Carriage of Malassezia spp. yeasts in Cornish Rex, Devon Rex and Domestic short-haired cats: a cross-sectional survey
Abstract
Carriage of Malassezia spp. yeasts in healthy Cornish Rex cats (CRC) was compared with that in Devon Rex (DRC) and Domestic short-haired (DSH) cats. Samples obtained from the left external ear canal, anus and claw fold of digit III of the left fore foot by swabbing, and the axilla and groin using contact plates, were incubated for yeasts on modified Dixon's agar at 32 °C for 7 days. Malassezia species were isolated from 90% of the DRC, but from only 39% of the CRC and 50% of the DSH cats. M. pachydermatis accounted for 121 of 141 Malassezia spp. isolates. Five CRC were colonized by M. pachydermatis alone, one CRC yielded only M. nana, and one cat yielded only M. slooffiae, whereas five CRC were colonized by both M. pachydermatis and M. nana and another yielded M. pachydermatis, M. slooffiae and M. nana. M. nana was primarily isolated from the ear canal, whereas M. slooffiae was most often isolated from the claw. Both the frequencies of isolation and the population sizes of M. pachydermatis at all sites sampled in the CRC were comparable to those of 10 healthy DSH cats. Populations of M. pachydermatis in the left axilla and left and right groin in the CRC were significantly lower when compared with counts in a group of 21 healthy DRC, a breed with very similar coat characteristics but prone to seborrheic dermatitis caused by M. pachydermatis.
Source of Funding
Janssen Animal Health kindly provided support for the laboratory consumables used in this project.
Conflict of Interest
No conflict of interest has been declared.
Introduction
In common with many mammalian species, the skin of healthy cats is often colonized by yeasts of the genus Malassezia.1–3 Malassezia spp. isolated from cats include M. pachydermatis,1 M. sympodialis,2,3 M. globosa,3 M. furfur,4 M. nana,5,6 and more recently, M. slooffiae,7,8 and healthy Devon Rex cats (DRC) seem to be more frequently colonized by larger populations of M. pachydermatis than healthy Domestic short-haired (DSH) cats.7 DRC may present with a greasy, seborrheic dermatitis associated with high populations of M. pachydermatis that significantly exceed those of both healthy DRC and healthy DSH,7 the clinical signs of which (and associated population densities) are dramatically reduced by oral itraconazole therapy.9
Four Rex mutations, each with subtle phenotypic differences in their soft, plush and wavy hair coats, have been reported in domestic cats, with the designations of Devon, Cornish, German and Oregon reflecting the place of origin.10,11 Mating studies have indicated that all are inherited as autosomal recessive traits, and that the Devon, Cornish and Oregon Rexes are genetically independent, whereas the Cornish and German Rex mutants may be identical or closely similar.10,11 Cornish Rex cats (CRC) share a similar coat type to DRC, characterized by short, dense, curled hair that is velvet-soft to the touch, and hence carriage rates of Malassezia spp. yeasts like those in DRC might be expected to increase in comparison to those of DSH cats. Thus, the purpose of this study was to compare the frequencies of isolation and population sizes of Malassezia spp. in healthy CRC with those of healthy DSH cats and healthy and seborrheic DRC.
Materials and methods
Cats
The use of the cats was approved by the Ethics and Welfare Committee of the Royal Veterinary College, and written informed consent from the owner was obtained before sampling. Thirty-three CRC (aged 2 months – 16 years, 28 females, five males) that were in good general health and free of skin disease were sampled in five different homes; none had been shampooed or received antifungal or immunosuppressive therapy in the prior6months. Data from these cats (CRC) were compared with those obtained from 21 healthy DRC (aged 1–13 years, 15 females, six males), nine DRC with clinical signs of localized or generalized seborrheic skin disease (aged 1–13 years, five females, six males) and 10 healthy DSH cats, comprising one neutered male and nine neutered females, aged 4–12 years.7
Skin sample collection
The skin of the left and right axillae and groin was sampled using contact plates comprising bijou bottle lids filled to the meniscus with modified Dixon's agar and processed as previously described;3 yeast counts were expressed as colony-forming units (CFU) per cm2 of skin. The anus, left external ear canal, and a claw fold on digit III on the left forefoot were swabbed for 5 s using mini-tipped swabs and the samples processed as previously described;3 yeast counts were expressed as log10(CFU swab−1 + 1).
Yeast identification
M. pachydermatis was identified by gross colonial and microscopical morphology, and on the ability to grow when subcultured on Sabouraud's dextrose agar (65 g L−1, Oxoid CM0041, Basingstoke, UK) at 32 °C. Lipid-dependent Malassezia spp. were identified again by gross colonial and microscopical morphology, and in this instance failed to grow when subcultured on Sabouraud's dextrose agar at 32 °C. Lipid-dependent isolates were further evaluated using the polymerase chain reaction–restriction enzyme analysis (PCR-REA) method of Guillot et al.12 with minor modifications.8 The identities of the lipid-dependent isolates obtained from the CRC and DRC were confirmed by sequencing the D1/D2 regions of the 26S rRNA gene using the conserved fungal primers NL1 and NL4.13 The amplification process consisted of 15 min at 95 °C, followed by 30 cycles of denaturation at 94 °C for 45 s, annealing at 51 °C for 1 min, and extension at 72 °C for 2 min plus a final extension period at 72 °C for 10 min. Sequences were aligned using the Staden Package software (http://staden.sourceforge.net) and compared with those available in GenBank using the BLAST program (http://www.ncbi.nlm.nih.gov/blast). GenBank accession numbers for the nucleotide sequences of the D1/D2 region of the 26S rRNA gene of the isolates obtained from the Rex cats are listed in Table 1.
| Strain | Source | Identification | D1/D2 GenBank accession no. |
|---|---|---|---|
| CR12LG | CRC, groin | M. nana | EU687496 |
| CR12E | CRC, ear | M. nana | EU687497 |
| CR12A | CRC, anus | M. nana | EU687498 |
| CR13E | CRC, ear | M. nana | EU687499 |
| CR14E | CRC, ear | M. nana | EU687500 |
| CR17E | CRC, ear | M. nana | EU687501 |
| CR18E | CRC, ear | M. nana | EU687502 |
| CR19E | CRC, ear | M. nana | EU687503 |
| CR32C | CRC, claw fold | M. nana | EU687504 |
| CR13LAX | CRC, axilla | M. slooffiae | EU687505 |
| CR27C | CRC, claw fold | M. slooffiae | EU687506 |
| DR1C | DRC, claw fold | M. slooffiae | EU687507 |
| DR2C | DRC, claw fold | M. slooffiae | EU687508 |
| DR6C | DRC, claw fold | M. slooffiae | EU687509 |
| DR7C | DRC, claw fold | M. slooffiae | EU687510 |
| DR9C | DRC, claw fold | M. slooffiae | EU687511 |
| DR11C | DRC, claw fold | M. slooffiae | EU687512 |
| DR14C | DRC, claw fold | M. slooffiae | EU687513 |
| DR23C | DRC, claw fold | M. slooffiae | EU687514 |
Statistical analyses
Population sizes of M. pachydermatis, M. slooffiae and M. nana were compared between breeds, body regions and between healthy and seborrheic cats using the Mann–Whitney U-test of association and the statistical software SPSS 15 for Windows (SPSS Inc., Chicago, IL, USA). Contingency tables and the survey commands of STATA Intercooled 9.2 for Windows (Stata Corporation, College Station, TX, USA) were used to identify associations between the frequency of isolation of M. pachydermatis, M. slooffiae and M. nana isolates and breeds, body regions sampled, and healthy or seborrheic cats.
Results
Malassezia isolates
Of the 141 Malassezia spp. isolates obtained, 121 were examples of M. pachydermatis. Eleven lipid-dependent isolates that formed small, yellow, domed colonies, without precipitates on modified Dixon's agar but failed to grow on Sabouraud's dextrose agar at 32 °C were obtained from the CRC, and a single lipid-dependent isolate was obtained from a DSH cat. Two isolates from CRC and one from a DSH cat had PCR-REA patterns indistinguishable from that of the M. slooffiae CBS 7956 type culture, and nine isolates had PCR-REA patterns that matched that of M. nana CBS 9557 type culture (Fig. 1a–c). These nine M. nana isolates had identical D1/D2 nucleotide sequences that also matched (577/577 bp) those of lipophilic isolates obtained from the ears of six cats and one dog by Cabañes et al.6,14 (GenBank accession numbers AY743608, AY743609, AY743610, AY743611, AY743612, AY743613, AY743614), and differed from the M. nana type culture CBS 9557 sequence (GenBank accession number EF140671) by only two of 545 bp. The two M. slooffiae isolates from CRC had identical D1/D2 nucleotide sequences that showed 99.3% (581/585 bp) similarity to M. slooffiae CBS 7956 (GenBank accession number AY743606). Minor variations (typically four or five of 560 bp) in D1/D2 sequence were observed when compared with a collection of M. slooffiae isolates obtained from humans with various skin diseases.15 The lipid-dependent isolates obtained from the DRC had PCR-REA patterns that matched the M. slooffiae CBS 7956 type culture.7 The D1/D2 nucleotide sequences for seven of eight of the DRC isolates were identical to those of the CRC M. slooffiae isolates, whereas the sequence for the remaining isolate (DR2C) varied from M. slooffiae CBS 7956 (GenBank accession number AY743606) by one bp, but was identical to that of M. slooffiae CBS 7971 (AY387248).15
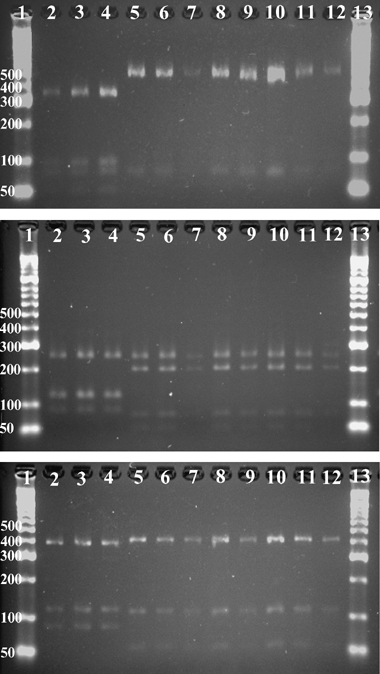
Restriction patterns obtained by (a) BanI, (b) HaeII, and (c) MspI digestion of an amplicon of the large subunit rRNA gene of CBS strains and field isolates of lipid-dependent Malassezia obtained from Cornish Rex cats. Tracks 1 and 13, molecular weight marker (base pairs); 2, M. slooffiae CBS 7956; tracks 3–4, field isolates of M. slooffiae; track 5, M. nana CBS 9557; tracks 6–12, field isolates of M. nana.
Association between Malassezia species and breed
Malassezia species were isolated from 90% of the DRC, but from only 39% of the CRC and 50% of the DSH cats (Table 2). M. pachydermatis was the most frequently isolated yeast in all three breeds. M. nana was isolated from CRC, either alone (3%), along with M. pachydermatis (15%), or along with both M. pachydermatis and M. slooffiae (3%) (Table 2). A combination of M. pachydermatis and M. slooffiae was isolated from the same anatomical site only in DRC (23%). The frequency of isolation of M. pachydermatis was significantly associated with breed (χ2 = 69.61, d.f. = 2, P = 0.003), whereas the frequencies of isolation of M. slooffiae (χ2 = 4.72, d.f. = 2, P = 0.356) and M. nana (χ2 = 11.10, d.f. = 2, P = 0.577) were not.
| Malassezia spp. | Devon RexN (%) | Cornish RexN (%) | Domestic short-hairedN (%) |
|---|---|---|---|
| No Malassezia spp. | 3 (10) | 20 (61) | 5 (50) |
| M. pachydermatis | 19 (63) | 5 (15) | 4 (40) |
| M. slooffiae | 1 (3) | 1 (3) | 1 (10) |
| M. nana | 0 | 1 (3) | 0 |
| M. pachydermatis and M. slooffiae | 7 (23) | 0 | 0 |
| M. pachydermatis and M. nana | 0 | 5 (15) | 0 |
| M. pachydermatis, M. slooffiae and M. nana | 0 | 1 (3) | 0 |
| Total | 30 (99) | 33 (100) | 10 (100) |
There was no significant difference in population size of M. pachydermatis isolates from the claw (P = 0.672), anus (P = 0.402) or left groin (P = 0.977) of healthy CRC and DSH cats. Similarly, there was no significant difference between population size of M. pachydermatis isolates from the claw (P = 0.967) or anus (P = 0.106) of healthy DRC and DSH cats, but population size of M. pachydermatis isolates from the left groin of healthy DRC was significantly larger than that isolated from the left groin of healthy DSH cats (P = 0.035; Table 3).
| Body region | Devon Rex cats | Cornish Rex cats | Domestic short-haired cats | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seborrheic | Healthy | Healthy | Healthy | |||||||||
| Median | Max¶ | IQR | Median | Max¶ | IQR | Median | Max¶ | IQR | Median | Max¶ | IQR | |
| Ear | 0 | 1.91 | 0 | 0 | 1.61 | 0 | 0 | 1.61 | 0 | not isolated | ||
| Claw | 3.90 | 5.38 | 0.85 | 0* | 5.18 | 0 | 0 | 3.43 | 0 | 0 | 3.08 | 0 |
| Anus | 0 | 3.22 | 1.28 | 0 | 1.61 | 0 | 0 | 3.86 | 0 | 0 | 2.08 | 1.91 |
| Left axilla | 35.99** | 79.62 | 75.80 | 0* | 26.43 | 0.48 | 0† | 2.55 | 0 | not isolated | ||
| Right axilla | 38.54 | 79.62 | 76.76 | 0* | 17.20 | 0.48 | 0 | 2.55 | 0 | not isolated | ||
| Left groin | 79.62 | 79.62 | 64.34 | 0*,**[link] | 66.24 | 0.64 | 0‡ | 6.37 | 0 | 0† | 0.32 | 0 |
| Right groin | 79.62 | 79.62 | 32.65 | 0.32* | 64.01 | 0.96 | 0§ | 3.18 | 0.16 | not isolated | ||
- Comparison with seborrheic Devon Rex cats, *P ≤ 0.001.
- Comparison with healthy Devon Rex cats,†P < 0.05,‡P = 0.001,§P = 0.007.
- ¶ Minimum population size for all body regions = 0 CFU cm2 or 0 log10[CFU swab−1 + 1], unless specified otherwise.
- ** Minimum population size = 0.32 CFU cm2.
Association between Malassezia species and body region
M. pachydermatis was isolated from all seven of the body regions sampled, M. nana from four (ear, claw, anus, left groin) and M. slooffiae from only two (claw, left axilla) (Table 4). The only site from which all three species were isolated was the claw, and ten (91%) of the eleven isolates of M. slooffiae were obtained from this site. Six of the nine (67%) M. nana isolates were obtained from the ear.
| Body region | MPN (%) | MSN (%) | MNN (%) |
|---|---|---|---|
| Ear | 4 (3) | not isolated | 6 (67) |
| Claw | 13 (11) | 10 (91) | 1 (11) |
| Anus | 10 (8) | not isolated | 1 (11) |
| Left axilla | 21 (17) | 1 (9) | not isolated |
| Right axilla | 22 (18) | not isolated | not isolated |
| Left groin | 23 (19) | not isolated | 1 (11) |
| Right groin | 28 (22) | not isolated | not isolated |
M. pachydermatis was most frequently isolated from the right groin (22% of isolates) and least often isolated from the ear (3%) (Table 4). Isolation frequency although similar for the left and right axillae and groins (17–22%) was significantly associated with body region (χ2 = 32.70, d.f. = 6, P = 0.002). No such regional relationship was found for the isolation frequencies of M. slooffiae (χ2 = 54.44, d.f. = 6, P = 0.133) or M. nana (χ2 = 21.71, d.f. = 6, P = 0.360).
The population sizes of M. pachydermatis isolated from the claw significantly exceeded those of the ear (P = 0.013), but the anal populations did not vary significantly from those of the ear (P = 0.074) or claw (P = 0.316) (Table 5). M. pachydermatis counts in the left and right axillae and groin were comparable (Mann–Whitney U-tests: P > 0.25 in each instance). Population sizes of M. nana and M. slooffiae were not compared statistically because of their infrequent isolation.
| Body region | MP | MS | MN | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Max‡ | IQR | Median | Max‡ | IQR | Median | Max‡ | IQR | |
| Ear | 0 | 1.91 | 0 | Not isolated | 0 | 3.12 | 0 | ||
| Claw | 0† | 5.38 | 0 | 0 | 3.85 | 0 | 0* | 2.86 | 0 |
| Anus | 0 | 3.86 | 0 | Not isolated | 0* | 1.61 | 0 | ||
| Left axilla | 0 | 79.62 | 0.32 | 0* | 0.64 | 0 | Not isolated | ||
| Right axilla | 0 | 79.62 | 0.32 | Not isolated | Not isolated | ||||
| Left groin | 0 | 79.62 | 0.32 | Not isolated | 0* | 1.59 | 0 | ||
| Right groin | 0 | 79.62 | 0.64 | Not isolated | Not isolated | ||||
- * n = 1;
- † comparison with ear counts of M. pachydermatis, P = 0.013.
- ‡ Minimum population size for all body regions = 0 CFU cm2 or 0 log10[CFU swab−1 + 1].
- CFU, colony-forming units; IQR, interquartile range; MP, M. pachydermatis; MS, M. slooffiae; MN, M. nana.
Association between Malassezia species and health status
M. pachydermatis was isolated from 70% of the seborrheic cats but from only 17% of the healthy cats. M. slooffiae was isolated from 4.8% of the seborrheic cats and 1.8% of the healthy cats. M. nana was isolated from 2.0% of the healthy cats and from none of the seborrheic cats. The frequency of isolation of M. pachydermatis was significantly associated with the presence of seborrheic dermatitis (χ2 = 84.73, d.f. = 1, P = 0.002), whereas frequency of isolation of M. slooffiae (χ2 = 2.32, d.f. = 1, P = 0.071) or M. nana (χ2 = 1.29, d.f. = 1, P = 0.521) was not significantly associated with health status.
Counts of M. pachydermatis isolated from the ear or anus of healthy and seborrheic cats did not vary significantly (P = 0.395 and P = 0.384, respectively) (data not shown). However, seborrheic (DRC) cats had significantly higher counts of M. pachydermatis on the claw, left and right axillae, and left and right groin (all P < 0.001). There were no significant differences in the population sizes of M. slooffiae between the claws of seborrheic and healthy cats (P = 0.082), or between those of M. nana in the left ear of seborrheic and healthy cats (P = 0.342).
Population sizes of M. pachydermatis in the seborrheic DRC significantly exceeded those of healthy DRC at the claw fold, left and right axillae (all P = 0.001), and left and right groin (both P < 0.001), whereas populations in the left ear and anus did not vary significantly (Table 3). Population sizes of M. pachydermatis were significantly greater in healthy DRC when compared with healthy CRC at the left axilla (P = 0.016), left groin (P = 0.001) and right groin (P = 0.007), whereas populations were comparable in these two breeds at the other sites (ear, P = 0.314; claw, P = 0.584; anus, P = 0.958; right axilla, P = 0.090) (Table 3).
Discussion
The results demonstrate that despite the similarity of coat appearance in CRC and DRC, only the DRC had high frequencies of isolation or population size of Malassezia spp. Similarly, the high prevalence of M. pachydermatis-associated seborrheic dermatitis seen in DRC3 was not found in the CRC. While larger surveys are required to confirm these findings, the data suggest that the Rex mutation alone might not favour susceptibility to skin infection by M. pachydermatis and that additional genetic factors may account for this susceptibility in DRC.
M. nana is a recently described yeast that has been isolated from the ear canals of cats from Japan and Europe, and those of Brazilian cattle with or without otitis externa.4–6 M. nana is closely related to M. sympodialis, and Cabañes et al.6 have questioned whether the genetic differences between them are sufficient to define them as separate species. The isolation of M. nana from the ear canal of six CRC not only supports a report of its isolation from the ear canal and claw fold of a hyperthyroid cat8 but interestingly demonstrates identical D1/D2 sequences to those isolated from cats’ ears by Cabañes et al.,6 suggesting that this yeast has adapted to the feline host. However, while the ear canal appears to represent an important anatomical location for this yeast in cats, the recovery of this species from the anus and groin of one CRC and from the claw fold of another demonstrates its capacity to colonize other skin and mucosal sites. Although DNA sequence analyses are now commonly used in phylogenetic studies of the genus Malassezia,6–8 the agreement between the PCR-REA and the D1/D2 nucleotide sequence identities obtained for all of the lipid-dependent isolates in this study provides further evidence of the value of the PCR-REA method of Guillot et al.12 for routine laboratory identification of Malassezia spp. from cats.8
The isolation of M. slooffiae from the axilla of one CRC adds to the list of anatomical sites from which this yeast may be found on feline skin; in recent studies M. slooffiae was isolated from the claw folds of healthy DRC, seborrheic DRC, from cats with diabetes mellitus and hyperthyroidism, and from the anus of a cat with multicentric lymphoma.7,8 While the D1/D2 nucleotide sequences of M. slooffiae in this study cluster with previous isolates obtained from humans and pigs, most of them showed minor differences from the strains previously deposited with GenBank. Further molecular analyses are required to determine whether these isolates represent a distinct genetic group that may be adapted to the feline host.
While there is a clear association between high counts of M. pachydermatis and the occurrence of an itraconazole-responsive seborrheic dermatitis in DRC, the pathogenic potential of M. nana and M. slooffiae in this species requires further assessment. Lipid-supplemented media appropriate for these lipophilic Malassezia species should be used when diagnostic samples are obtained from cats, especially when the ear canal or claw fold are sampled.
Acknowledgements
The authors thank Janssen Animal Health for generous financial support and the owners of the cats whose kind help made this study possible. Arthur House, Carole Thomas and Anna Riddle provided skilled technical assistance.
References
Résumé Le portage de levures Malassezia spp. a été comparé chez des chats Cornish Rex sains (CRC), des Devon Rex (DRC) et des Européens (DSH). Les prélèvements ont été obtenus à partir du conduit auditif gauche, de l’anus, de la griffe du doigt III (postérieur gauche) par écouvillonnage et de la zone axillaire et du l’aine en utilisant des plaques de contact. L’incubation a eu lieu sur une gélose modifiée de Dixon à 32 °C pendant 7 jours. Malassezia a été isolée de 90% des DRC, mais de seulement 39% des CRC et 50% des DSH. M. pachydermatis représentait 121 des 141 isolats. Cinq CRC étaient colonisés par M. pachydermatis seulement, un CRC hébergeait seulement M. nana, et un chat seulement M. slooffiae, alors que cinq CRC étaient colonisés par à la fois M. pachydermatis et M. nana et un autre par M. pachydermatis, M. slooffiae et M. nana. M. nana était surtout isolée du conduit auditif, alors que M. slooffiae était plus souvent isolée des griffes. Les fréquences d’isolement et les quantités de population étaient comparables pour les CRC et pour les chats Européens sains. Les populations de M. pachydermatis de la zone axillaire gauche et de l’aine droite étaient significativement plus faibles pour les CRC en comparaison du groupe DRC, une race à pelage semblable mais prédisposée à développer une dermatite séborrhéique due àM. pachydermatis.
Resumen Se compararon gatos Cornish Rex (CRC) portadores asintomáticos de Malassezia spp. con gatos Devon Rex (DRC) y gatos domésticos de pelo corto (DSH). Las muestras se obtuvieron del canal auditivo externo del lado izquierdo, del ano y del pliegue de la uña del dedo III de la pata delantera izquierda con hisopos, y de la axila e ingle mediante contacto en placas. Las muestras se incubaron para detectar la presencia de levaduras en agar modificado de Dixon a 32 oC durante 7 días. Especies del género Malassezia se aislaron en 90% de los DRC, pero sólo en un 39% de los CRC y 50% de los gatos DSH. M. pachydermatis se aisló en 121 de los 141 aislados de Malassezia spp. Cinco CRC estaban colonizados por M. pachydermatis sólamente, un CRC presentó sólamente M. nana y otro presentó sólamente M. slooffiae, mientras que cinco CRC estaban colonizados por M. pachydermatis y M. nana y otro por M. pachydermatis, M. slooffiae y M. nana. M. nana fue el aislado primario en el canal auditivo externo, mientras que M. slooffiae se aisló con más frecuencia de la uña. Tanto la frecuencia de aislamiento como el tamaño de la población de M. pachydermatis en todas las zonas muestreadas en CRC fueron comparables a los de los 10 gatos DSH sanos. Las poblaciones de M. pachydermatis en la axila izquierda e ingle derecha en CRC fueron significativamente menores comparados con los números en un grupo de 21 DRC sanos, una raza con características de pelo similares pero con tendendia a presentar dermatitis seborreica causada por M. pachydermatis.
Zusammenfassung Das Auftreten von Malassezia spp. Hefepilzen bei gesunden Cornish Rex Katzen (Cornish Rex cats – CRC) wurde mit dem von Devon Rex (Devon Rex cats – DRC) und Europäischen Kurzhaarkatzen (Domestic short-haired cats – DSH) verglichen. Proben, die aus dem linken externen Ohrkanal, vom Anus und vom Krallenfalz der dritten Zehe des linken Vorderfusses mittels Tupfer, und von der Axilla und der Leiste mittels Kontaktplatten genommen worden waren, wurden zur Untersuchung auf Hefepilze auf modifiziertem Dixon Agar bei 32 °C 7 Tage lang inkubiert. Malassezia species wurden von 90% der DRK, aber nur von 39% der CRC und 50% der EKH Katzen isoliert. M. pachydermatis machte 121 von 141 Malassezia spp. Isolaten aus. Fünf CRC wurden nur von M. pachydermatis kolonisiert, eine CRC wies nur M. nana auf, und bei einer Katze wurde nur M. slooffiae gefunden, während fünf Katzen von sowohl M. pachydermatis als auch von M. nana kolonisiert wurden und eine weitere Katze M. pachydermatis, M. slooffiae und M. nana hatte. M. nana wurde primär vom Ohrkanal isoliert, während M. slooffiae am häufigsten von der Kralle isoliert wurde. Sowohl die Häufigkeit mit der M. pachydermatis isoliert wurde als auch die Populationsgrößen, die aus Proben von allen Lokalisationen bei CRC stammten, waren mit denen von 10 gesunden DSH Katzen vergleichbar. Die Populationsgrößen von M. pachydermatis in der linken Axilla und in der linken und rechten Leiste von CRC waren signifikant niedriger im Vergleich zu den Zahlen einer Gruppe von 21 gesunden DRC, einer Rasse mit sehr ähnlichen Fellmerkmalen, die allerdings zu seborrhöischer Dermatitis durch M. pachydermatis neigt.




