned with a fluorescent image analyzer (Molecular Imager FX system; Bio-Rad Laboratories, Hercules, CA) and differentially expressed DNA bands (positive bands) were excised from the gel. DNA fragments of interest were eluted in 50 μl of TE buffer (10 mM Tris–HCl, pH 8.0, 1 mM EDTA) and re-amplified by a second round of PCR using the appropriate combination of arbitrary 10-mer upstream primers and fluorescein-labeled downstream primers for 17 cycles of 30 s at 94°C, 2 min at 38°C, and 1 min at 72°C. Because this resultant PCR reaction often contains equal-sized DNA fragments differing in base composition, DNA fragments were separated by electrophoresis with an agarose gel containing a base-specific ligand (HA-red or HA-yellow, Takara Shuzo, Kyoto, Japan). The pattern of DNA fragments was scanned with a fluorescent image analyzer, and each DNA band was excised from the gel and purified with QIAEX II Gel Extraction Kit (QIAGEN). Southern blot hybridization was carried out to identify the DNA fragment that corresponds to the one of interest. Each DNA was labeled with alkaline phosphatase enzyme with an AlkPhos DIRECT labeling kit (Amersham Pharmacia Biotech, Buckinghamshire, UK) and used for Southern blot hybridization. cDNA from the first round of PCR (aliquots removed) was subjected to electrophoresis on a 1.5% agarose gel. Bands of DNA were then transferred to a Hybond-N+ membrane (Amersham Pharmacia Biotech, Buckinghamshire, UK) by capillary action in 0.4 M NaOH, according to the manufacturer’s instructions. The DNA on the membrane was allowed to hybridize with an alkaline phosphatase–labeled DNA probe at 55°C for 17 h. The membrane was washed and signals were detected by chemiluminescence with CDP-Star (Tropix, Bedford, MA) according to the manufacturer’s instructions. The cDNA fragments that showed differential expression were cloned into the vector pCR II (Invitrogen, San Diego, CA) and sequenced.
Quantification of transcript levels using real-time PCR assay
Quantitative real-time PCR analyses were performed using a LightCycler system from Roche Diagnostics GmbH (Mannheim, Germany). Two hundred nanograms of Poly(A)+ RNA that had been extracted from each sample was reverse transcribed with AMV reverse transcriptase (Roche Diagnostics GmbH) using 1.6 μM of oligo d(T)15 primer. The reaction was incubated at 25°C for 10 min to anneal primer to the RNA template and then at 42°C for 60 min. The mixture was then heated to 99°C for 5 min to inactivate the reverse transcriptase. All PCR reactions were performed using LightCycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics GmbH), according to the protocol provided by the supplier. The reaction mixture contained one-tenth of the reverse transcription reaction, 1× LightCycler-FastStart DNA Master SYBR Green I, 4 mM MgCl2 and 500 nM of each forward and reverse gene-specific primer (F and R) in a 20 μl reaction mixture. The cycling conditions were as follows: initial denaturation at 95°C for 10 min, followed by denaturation of the template at 95°C for 15 s; annealing of primers at 54–62°C for 5 s; and extension at 72°C for 10 s, for a total of 45 cycles. The annealing temperature differed depending on the combinations of gene-specific primers. The F and R primers for each gene and the annealing temperature are as follows: Pm3 (water channel protein, MIP), 5′-GCGGTGTTGGGTTGGTGAAG-3′ (F), 5′-CTCTGGCGTTTCTCTTGGGG-3′ (R), 60°C; Pm8 (unknown function), 5′-CCACACAGCACATAACCCAG-3′ (F), 5′-GTTGCTTTCCAGTGCTCCAG-3′ (R), 63°C; Pm15 (cinnamyl-alcohol dehydrogenase), 5′-AGTGCGCGGATGATAAGCCT-3′ (F), 5′-CACCAGCCGAAACATAGCAC-3′ (R), 63°C; Pm21 (2-oxoacid-dependent dioxygenase), 5′-TGTCCCAGTTGATCCTTGCC-3′ (F), 5′-GCTTCCTGATCCTTTGGTCC-3′ (R), 60°C; Pm22 (1-acyl-sn-glycerol-3-phosphate acyltransferase), 5′-TGTTGCTTGCGTGTTCGTCA-3′ (F), 5′-AGCCTCACCATGTTCACTAC-3′ (R), 58°C; Pm27 (no homology), 5′-CTCTCCTATTGGTGGTTCGG-3′ (F), 5′-CAACAACACCCTGCCATGAC-3′ (R), 63°C; Pm38 (alcohol dehydrogenase), 5′-GTTGATGGGGCACAGTCTCT-3′ (F), 5′-GAGATGTGGAGTGTAGGGTG-3′ (R), 61°C; Pm41 (no homology), 5′-GTTATTTATCTGCCCTTAGC-3′ (F), 5′-GAACATAATGAAACAGAGCC-3′ (R), 58°C; Pm52 (unknown function), 5′-GTGCAGAAGAGGAAGAGAGCGC-3′ (F), 5′-GCCCTTTTGCACAGCCTCGTTC-3′ (R), 68°C; Pm65 (pectate lyase), 5′-ATATAGGTGGAGGGCAGTGA-3′ (F), 5′-ACCCTATTGCTGCCTAAGAG-3′ (R), 61°C; Pm68 (expansin), 5′-AGCCAGGGTAGATTAGGAGA-3′ (F), 5′-TAGCTCACAATCTCCACCGT-3′ (R), 61°C; Pm69 (serine carboxypeptidase), 5′-ACGATCTGCTTGTCCATAAC-3′ (F), 5′-GTGAAATTGTATAGCCAGCC-3′ (R), 60°C; Pm71 (no homology), 5′-CGATTTGGGGACGAAGCTGC-3′ (F), 5′-ATCCAATCATCCCCCGCCAC-3′ (R), 64°C; Pm74 (NAC family protein), 5′-GGAAGGAGTGGGAGAAGGCC-3′ (F), 5′-TCTCCCGTTGCCGTCCGATG-3′ (R), 62°C; Pm94 (alcohol acyltransferase), 5′-TTGAGGGGCTGGCTTTCGTA-3′ (F), 5′-GCACTCGGAAAGACCATATC-3′ (R), 61°C; PmACS1, 5′-CCCAATGTCTCGCCAGGGTC-3′ (F), 5′-CTTCCCCGGGCGTACAAATG-3′ (R), 65°C; PmACO1, 5′-GATCAAGGGTCTCCGGGCTC-3′ (F), 5′-AGTGGCGCATGGGAGGCACA-3′ (R), 67°C; PmER1 (used as a control gene), 5′-ACACTCCGTCAGCAGAATCCAGTA-3′ (F), 5′-TAGAGAGATGGAGGAGAGGGACA-3′ (R), 66°C. Quantitative real-time PCR reactions were conducted at least five times with five fruits in each treatment, and the mean copy numbers of individual cDNAs corresponding to mRNA species of interest were estimated using standard cDNA preparations of known molar concentrations.
Rapid amplification of cDNA ends
The 5′ ends of the cDNA fragments (Pm3, Pm15, Pm22, Pm27, Pm38, Pm52, Pm65, Pm68, Pm69, Pm94), which remained undetermined after fluorescent differential display, were isolated by the 5′ rapid amplification of cDNA ends (RACE) method. All reactions were performed with the Marathon cDNA Amplification kit (Clontech Laboratories, Palo Alto, CA) in accordance with the manufacturer’s instructions. Poly(A)+ RNA was isolated from the pericarp of ripened fruit or wounded fruit, and cDNA was reverse transcribed from it. The cDNA was then ligated to the Marathon cDNA adaptor. The 5′ cDNA fragment for each cDNA was amplified by PCR with the gene-specific reverse primer that was also used for quantitative real-time PCR assay and adaptor primer 1 as primers and the adaptor-ligated cDNA as template. The 5′ end of the Pm3 cDNA could be isolated by this PCR (gene-specific primer; 5′-CTCTGGCGTTTCTCTTGGGG-3′). The cDNA for other genes in a portion of the mixture after PCR was re-amplified with the gene-specific nested primer and nested adaptor primer 2. The nucleotide sequences of the gene-specific nested primer for each gene are as follows: Pm15, 5′-TCTGCCACTAGCTGAAGGAATCTC-3′; Pm22, 5′-CCAACCAAAAGGGCTGAGGGAAGT-3′; Pm27, 5′-CGCCATCCTCTTCGTCTGTATCAA-3′; Pm38, 5′-TCACATATAAGAGCGCATCCGAGG-3′; Pm52, 5′-CTCCACCATCAGCAGCAGCCATTT-3′; Pm65, 5′-ATCTATTCCCTTGGCTATTGATTG-3′; Pm68, 5′-CCCCAGTTCCTTGACATGGCTTGC-3′; Pm69, 5′-TGAGATTGTACAGCCAGCCATTTTG-3′; Pm94, 5′-GGCATGTCGGTTCCTTATATGTCG-3′. The parameters for the first round of PCR were as follows: denaturation at 94°C for 30 s, annealing at 60°C for 30 s and extension at 68°C for 4 min, for a total of 30 cycles. Fifteen cycles of nested PCR were performed under the same conditions as the first PCR. The 5′ ends of the fragments of Pm8, Pm41 and Pm71 could not be isolated. The full-length open reading frames (ORFs) of Pm21 and Pm74 were included in the first DNA fragments obtained in the course of fluorescent differential display.
DNA sequence analysis
The nucleotide sequence of each cloned cDNA was determined by the dideoxy sequencing method (Sanger et al. 1977). The sequences were edited to remove vector sequences and aligned using GENETYX-MAC ver. 11 (Software Development, Tokyo, Japan). DNA sequences were compared with all known DNA sequences in the GenEMBL database using GENETYX-MAC and FASTA (Genetics Computer Group, Madison, WI). Non-redundant sequences from the pericarp of mume clones have been submitted to the database, and the accession numbers are listed in Table 1.
| Clone | Accession no. for mume clone | cDNA size (bp) | Transcript size (kb) | Putative identity | Related sequence and accession no. | Identity/aa overlap | |
|---|---|---|---|---|---|---|---|
| % | aa | ||||||
| Pm3 | AB218716 | 1278 | 1.5 | Water channel protein, MIP | Radish AB030698 | 82 | 282 |
| Pm8 | AB218717 | 1161 | 3.5 | Unknown function | Soybean BE611556 | 58 | 175 |
| Pm15 | AB218718 | 1321 | 1.6 | Cinnamyl alcohol dehydrogenase | Apple T16995 | 94 | 326 |
| Pm21 | AB218719 | 1172 | 1.4 | 2-Oxoacid-dependent dioxygenase | Apple AJ225045 | 82 | 307 |
| Pm22 | AB218780 | 1741 | ND | 1-Acyl-sn-glycerol-3-phosphate acyltransferase | Limnanthes Z48730 | 71 | 387 |
| Pm27 | AB218781 | 1622 | 1.8 | No significant homology | |||
| Pm38 | AB218782 | 1119 | 1.3 | Alcohol dehydrogenase | Strawberry AX025497 | 83 | 176 |
| Pm41 | AB218783 | 376 | ND | No significant homology | |||
| Pm52 | AB218785 | 541 | ND | Unknown function | Arabidopsis AC003981-6 | 53 | 131 |
| Pm65 | AB218786 | 1695 | 1.6 | Pectate lyase | Tomato X55193 | 74 | 414 |
| Pm68 | AB218787 | 1258 | 1.3 | Expansin | Strawberry AF159563 | 91 | 253 |
| Pm69 | AB218788 | 1755 | 1.8 | Serine carboxypeptidase | Arabidopsis T48977 | 65 | 506 |
| Pm71 | AB218790 | 347 | ND | No significant homology | |||
| Pm74 | AB218789 | 1295 | 1.4 | NAC family protein | Arabidopsis X74755 | 71 | 295 |
| Pm94 | AB218791 | 1695 | 1.8 | Alcohol acyltransferase | Mango AX025510 | 78 | 430 |
Preparation of digoxigenin-UTP-labeled RNA probes
Digoxigenin (DIG)-labeled single-stranded antisense RNA probes were prepared from recombinant plasmids, into which the 3′ cDNA fragments obtained by RACE (Mita et al. 1999) had been cloned, with a DIG RNA-Labeling kit (Roche Diagnostics, Mannheim, Germany) according to the manufacturer’s instructions.
Northern blotting analysis
Northern blotting analysis was performed as previously described (Mita et al. 1999). Two micrograms of Poly(A)+ RNA was subjected to electrophoresis on a 1.2% agarose gel that contained 0.66 M formaldehyde.
Results and discussion
Negative regulation of wound-inducible expression of PmACS1 by ethylene
As previously described (Mita et al. 1999), we could isolate only one cDNA for the gene that encodes ACC synthase, PmACS1, and only one cDNA for the gene that encodes ACC oxidase, PmACO1, which were expressed within fruit after extensive reverse transcription–PCR (RT-PCR) and RACE. Expression of mume fruit PmACS1 is enhanced during ripening and is responsive to ethylene and wounding (Mita et al. 1999). Because of the effect of exogenous application of ethylene to the pericarp after wounding (Sawamura and Miyazaki 1989), it has been proposed that ethylene negatively regulates the wound-induced increase in the activity of ACC synthase. As shown in Fig. 1, wound-inducible accumulation of PmACS1 mRNA was markedly repressed by the simultaneous application of 20 μl l−1 of ethylene. Exposure of wounded pericarp to NBD, a competitive inhibitor of ethylene action, at 4000 μl l−1 does not significantly affect the amount of PmACS1 mRNA in wounded pericarp (Fig. 1). The wound-inducible accumulation of PmACS1 mRNA should not require the action of ethylene. Ethylene had a negative effect on wound-inducible accumulation of PmACS1 mRNA, because simultaneous application of 100 μl l−1 ethylene with 4000 μl l−1 NBD markedly repressed the wound-inducible accumulation of PmACS1 mRNA (Fig. 1). It is known that 100 μl l−1 ethylene overcomes the inhibitory effect 4000 μl l−1 NBD (Mita et al. 1999). This result is consistent with the previous observation described by Sawamura and Miyazaki (1989). However, this result is troubling because exposure of freshly harvested mature green mume fruit to ethylene increased the amount of PmACS1 mRNA (Mita et al. 1999; Fig. 2[link]). Thus, the ethylene and wounding signals act as positive regulators of PmACS1 when they act independently, but may be regulatory antagonists when simultaneously acting in mume fruit. On the other hand, it should be noted that ethylene exposure times varied from experiment to experiment (i.e. 7 h in Fig. 1, 20 h in Fig. 2 and 12 h in Mita et al. 1999). We cannot exclude the possibility that ethylene also negatively regulates the expression of PmACS1 when freshly harvested mature green mume fruit are exposed to ethylene for 7 h or below.
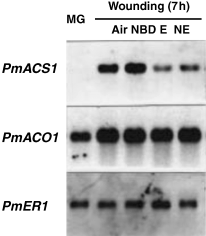
Effects of ethylene and 2,5-norbornadiene (NBD) on wound-inducible accumulation of PmACS1 messenger RNA (mRNA). Mature green fruit were sliced into cubes and incubated under one of the following four sets of conditions for 7 h: in air, in the presence of 4000 μl l−1 NBD (NBD), in the presence of 20 μl l−1 ethylene (E) and in the presence of 4000 μl l−1 NBD plus 100 μl l−1 ethylene (NE). Two micrograms of sample of Poly(A)+ RNA obtained from pericarp was analyzed by Northern blot hybridization. Ethylene receptor mRNA (PmER1) was used as the control.
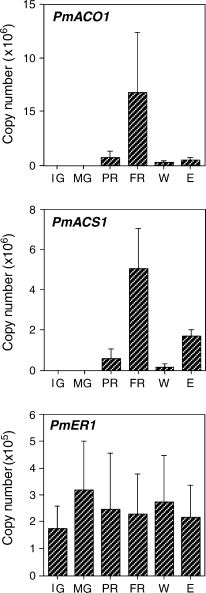
1-Aminocyclopropane-1-carboxylic acid (ACC) synthase (PmACS1) and ACC oxidase (PmACO1) expression as studied by real-time PCR. Immature green fruit (IG) and mature green fruit (MG) were harvested. MG fruit were held at 20°C and allowed to partially ripen (2 days after harvest; PR) or to fully ripen (5 days after harvest; FR). Intact MG fruit were sliced into cubes and incubated in air for 7 h (W) or treated with ethylene at 20 μl l−1 for 20 h (E). Expression of the ethylene receptor (PmER1) was used as the control. The data represent means obtained from at least five experiments with five fruits in each sample. Error bars indicate the standard deviation of the mean.
Although the mRNA band of PmACO1 was barely detectable in previous experiments (Mita et al. 1999), exposure to X-ray film for a longer period made it detectable. In previous experiments, wounding of the pericarp was limited to 5 h, with no effect on PmACO1 (Mita et al. 1999). Wounding the pericarp of mume fruit also increased the mRNA level of PmACO1 within 7 h, but in contrast to expression of PmACS1, the wound-enhanced accumulation of PmACO1 mRNA was not affected by ethylene, suggesting that PmACS1 and PmACO1 are regulated independently. These results also suggest that the mechanisms regulating the perception and transduction of the ethylene signal in wounded fruit are different from those in intact ripening fruit where expression of PmACS1 is upregulated by ethylene (Mita et al. 1999, Sawamura and Miyazaki 1989). The effects of ethylene on wounded plant tissues appear to differ (Hyodo et al. 1993, Kim and Yang 1994, Nakajima et al. 1990). Wounding induces a rapid increase in the activities of ACC synthase and ACC oxidase in the mesocarp tissue of winter squash, with a resultant increase in the rate of ethylene production (Hyodo et al. 1993). It was suggested that ethylene, produced after wounding, might regulate the rate of ethylene production by suppressing the activity of ACC synthase and enhancing that of ACC oxidase (Hyodo et al. 1993, Nakajima et al. 1990). On the other hand, wound-induced expression of the gene for ACC oxidase in mung bean hypocotyls requires the action of ethylene (Kim and Yang 1994).
Isolation of cDNA clones for genes whose expression is modulated during ripening and in response to wounding
As a first step toward understanding the molecular aspects of the ripening of mume fruit, and its response to ethylene and wounding signals, we isolated 15 cDNA clones of genes that are differentially expressed in response to ripening, ethylene and wounding. We compared cDNA derived from the pericarp of mature green fruit and cDNA derived from the pericarp of ripe fruit using differential display RT-PCR. Eleven cDNA fragments were identified (Pm15, Pm21, Pm22, Pm27, Pm38, Pm41, Pm52, Pm65, Pm68, Pm69 and Pm94) that correspond to ripening-enhanced genes and that have altered expression during ripening, and two (Pm3 and Pm8) that correspond to ripening-repressed genes. We also compared cDNA derived from the pericarps of mature green fruit with cDNA from the pericarps of wounded fruit, and obtained two cDNA fragments (Pm71 and Pm74) corresponding to genes whose expression is inducible in response to wounding.
Analyses of expression patterns during fruit ripening and in response to wounding and ethylene with real-time PCR assay
It has been reported that several genes that are regulated during ripening are also regulated by wounding and ethylene signals (Balagué et al. 1993, Barry et al. 1996, Callahan et al. 1992, Lincoln et al. 1993, Miki et al. 1995, Mita et al. 1999, Ross et al. 1992). Although several ACC oxidase genes, whose expression in fruit is enhanced during ripening, are also expressed in response to wounding (Balagué et al. 1993, Barry et al. 1996, Callahan et al. 1992), wound-inducible expression has been confirmed mainly in organs other than fruit. Further analysis is required to determine whether the expression of the ACC oxidase genes is also wound inducible in fruit. Expression of PmACO1 and PmACS1 was examined at an early stage of ripening (2 days after harvest) and at the fully ripened stage (5 days after harvest) with real-time PCR. As previously reported (Mita et al. 1999), expression of both genes was markedly enhanced during ripening (Fig. 2). Fruit that had been wounded for 7 h or treated with ethylene (20 μl l−1) for 20 h also had higher expression levels of PmACO1 and PmACS1 than unripened mature green fruit, but the levels were considerably lower than in fully ripened fruit (Fig. 2). Likewise, pectate lyase transcripts were considerably lower in ethylene-treated fruit than in ripened fruit (Domínguez-Puigjaner et al. 1997). Expression of the control gene PmER1 was unaffected (Fig. 2). Standard deviations are relatively large, possibly because fruits used for this study were collected in different years, from 1999 to 2003. We also examined the expression patterns of genes corresponding to cDNAs isolated by differential display in detail using real-time PCR. Expression of these 15 genes was differentially regulated during ripening and in response to ethylene and wounding (3-5). Among genes corresponding to the isolated cDNAs, expression of 11 genes was upregulated during ripening (Fig. 3). The expression levels of Pm22, Pm27, Pm41, Pm68 and Pm94 at an early stage of ripening were comparable to the fully ripened stage (Fig. 3). Expression of genes corresponding to Pm15, Pm21, Pm22, Pm27, Pm38, Pm41, Pm65, Pm68, Pm69 and Pm94 was also enhanced after ethylene treatment, but the expression of Pm52 increased only slightly after ethylene treatment (Fig. 3). Ripening-enhanced expression of Pm52 may be ethylene independent. Expression levels of genes corresponding to Pm3 and Pm8 decreased during ripening and after ethylene treatment (Fig. 4).
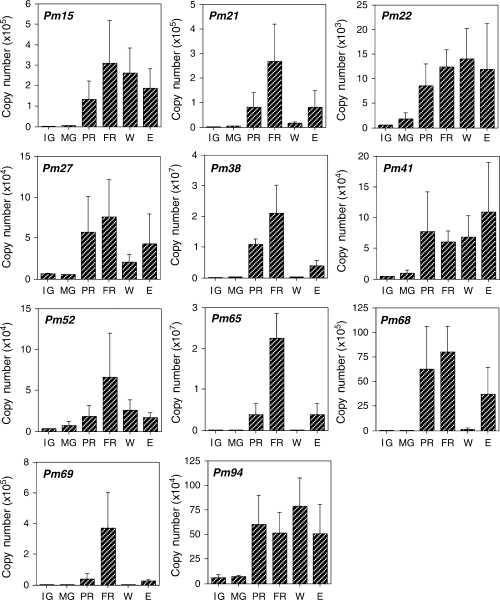
Expression patterns of 11 isolated genes as studied by real-time PCR. Immature green fruit (IG) and mature green fruit (MG) were harvested. MG fruit were held at 20°C and allowed to partially ripen (2 days after harvest; PR) or to fully ripen (5 days after harvest; FR). Intact MG fruit were sliced into cubes and incubated in air for 7 h (W) or treated with ethylene at 20 μl l−1 for 20 h (E). Expression of the ethylene receptor (PmER1) was used as the control, shown in Fig. 2. The data represent mean values obtained from at least five experiments with five fruits in each sample. Error bars indicate the standard deviation of the mean.
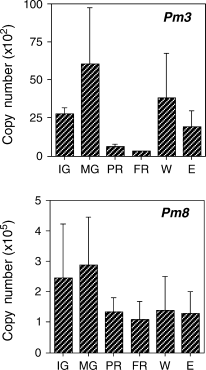
Expression patterns of Pm3 and Pm8 as studied by real-time PCR. Immature green fruit (IG) and mature green fruit (MG) were harvested. MG fruit were held at 20°C and allowed to partially ripen (2 days after harvest; PR) or to fully ripen (5 days after harvest; FR). Intact MG fruit were sliced into cubes and incubated in air for 7 h (W) or treated with ethylene at 20 μl l−1 for 20 h (E). Expression of the ethylene receptor (PmER1) was used as the control, shown in Fig. 2. The data represent mean values obtained from at least five experiments with five fruits in each sample. Error bars indicate the standard deviation of the mean.
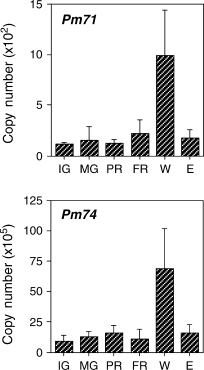
Expression patterns of Pm71 and Pm74 as studied by real-time PCR. Immature green fruit (IG) and mature green fruit (MG) were harvested. MG fruit were held at 20°C and allowed to partially ripen (2 days after harvest; PR) or to fully ripen (5 days after harvest; FR). Intact MG fruit were sliced into cubes and incubated in air for 7 h (W) or treated with ethylene at 20 μl l−1 for 20 h (E). Expression of the ethylene receptor (PmER1) was used as the control, shown in Fig. 2. The data represent means from at least five experiments with five fruits in each sample. Error bars indicate the standard deviation of the mean.
Wounding the pericarp of mume fruit significantly induced the expression of genes corresponding to Pm15, Pm22, Pm27, Pm41, Pm52 and Pm94 within 7 h but did not affect Pm21, Pm38, Pm65, Pm68 nor Pm69. It appears that the cDNA clones in Fig. 3 are grouped into at least two expression pattern groups in response to wounding and ethylene. One group includes Pm15, Pm22, Pm27, Pm41 and Pm94. Another group includes Pm21, Pm38, Pm65, Pm68 and Pm69. Expression of the genes pT52 and pT53 in tomato fruit is inducible in response to wounding and ethylene (Parsons and Mattoo 1991); thus, regulatory mechanisms governing the response to wounding and ethylene might be conserved beyond plant species. On the other hand, mRNA levels of Pm71 and Pm74 increased after wounding but were unchanged during ripening and after ethylene treatment (Fig. 5).
cDNA identification and sequence analysis
The 5′ ends of cDNA fragments, which remained undetermined after differential display, were isolated by 5′ RACE for more accurate identification of each cDNA. As a result, we determined the sequences of 12 perfect ORFs from cDNAs other than Pm8, Pm41, and Pm71. Each clone was then analyzed by comparison with sequences on nucleic acid databases as described in Materials and methods. Among the 15 clones, Pm3, Pm15, Pm21, Pm22, Pm38, Pm65, Pm68, Pm69, Pm74 and Pm94 show significant sequence similarity to a deduced protein in the nucleic acid databases. A summary of these cDNA clones is given in Table 1.
Pm3–water channel protein, MIP
The deduced amino acid sequence of Pm3 was 84% homologous to PAC2C, a water channel protein in the plasma membrane of radish (AB030698; Suga et al. 2001) and 82% homologous to water channel protein in the plasma membrane, MIP, of Arabidopsis (D13254; Yamaguchi-Shinozaki et al. 1992). The deduced amino acid sequence of Pm3 has six membrane-spanning domains and two NPA motifs, which are conserved within all water channels. The first and fourth membrane-spanning domains also include two conserved glutamic acid residues that are the only two conserved charged residues in the transmembrane domains, and are thought to be involved in the recognition and transport of water molecules across the water channel together with the NPA-containing loops (Suga et al. 2001). The expression levels of tonoplast water channel in pear fruit were high at the middle and end of the cell-division stage of fruit development and decreased during the ripening stage (Shiratake et al. 2001b). Cell division in mume fruit comes to an end around the middle of April (Arai 1988). Fruit at the immature green stage was harvested in the middle of May, whereas fruit at the mature green stage was harvested in the middle of June, indicating that cell expansion rather than cell division occurs during the immature green stage. Expression of Pm3 was high in both the immature green and mature green stages but decreased during ripening. Expression of Pm3 might decrease as cell expansion comes to an end. Expression of water channels in the plasma membrane is almost constant during grape berry development (Shiratake et al. 2001a)
Pm15–cinnamyl alcohol dehydrogenase and Pm38–alcohol dehydrogenase
The deduced amino acid sequence of Pm15 was 94 and 82% homologous to cinnamyl alcohol dehydrogenase from apple (T16995) and Eucalyptus gunnii (T10736; Goffner et al. 1998), respectively. The cinnamyl alcohol dehydrogenase of E. gunnii (CAD1) is a novel aromatic alcohol dehydrogenase and recombinant CAD1 exhibits high affinity to lignin precursors, including the 4-coumaraldehyde range of aromatic substrates (Goffner et al. 1998). A putative NAD/NADP(H)-binding site is well conserved at the amino terminus (residues 11–31 of Pm15). Pm38, was presumed to encode an alcohol dehydrogenase that is homologous to strawberry alcohol dehydrogenase (AX025497), possibly involved in flavor biogenesis during fruit ripening, but structurally distinct from the putative cinnamyl alcohol dehydrogenase encoded by Pm15. The upregulation of cinnamyl alcohol dehydrogenase during fruit ripening seems to be inconsistent with the observation that lignin does not increase during ripening. The expression of Pm15 in non-lignifying tissue provides indirect evidence for non-lignin end products (Goffner et al. 1998, O’Malley et al. 1992). Wound-induced Pm15 may contribute to the synthesis of “stress-lignin” after wounding. Cinnamyl alcohol dehydrogenase is thought to contribute to the biogenesis of flavor in Onagrace (Wang et al. 1997). Therefore, enhanced expression of cinnamyl alcohol dehydrogenase may be involved with Pm38 to evolve flavor during ripening, although there are no data at present to support this conclusion.
Pm21–2-oxoacid-dependent dioxygenases
The tomato negative regulatory protein of ethylene synthesis, E8, is upregulated during fruit ripening (Lincoln et al. 1987, Peñarrubia et al. 1992). E8 is structurally related to ACC oxidase and to a large family of 2-oxoacid-dependent dioxygenases. The deduced amino acid sequence of Pm21 was 82% homologous to 2-oxoacid-dependent dioxygenase of apple (ARRO-1; AJ225045; Butler and Gallagher 1999) but not homologous to E8 of tomato. Putative Fe2+-binding residues are conserved in Pm21. ARRO-1 is inducible by auxin. It is unknown whether expression of Pm21 is also auxin induced. In addition, it is also still unknown whether Pm21 affects ethylene synthesis as E8 does. Expression of 2-oxoacid-dependent dioxygenases was induced during leaf senescence in Arabidopsis (Fujiki et al. 2001). Reactions catalyzed by dioxygenase may be important for ageing beyond the various plant tissues and organs.
Pm22–1-acyl-sn-glycerol-3-phosphate acyltransferase
The deduced amino acid sequence of Pm22 was 71 and 67% homologous to 1-acyl-sn-glycerol-3-phosphate acyltransferases from Limnanthes douglasii (pLAT1; Z48730) and Zea mays (pMAT1; Z29518; Brown et al. 1994), respectively. pMAT1 cDNA complemented a 1-acyl-sn-glycerol-3-phosphate acyltransferase mutant of Escherichia coli (Brown et al. 1994). We found six putative transmembrane domains composed of stretches of hydrophobic amino acids and searching with the TargetP program (http://www.cbs.dtu.dk/services/TargetP/) (Emanuelsson et al. 2000) predicted that the protein encoded by Pm22 is part of the secretory pathway. It is possible that the Pm22 protein is localized at the ER membrane, where phospholipid synthesis occurs. 1-Acyl-sn-glycerol-3-phosphate acyltransferase has an important role for synthesis of the second messenger phosphatidic acid (PA). Evidence is accumulating that not only lysoPA but also PA works as a second messenger (Munnik 2001). It is generally accepted that PA formation occurs via two major independent pathways; one is via phospholipase D and another is via phospholipase C and diacylglycerol kinase (Munnik 2001). However, the pathway via 1-acyl-sn-glycerol-3-phosphate acyltransferase must be considered as important to the cell. In fact, overexpression of human 1-acyl-sn-glycerol-3-phosphate acyltransferase cDNA in mammalian cells leads to increased 1-acyl-sn-glycerol-3-phosphate acyltransferase activity and enhanced cytokine-induced signaling response in cells (West et al. 1997). In addition, treatment of mammalian cells with an inhibitor of 1-acyl-sn-glycerol-3-phosphate acyltransferase prevents hypoxia-induced PA and cytokine increases (Abraham et al. 1995). PA might act as a second messenger during ripening, and in response to ethylene and wounding signals (Fig 3; Munnik 2001[link]). In tomato, expression of phospholipase D was enhanced during fruit ripening (Bruce et al. 2001).
Pm65–pectate lyase and Pm68–expansin
Fruit ripening is associated not only with the burst of ethylene production and increased activity of respiration, but also with prominent fruit softening. The deduced amino acid sequence of Pm65 was 74 and 60% homologous to the pectate lyases of tomato (X55193) and strawberry (U63550; Medina-Escobar et al. 1997a), respectively. The deduced amino acid sequence of Pm68 was highly related to strawberry expansin, FaEXP2 (91%; AF159563), whose expression is also enhanced during fruit ripening (Civello et al. 1999). Genes for expansin whose expression is upregulated during ripening have been isolated in tomato and strawberry (Civello et al. 1999, Rose et al. 1997). Repression of a fruit ripening-specific expansin (Exp1) in tomato resulted in reduced softening, whereas overexpression of Exp1 resulted in enhanced softening (Brummell et al. 1999). In addition, fruit-specific expression of cDNA clones with homology to bacterial pectate lyases that were proven to break down the pectic substances of the plant cell wall have been reported in banana (Domínguez-Puigjaner et al. 1997). Transgenic strawberry plants with pectate lyase antisense sequences have been constructed (Jimenez-Bermudez et al. 2002). Fruit from these transgenic plants was firmer than that from controls. The postharvest softening of transgenic fruit was also diminished. These results indicated that the pectate lyase gene is an excellent candidate for biotechnological improvement of fruit softening in strawberry.
Fruit softening of mume occurs very rapidly after mature green unripened fruit are harvested. We isolated cDNAs that encode pectate lyase (Pm65) and expansin (Pm68). Among the two genes, prominent enhancement of expression of the gene for expansin was already observed at the early stage of ripening (Fig. 3). Ripening-specific expression of pectate lyase and expansin has been reported in several plants (Domínguez-Puigjaner et al. 1997, Giovannoni 2001, Medina-Escobar et al. 1997a). Ripening induction of Pm65 (Pectate lyase) and Pm68 (Expansin) may be responsible for fruit softening during mume fruit ripening. On the other hand, neither Pm65 nor Pm68 is inducible in response to wounding, suggesting that neither had any role in the pericarp after wounding.
Pm69–serine carboxypeptidase
Several cDNAs corresponding to genes involved in the metabolism of protein and amino acids during fruit ripening have been isolated (Alonso and Granell 1995, Itai et al. 2000, Nam et al. 1999, Praekelt et al. 1988). The deduced amino acid sequence of Pm69 was 61% homologous to the serine carboxypeptidase of Matricaria chamomilla (Kohchi et al. 1999). Comparison of sequences and searching with the TargetP program (http://www.cbs.dtu.dk/services/TargetP/) (Emanuelsson et al. 2000) predicted that the N-terminal signal peptide of the Pm69 protein that would direct the protein to the secretory pathway is composed of 24 amino acid residues. It has been reported that expression of proteases is upregulated during ripening (Alonso and Granell 1995, Itai et al. 2000). The physiological role of serine carboxypeptidase remains unknown in plants, except that serine carboxypeptidase is involved in brassinosteroid signal transduction (Li et al. 2001). To our knowledge, this is the first report of cloning of a serine carboxypeptidase whose expression is upregulated during fruit ripening.
Pm74–NAC transcription factor
Wounding significantly increased expression of Pm74 (Fig. 5). The deduced amino acid sequence of Pm74 was 71% homologous to the ATAF1 transcription factor of Arabidopsis, which contains a NAC domain in the N-terminal region (Collinge and Boller 2001). Expression of Arabidopsis ATAF1 is also wound inducible (Collinge and Boller 2001). In addition, a glutamine-rich region with 11 consecutive glutamine residues was also found in the middle part of Pm74. Glutamine-rich regions work as a transactivation domain in many transfactors. The glutamine-rich region was absent from the protein sequence of ATAF1. NAC transcription factors are structurally distinct and are functionally diverse (Olsen et al. 2005). Expression of many NAC domain types of transcription factors is responsive to both biotic and abiotic stresses (Olsen et al. 2005). Pm74 transcription factors might be involved in the regulatory mechanisms of expression of other genes whose expression is inducible in response to wounding.
Pm94–alcohol acyltransferase
The deduced amino acid sequence of Pm94 was 78% homologous to the putative alcohol acyltransferase in mango. In melon, expression of alcohol acyltransferase (CM-AAT1; previously named Mel2) is specifically expressed in fruit at increasing rates in the early and middle phases of ripening (Aggelis et al. 1997, Yahyaoui et al. 2002). Despite the observation that Pm94 and CM-AAT1 are structurally divergent, H-x-x-x-DG and DFGWG motifs are thought to play a role in activity and are well conserved. CM-AAT1 is involved in the generation of aroma volatile esters during melon ripening (Yahyaoui et al. 2002). Pm94 may be involved in the synthesis of flavor during mume fruit ripening. Expression of the gene corresponding to Pm94 was also wound inducible. It has been reported that several acyltransferases are responsive to biotic stresses (Czernic et al. 1996, Yang et al. 1997), and are involved in the synthesis of phytoalexins as a response to biotic stresses. The physiological meaning of wound-responsive induction of Pm94 remains unknown.
Conclusions
In this study we examined the effects of ethylene on wound-inducible expression of ACC synthase (PmACS1) and proposed that molecular regulation in response to ethylene and wounding, like many plant hormone interactions, is complicated. Intact and wounded fruit may respond in opposite ways to the ethylene signal, at least for expression of PmACS1 (1, 2[link]). Future work will be performed to clarify the factor(s) involved in wound response interactions with the ethylene signal in the regulation of PmACS1. A number of ripening-regulated genes have been isolated by differential screening or differential display in both climacteric and non-climacteric fruit. However, there have been only a few experiments that address whether expression of these genes is also responsive to the wounding signal in fruit tissues. In addition, simultaneous comparisons of ACC synthase or ACC oxidase expression between ripe, wounded and ethylene-treated fruit have yet to be fully exploited in gene regulation studies. We used a differential display method to isolate cDNAs that had markedly different expression levels during ripening and in response to ethylene and wounding signals. As a result, we isolated 15 cDNAs that are differentially regulated and have discussed their physiological roles based on individual sequences and expression patterns. Further experiments will be required to confirm the function of each cDNA. We are in the process of constructing transgenic tomato plants in which each of these cDNAs is overexpressed. These plants may allow us to determine the function of each of the gene products during ripening, and in response to ethylene and wounding signals. Ripening is a kind of genetically programmed senescence, and many stress-related genes, such as pathogen resistance genes, that are regulated during ripening have been isolated (Clendennen and May 1997, Fils-Lycaon et al. 1996, Itai et al. 2000, Ledger and Gardner 1994, Medina-Suárez et al. 1997, Nam et al. 1999, Tattersall et al. 1997, Zegzouti et al. 1999). However, we could not isolate stress-related genes that are modulated during ripening in this study. In addition, neither PmACS1 nor PmACO1 was identified by this differential display experiment. More effective methods such as expression analysis of the expressed sequence tags and cDNA microarrays should be considered to identify more genes of interest. Quantitative real-time PCR analysis showed that the isolated genes are differentially regulated, but some genes seem to be similarly regulated. Therefore, although more than one stimulus pathway controlled their expression, it is likely that a common pathway also regulates expression of a subset of these genes. Because most of the ripening-inducible genes are also upregulated by ethylene to a greater or lesser extent, it is expected that ripening-inducible expression is dependent on ethylene in climacteric fruit.
A subset of ripening-enhanced genes is also induced by wounding. The same regulatory mechanisms may work to control them in response to both ripening and wounding signals. Because wounding is associated with plant defense responses, wounding-enhanced genes may have some role in plant–pathogen interactions. Phytohormones are involved in response to wounding in many cases (Delessert et al. 2004, Lincoln et al. 1993, Olson et al. 1991, Park et al. 2002, Royo et al. 1996, Yamada et al. 2004). We have not yet performed experiments to confirm whether phytohormones are involved in the wound-enhanced expression of genes that were isolated in this study. In addition, it is also unknown whether wound-inducible expression of genes other than PmACS1 is affected by ethylene. Further study should unfold the underlying molecular mechanisms that allow the plant to respond to ripening, ethylene and wounding signals.
Edited by D. Van Der Straeten




