Synthesis and AT2 receptor-binding properties of angiotensin II analogues
Abstract
Abstract: The present study investigates the importance of the amino acid side chains in the octapeptide angiotensin II (Ang II) for binding to the AT2 receptor. A Gly scan was performed where each amino acid in Ang II was substituted one-by-one with glycine. The resulting set of peptides was tested for affinity to the AT2 receptor (porcine myometrial membranes). For a comparison, the peptides were also tested for affinity to the AT1 receptor (rat liver membranes). Only the substitution of Arg2 reduced affinity to the AT2 receptor considerably (92-fold when compared with Ang II). For the other Gly-substituted analogues the affinity to the AT2 receptor was only moderately affected. To further investigate the role of the Arg2 side chain for receptor binding, we synthesized some N-terminally modified Ang II analogues. According to these studies a positive charge in the N-terminal end of angiotensin III [Ang II (2–8)] is not required for high AT2 receptor affinity but seems to be more important in Ang II. With respect to the AT1 receptor, [Gly2]Ang II and [Gly8]Ang II lacked binding affinity (Ki > 10 μm). Replacement of the Val3 or Ile5 residues with Gly produced only a slight decrease in affinity. Interestingly, substitution of Tyr4 or His6, which are known to be very important for AT1 receptor binding, resulted in only 48 and 14 times reduction in affinity, respectively.
Abbreviations:
-
- Ac
-
- acetyl
-
- BSA
-
- bovine serum albumin
-
- t-Bu
-
- tert-butyl
-
- DMF
-
- N,N-dimethylformamide
-
- ESI
-
- electrospray ionization
-
- Fmoc
-
- 9-fluorenylmethyloxycarbonyl
-
- HBTU
-
- 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
-
- LC-MS
-
- liquid chromatography mass spectrometry
-
- NMM
-
- N-methyl morpholine
-
- Pbf
-
- 2,2,4,6,7-pentamethyldihydro-benzofuran-5-sulfonyl
-
- RP-HPLC
-
- reversed phase high-performance liquid chromatography
-
- SAR
-
- structure–activity relationship
-
- TFA
-
- trifluoroacetic acid
-
- Trt
-
- triphenylmethyl
Introduction
The octapeptide angiotensin II (Ang II) is the main effector component of the renin-angiotensin system. Two major subtypes of Ang II receptors, AT1 and AT2, have been identified. The AT1 subtype is involved in the well-known biological functions of Ang II, which include vasoconstriction, aldosterone release, stimulation of sympathetic transmission and cellular growth (1–3). The role of the AT2 receptor is still somewhat unclear but a large body of evidence now supports its role in a number of biological processes, such as antiproliferation, cellular differentiation, programmed cell death (apoptosis) and vascular relaxation (4–6). The AT2 receptor has recently attracted considerable interest as a new potential therapeutic target.
To understand the actions of the AT2 receptor, the selective agonists CGP-42112 and [4-NH2-Phe6]Ang II and the antagonist PD123,319 have most often been used as pharmacological tools. However, there is a need for novel agonists and antagonists as AT2 receptor ligands (7). We have approached this need by modifying Ang II using the peptidomimetic strategy (8). A key component in this approach is to understand which amino acid residues are responsible for the activity. Recently, Miura et al. (9) reported various modifications of Ang II side chains and studied their influence on the affinity to the AT2 receptor. In six of the eight positions in Ang II, substitution with Ala was carried out and in one position with Gln. Surprisingly, only a minor decrease in affinity to the AT2 receptor was observed. This is different from what is observed at the AT1 receptor where the side chains on residues 2, 4, 6 and 8 are key elements for binding (10). Therefore, as a first step, we wanted to confirm and further explore these unexpected findings at the AT2 receptor.
Another component in the peptidomimetic strategy is to transform peptides to nonpeptides by incorporation of well-defined secondary structure mimetics in the target peptides. We have previously utilized this approach and performed several mono- and bicyclizations in Ang II and studied the binding affinity of the resulting analogues (11–14). Building on the hypothesis that Ang II adopts a γ-turn around Tyr4 when binding to the AT1 receptor, we recently synthesized pseudopeptide analogues of Ang II in which this peptide segment was replaced by a benzodiazepine-based γ-turn like scaffold (Fig. 1) (15). Interestingly, one of these compounds displayed high affinity to the AT2 receptor (Ki = 3 nm). We hypothesized that the γ-turn like region around Tyr4, the guanidino group of the Arg2 residue, the N-terminal end and their relative orientations are critical for AT2 receptor recognition. It was shown that when the Arg residue was substituted by Ala, the affinity dropped from 3.0 nm to more than 10 μm (i.e., 17 to 18). This loss of affinity is much larger than the 10-fold affinity drop upon Gln substitution at the Arg2 position in Ang II (9). We therefore also decided to study the importance of the N-terminal end of Ang II for AT2 receptor affinity in further detail.
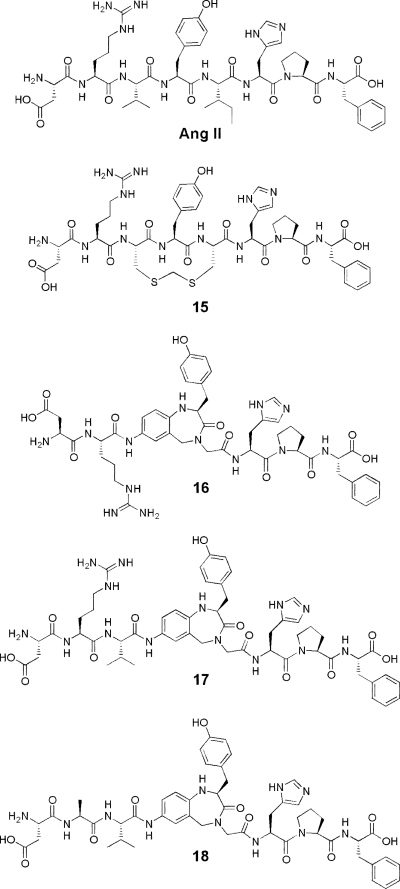
Angiotensin II (Ang II) and some previously synthesized pseudopeptide analogues of Ang II.
Experimental
General
Preparative reversed phase high-performance liquid chromatography (RP-HPLC) was performed on a Vydac 10-μm C18 column (22 × 250 mm) using a CH3CN/H2O gradient with 0.1% trifluoroacetic acid (TFA) at a flow rate of 5 mL/min and with UV detection at 230 nm. Analytical RP-HPLC was carried out on a Zorbax 5-μm SB-C8 column (4.6 × 50 mm) or a YMC 5-μm ODS-AQ column (4.6 × 50 mm) using the same buffer system at a flow rate of 2 mL/min and detection at 220 nm. Liquid chromatography mass spectrometry (LC-MS) was performed on a Gilson-Finnigan AQA system (Gilson, Middletown, WI, USA and Thermo Electron, Woburn, MA, USA) in ESI mode using a Merck Chromolith RP-18e column (4.6 × 100 mm) and a CH3CN/H2O gradient with 0.05% HCOOH at flow rate of 4 mL/min and with UV (214 and 254 nm) and MS detection. Amino acid analysis was carried out at the Department of Biochemistry, Uppsala University, Uppsala, Sweden, using an LKB 4151 alpha plus analyzer (Biochrom, Cambridge, UK) with ninhydrin detection. Samples were hydrolysed with 6 m HCl at 110 °C for 24 h.
Materials
Amino acid derivatives were obtained from Novabiochem (Läufelfingen, Switzerland), Alexis Corporation (Läufelfingen, Switzerland) or Senn Chemicals (Dielsdorf, Switzerland). Fmoc-Phe-Wang resin and 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate (HBTU) were purchased from Senn Chemicals. H-Gly-2-Cl-trityl resin was prepared from 2-Cl-tritylchloride resin (Alexis Corporation) as described in the literature (16). N,N-Dimethylformamide (DMF) (analytical grade) was obtained from Fischer Chemicals (Loughborough, UK) and was used without further purification.
Solid-phase peptide synthesis
The peptides were synthesized on a 100-μmol scale with a Symphony instrument (Protein Technologies, Inc., Tucson, AZ, USA) using Fmoc/tert-butyl (t-Bu) protection. The starting polymer was Fmoc-Phe-Wang resin (1.00 mmol/g) or H-Gly-2-Cl-trityl resin (0.75 mmol/g), and for the Fmoc amino acids the side chain protection was as follows: Asp(Ot-Bu), Arg(Pbf), Tyr(t-Bu) and His(Trt). The Fmoc group was removed by treatment with 20% piperidine in DMF (2 × 2.5 mL) for 5 + 10 min. The amino acids (0.25 mmol) were coupled in DMF (2.5 mL) using HBTU (0.25 mmol) in the presence of N-methyl morpholine (NMM; 0.50 mmol). Double couplings (2 × 30 min) were used for all amino acids. N-terminal acetylation, when applied, was achieved by treatment of the resin immediately after deprotection, i.e. without washing, with 20% acetic anhydride in DMF (2 × 2.5 mL; 2 × 15 min). At the end of each coupling cycle, remaining amino groups were capped by addition of the acetic anhydride solution (1.25 mL) to the coupling mixture and allowing the reaction to proceed for 5 min. After completion of the synthesis the Fmoc group, if present, was removed and the partially protected peptide resin was washed with several portions of DMF and CH2Cl2 and dried in a stream of nitrogen and in vacuo.
Part of the resin (approximately 100 mg) was transferred to a small centrifuge tube. Triethylsilane (50 μL) and 95% aqueous TFA (2.0 mL) were added and the mixture was agitated by rotation for 1.5 h at room temperature. The resin was removed by filtration through a small plug of glass wool in a Pasteur pipette and washed with TFA (3 × 0.3 mL). The volume of the filtrate was reduced in a stream of nitrogen to approximately 2 mL, and the product precipitated by addition of cold, anhydrous diethyl ether (12 mL). The precipitate was collected by centrifugation, washed with diethyl ether (4 × 7 mL), and dried in a stream of nitrogen and then in vacuo overnight.
The crude peptide (approximately 25 mg) was dissolved in 0.1% TFA – 10% CH3CN – H2O (2.2 mL), filtered through a 0.45-μm nylon membrane, and purified by RP-HPLC. Selected fractions were analysed by RP-HPLC and/or LC-MS. Those containing pure material were pooled, lyophilized and dissolved in water (occasionally supplemented with CH3CN). The peptide content, usually 70–80% on a weight basis, was then determined by amino acid analysis of a withdrawn aliquot. Analytical values were within the expected range (±2%), except for the dipeptide sequence Ile-His, which was found to require 72 h heating for complete hydrolysis. The HPLC purity (at 214 nm) of the peptides ranged from 97 to 99%. Yields of the purified peptides and their analytical data are shown in Table 1.
| Amino acid analysisa | Yield (%)b | Molecular weight (MW) | M + H+, (M + 2H+)/2 | |
|---|---|---|---|---|
| 1 | Gly 1.00, Arg 1.02, Val 0.97, Tyr 1.01, Ile 0.90, His 0.90, Pro 1.00, Phe 1.01 | 35.3 | 987.53 | 988.2, 494.9 |
| 2 | Asp 1.01 Gly 0.99, Val 0.98, Tyr 1.01, Ile 0.91, His 0.90, Pro 1.00, Phe 1.02 | 42.8 | 946.45 | 947.1, 474.4 |
| 3 | Asp 0.99, Arg 1.00, Gly 1.01, Tyr 1.00, Ile 0.90, His 0.92, Pro 1.00, Phe 1.00 | 57.3 | 1003.49 | 1004.2, 502.9 |
| 4 | Asp 1.01, Arg 1.00, Val 0.99, Gly 1.01, Ile 0.83, His 0.83, Pro 1.00, Phe 0.99 | 34.6 | 939.49 | 940.2, 470.9 |
| 5 | Asp 0.99, Arg 0.98, Val 0.97, Tyr 0.99, Gly 1.02, His 1.01, Pro 1.02, Phe 1.00 | 29.4 | 989.47 | 990.2, 495.9 |
| 6 | Asp 1.01, Arg 1.00, Val 0.96, Tyr 0.99, Ile 0.99, Gly 1.02, Pro 1.01, Phe 1.01 | 34.8 | 965.50 | 966.2, 483.9 |
| 7 | Asp 1.01, Arg 1.01, Val 0.95, Tyr 0.99, Ile 0.89, His 0.90, Gly 1.02, Phe 1.01 | 29.6 | 1005.50 | 1006.3, 503.9 |
| 8 | Asp 1.01, Arg 1.01, Val 0.97, Tyr 0.99, Ile 0.89, His 0.90, Pro 1.01, Gly 1.01 | 33.5 | 955.49 | 956.2, 478.9 |
| 9 | Arg 0.99, Val 1.00, Tyr 1.00, Ile 0.90, His 0.90, Pro 1.01, Phe 1.01 | 56.1 | 930.51 | 931.5, 466.5 |
| 10 | Gly 1.02, Val 0.98, Tyr 1.00, Ile 0.91, His 0.91, Pro 1.00, Phe 1.00 | 37.3 | 831.43 | 832.1, 416.8 |
| 11 | Gly 1.02, Val 0.98, Tyr 1.00, Ile 0.92, His 0.91, Pro 1.00, Phe 1.01 | 48.1 | 873.44 | 874.6, 438.0 |
| 12 | Asp 0.99, Arg 1.00, Val 1.00, Gly 1.00, Tyr 1.01, Ile 0.91, His 0.93, Pro 1.01, Phe 1.00 | 50.1 | 1102.56 | 1103.3, 552.4 |
| 13 | Asp 1.00, Arg 1.00, Gly 1.02, Val 0.99, Tyr 0.98, Ile 0.89, His 0.90, Pro 0.99, Phe 1.01 | 45.5 | 1102.56 | 1103.4, 552.5 |
| 14 | Asp 1.00, Arg 1.02, Tyr 1.00, Ile 0.90, His 0.91, Pro 1.00, Phe 0.99 | 47.8 | 946.47 | 947.7, 474.4 |
- a. Samples were hydrolysed with 6 m HCl at 110 °C for 24 h. The dipeptide sequence Ile-His was found to require 72 h heating for complete hydrolysis.
- b. After purification. Corrected for peptide content according to amino acid analysis.
Rat liver membrane AT1 receptor binding assay
Rat liver membranes were prepared according to the method of Dudley et al. (17). Binding of [125I]Ang II to membranes was conducted in a final volume of 0.5 mL containing 50 mm Tris-HCl (pH 7.4), 100 mm NaCl, 10 mm MgCl2, 1 mm ethylenediaminetetraacetic acid (EDTA), 10 μm bacitracin, 10 μm pepstatin A, 10 μm bestatin, 10 μm captopril, 0.2% bovine serum albumin (BSA), liver homogenate corresponding to 5 mg of the original tissue weight, [125I]Ang II (80 000 cpm, 0.03 nm) and variable concentrations (0.01 nm–1.0 μm) of test substance. Samples were incubated at 25 °C for 2 h, and binding was terminated by filtration through Whatman GF/B glass-fiber filter sheets using a Brandel cell harvester. The filters were washed with 3 × 3 mL of Tris-HCl (pH 7.4) and transferred to tubes. The radioactivity was measured in a γ-counter. Nonspecific binding was determined in the presence of 1 μm Ang II. The specific binding was determined by subtracting the nonspecific binding from the total bound [125 I]Ang II. The dissociation constant (Kd = 1.7 ± 0.1 nm, [L] = 0.057 nm) was determined by Scatchard analysis of data obtained with Ang II by using grafit (Erithacus Software, Horley, UK). The binding data were best fitted with a one-site fit. All experiments were performed in triplicate. Ki values were calculated using the Cheng-Prusoff equation.
Porcine (pig) myometrial membrane AT2 receptor binding assay
Myometrial membranes were prepared from porcine uteri according to the method of Nielsen et al. (18). Potential interference by binding to AT1 receptors was blocked by the addition of 1 μm losartan. Binding of [125I]Ang II to membranes was conducted in a final volume of 0.5 mL containing 50 mm Tris-HCl (pH 7.4), 100 mm NaCl, 10 mm MgCl2, 1 mm EDTA, 10 μm bacitracin, 10 μm pepstatin A, 10 μm bestatin, 10 μm captopril, 0.2% BSA, homogenate corresponding to 10 mg of the original tissue weight, [125I]Ang II (80 000 cpm, 0.03 mm), and variable concentrations (0.01 nm–1.0 μm) of test substance. Samples were incubated at 25 °C for 1.5 h, and binding was terminated by filtration through Whatman GF/B glass-fiber filter sheets using a Brandel cell harvester. The filters were washed with 3 × 3 mL of Tris-HCl (pH 7.4) and transferred to tubes. The radioactivity was measured in a γ-counter. Nonspecific binding was determined in the presence of 1 μm Ang II. The specific binding was determined by subtracting the nonspecific binding from the total bound [125I]Ang II. The dissociation constant (Kd = 0.7 ± 0.1 nm, [L] = 0.057 nm) was determined by Scatchard analysis of data obtained with Ang II by using grafit (Erithacus Software). The binding data were best fitted with a one-site fit. All experiments were performed in triplicate. The Ki-values were calculated using the Cheng-Prusoff equation.
Results
The Gly scan produced compounds (1–8) with relatively high affinity to the AT2 receptor (Table 2). The binding was only substantially affected when the Arg side chain was removed, leading to an almost 100-fold affinity loss for compound 2. For the remaining analogues, the affinity was reduced by a factor of 13 or less. The binding constants of these peptides to the AT1 receptor were markedly different (Table 2). The [Gly2]Ang II and the [Gly8]Ang II analogues completely lacked affinity to the AT1 receptor (Ki > 10 μm). Replacement of Pro with Gly resulted in 7 with a Ki of 146 nm. Substitution of Tyr4 or His6 with Gly gave 4 and 6, respectively, with <50-fold affinity reduction when compared with Ang II. Replacement of the Val3 or Ile5 residue with Gly gave 3 and 5, respectively, with a binding affinity similar to Ang II.
| Number | Compound | AT2KI (nm), (±SEM) | AT1Ki (nm), (±SEM) |
|---|---|---|---|
| Ang II | 0.6 | 1.0 | |
| [4-NH2-Phe6]Ang II | 0.8 | – | |
| Glycine-substituted peptides | |||
| 1 | Gly-Arg-Val-Tyr-Ile-His-Pro-Phe | 4.0 ± 0.6 | 6.1 ± 0.2 |
| 2 | Asp-Gly-Val-Tyr-Ile-His-Pro-Phe | 55 ± 5 | >10 000 |
| 3 | Asp-Arg-Gly-Tyr-Ile-His-Pro-Phe | 1.2 ± 0.2 | 1.6 ± 0.1 |
| 4 | Asp-Arg-Val-Gly-Ile-His-Pro-Phe | 5.2 ± 0.8 | 48 ± 2.0 |
| 5 | Asp-Arg-Val-Tyr-Gly-His-Pro-Phe | 2.3 ± 0.1 | 1.6 ± 0.1 |
| 6 | Asp-Arg-Val-Tyr-Ile-Gly-Pro-Phe | 7.6 ± 1.0 | 14.3 ± 0.06 |
| 7 | Asp-Arg-Val-Tyr-Ile-His-Gly-Phe | 4.3 ± 0.6 | 146 ± 7.0 |
| 8 | Asp-Arg-Val-Tyr-Ile-His-Pro-Gly | 1.1 ± 0.1 | >10 000 |
| N-terminally modified peptides | |||
| 9 | Arg-Val-Tyr-Ile-His-Pro-Phe | 2.2 ± 0.2 | 10.5 ± 0.3 |
| 10 | Gly-Val-Tyr-Ile-His-Pro-Phe | 5.4 ± 0.4 | >10 000 |
| 11 | Ac-Gly-Val-Tyr-Ile-His-Pro-Phe | 2.8 ± 0.3 | 17.3 ± 0.2 |
| 12 | Asp-Arg-Val-Gly-Tyr-Ile-His-Pro-Phe | 255 ± 12 | >10 000 |
| 13 | Asp-Arg-Gly-Val-Tyr-Ile-His-Pro-Phe | 2.9 ± 0.3 | >10 000 |
| 14 | Asp-Arg-Tyr-Ile-His-Pro-Phe | 238 ± 7 | 204 ± 10 |
| Pseudopeptides | |||
| 15 | Fig. 1 | 0.62 ± 0.04a | 44 ± 1a |
| 16 | Fig. 1 | >10 000b | >10 000b |
| 17 | Fig. 1 | 3.0 ± 1.1b | >10 000b |
| 18 | Fig. 1 | >10 000b | >10 000b |
Among the N-terminally modified peptides (compounds 9–14) the affinity to the AT2 receptor ranged between 2.2 and 255 nm (Table 2). Removal of the Asp1 residue gave 9 [Ang III, Ang II (2–8)], a metabolite of Ang II, with a Ki of 2.2 nm, which is similar to previously reported data (17, 19, 20). Substitution of Arg with Gly in 9 gave 10 with 5.4 nm affinity. N-terminal acetylation of 10 produced 11 with a Ki of 2.8 nm. Increasing the distance between Arg2 and Tyr4 by insertion of a Gly residue between Val and Tyr gave 12 that had a low affinity to AT2 receptors (Ki = 255 nm). In contrast, when the Gly residue is introduced between Arg and Val (13) relatively high affinity is maintained (Ki = 2.9 nm). Shortening the distance between Arg and Tyr by removal of Val3 produced peptide 14 that binds with a Ki of 238 nm. The highly AT2 receptor selective [4-NH2-Phe6]Ang II was used as a reference peptide in the AT2 receptor assay.
The affinity of compounds 9–14 to the AT1 receptor ranged between 10.5 nm and 10 μm. (Table 2). Ang III (9) had similar affinity when compared with what has previously been reported (17, 20). Compound 10 and the backbone elongated compounds 12 and 13 lacked affinity to the AT1 receptor (Ki > 10 μm). Compounds 11 and 14 had 17.3 nm and 204 nm affinity, respectively.
Discussion
Many Ang II peptide analogues have been synthesized and screened for affinity and functional activity at Ang II receptors. However, most of these studies were performed before investigators were aware of receptor heterogeneity. Thus, AT1 and AT2 subtype specificity was not considered. Although structure–activity relationship (SAR) studies have appeared where binding to the AT2 receptor has been investigated specifically, they unfortunately lack information on the functional properties of the ligands. A robust and general in vitro assay for exploring functional properties of AT2 receptors has not yet emerged. Most of the binding affinity studies performed to date show that modification of the linear peptides Ang II or [Sar1]Ang II is well tolerated by the AT2 receptor (9, 10, 19, 21–24). Several 3–5 monocyclized analogues of Ang II have also been synthesized and show high affinity to AT2 receptors (11, 20). Using rat AT2 receptors expressed in COS1 cells, Miura et al. (9) recently investigated which side chains of Ang II were essential for high AT1 and AT2 receptor-binding affinity. Surprisingly, they found that each of the side chains contributed only to a small degree to the AT2 receptor binding.
We were intrigued by this finding and decided to repeat the amino acid scan, although with pig uterine membranes instead of transfected COS1 cells, as in our standard assay for AT2 receptor binding. Furthermore, we used Gly substitution instead of Ala substitution to avoid any possible influence of the methyl group on receptor recognition. It should be recognized that Ala scans are preferred when attempting to identify which side chains are important for binding. In an Ala scan, the conformation of the peptide backbone more resembles that of the native peptide. In a Gly scan, the peptide has a larger conformational freedom when compared with the Ala-substituted analogue. Since the aim in this study was also to examine if the extra steric bulk of the methyl group contributes favourably to binding, a Gly scan was performed. Miura et al. (9) performed the Ala scan using [Sar1]Ang II instead of Ang II. Furthermore, Gln2 was used instead of Ala2 and position 5 was not substituted. A comparison of the AT2 receptor binding affinities obtained by Miura et al. (9) with our results in Table 2 showed that the analogues had comparable affinity. Thus, the use of Gly instead of Ala/Gln did not seem to affect AT2 receptor affinity notably.
In the Gly scan compound 2 showed a marked decrease in affinity, almost 100-fold when compared with Ang II. The other analogues retained a relatively high affinity, reduced only by a factor of 2–13. Thus, the Arg2 side chain seems to be the most important for binding to AT2 receptors, a finding in line with the observation by Miura et al. (9). Further support for a role of Arg2 in receptor binding comes from binding studies of Ang IV [Ang II (3–8)]. Ang IV lacks the Arg residue and has approximately 2400 times lower affinity to the AT2 receptor than Ang II (19). Mutagenesis studies of the AT2 receptor have also indicated that Arg2 in Ang II is important for binding and it is hypothesized that it interacts with Asp297 in the third extracellular loop of the receptor (25, 26).
We recently synthesized pseudopeptide analogues of Ang II in which amino acid residues Val3-Ile5 were replaced by a γ-turn like scaffold (15). The design of these analogues was based on the observation that the 12-membered methylenedithioether analogue 15 had binding preference for the AT2 receptor (see Fig. 1 and Table 2). Theoretical conformational analysis of a model tripeptide of this analogue indicated that it preferentially adopts low energy inverse γ-turn conformations centred at Tyr4. The bicyclic γ-turn mimicking benzodiazepine scaffold shown in Fig. 1 was therefore introduced in this region. In the synthesized pseudopeptides, it was shown that both the presence of the Arg side chain and the distance between the Arg residue and the Tyr moiety were important for obtaining ligands with high affinity to the AT2 receptor. Compound 16 lacked affinity (Ki > 10 μm) to the AT2 receptor while compound 17 with the Val residue inserted between Arg and the bicyclic scaffold, had 3 nm affinity to the receptor. We hypothesized that the extra Val residue in 17 served as a spacer to allow the guanidino group of the Arg2 residue and the N-terminal part of the active analogues 15 and 17 to access common regions of space relative to the bicyclic scaffold inaccessible to the compound that lacked affinity (16). Furthermore, substituting the Arg2 residue by Ala in compound 17 gave compound 18 with no affinity for the AT2 receptor, again indicating the importance of the guanidino group for receptor binding. Thus, there seems to be a difference in binding mode for the peptides and the pseudopeptides: when Arg2 is substituted by Gly in Ang II only a 100-fold loss in affinity is seen, whereas substitution of Arg2 by Ala in the pseudopeptide results in more than a 10 000-fold loss of affinity. This is most likely due to the more conformationally restricted bicyclic scaffold, which may not mimic the more flexible peptide optimally.
To further study the importance of the Arg side chain for AT2 receptor binding we synthesized a number of N-terminally modified Ang II analogues including some where the distance between Arg2 and the Tyr4 was varied. Ang III (9) in accordance with earlier reports (17, 19, 20) had similar affinity as Ang II to AT2 receptors. Interestingly, compound 10 lacking the Arg side chain had only nine times lower affinity to AT2 receptors than Ang II, and two times lower affinity than Ang III to the AT2 receptors. We therefore speculated that the charged N-terminal primary amine of 10 could reach the same region of space as that occupied by the Arg-side chain in 9 (and potentially interact with Asp297 or another Asp/Glu residue in this region of the AT2 receptor). To address this hypothesis we acetylated the primary amine of the Gly residue of 10 to obtain 11. Also this compound had a high affinity for the AT2 receptor (Ki = 2.8 nm). Thus, from these studies it is obvious that a charged group in the N-terminal end of Ang III is not necessary for binding. This is different from what is observed for [Gly2]Ang II where a 100-fold drop in affinity was seen, which suggests that Ang II and Ang III may bind differently to the AT2 receptor. It also seems that the pseudopeptide analogues and Ang II interact with the AT2 receptor differently since all affinity is lost in the pseudopeptide when Arg is substituted for Ala.
We synthesized the internally elongated nonapeptides 12 and 13 in order to address the importance of the distance between Arg2 and Tyr4 for binding. Interestingly, both compounds bind to the AT2 receptor, albeit with a 100-fold difference in strength. One reason for the lower affinity of 12 when compared with 13 may be that the Val-Gly and Gly-Val spacers induce different conformational preferences. We also synthesized the deletion analogue 14 where Arg and Tyr are adjacent to each other. This compound also binds with low affinity (Ki = 238 nm). It thus seems that chain elongation or deletion in the N-terminal region also influences the binding affinities to the AT2 receptor.
The binding affinity to the AT1 receptor of the Ang II analogues was also studied to enable a comparison between the two receptor subtypes (Table 2). It is well known that positions 2, 4, 6 and 8 in Ang II correspond to residues of importance for binding to the AT1 receptor (10). When Arg2 or Phe8 was substituted for Gly to give 2 and 8 all affinity was lost. Substitution of Tyr4 or His6 to give 4 and 6 gave only a slight decrease in affinity of 48 and 14 times, respectively. Surprisingly [Gly7]Ang II had lower binding affinity than 4 and 6 to the AT1 receptor. Overall, these results are similar to those obtained in the Ala scan performed by Miura et al. (9). The main difference is the wider range of affinities in the present study as well as the lack of affinity of [Gly8]Ang II (Ki > 10 μm) when compared with [Ala8]Ang II (Ki = 1 nm). Many analogues with modifications at position 8 in Ang II have been synthesized and tested for the pressor and myotropic response (10, 27). It is therefore well known that when the aromatic ring in Ang II is replaced with an aliphatic substituent, the agonistic response is turned into an antagonistic response. [Gly8]Ang II lacks the aliphatic side chain and this may be one reason for its lack of affinity. Ang III (9), in accordance with earlier reports (17, 20), had similar affinity as Ang II to AT1 receptors. Substituting the Arg residue in 9 for Gly gave 10, which lacked affinity to AT1 receptors. When the terminal amino group of 11 was acetylated to give 10, the affinity was recovered (17.3 nm). In contrast, Jorgensen et al. (28) found that 10 had lower pressor activity than 11. Thus, it seems that affinity and functional activity is not correlated in these peptides. The internally elongated nonapeptides 12 and 13 lacked affinity to AT1 receptors indicating that the position of Arg2 relative to Tyr4, His6 and Phe8 has been perturbed. The importance of the relative position of the side chains for AT1 receptor binding affinity is also illustrated by the deletion analogue 14, which has 200 times lower affinity than Ang II. Overall, the AT2 receptor seems less sensitive to Ang II modifications than the AT1 receptor.
Conclusion
A Gly scan of Ang II produced analogues with relatively high affinity to the AT2 receptor. Only removal of the Arg side chain affected the binding substantially, leading to an almost 100-fold affinity loss. Therefore, the charged group seems to be important for binding in Ang II. However, analysis of peptides modified in the N-terminal end revealed that a charged group is not necessary in the Ang III peptide analogues. We therefore conclude that the SAR in these compounds differs, possibly because they interact with the AT2 receptor somewhat differently.
Acknowledgments
Acknowledgements: Authors thank the Swedish Foundation for Strategic Research (SSF) and the Swedish Research Council (VR) for financial support.




