Microbiological and histopathological features of canine acral lick dermatitis
Abstract
The purpose of this study was to investigate microbiological and histopathological features of canine acral lick dermatitis (ALD). Microbial characteristics of ALD are poorly described in current literature. If infection is recognized, antimicrobial selection is usually empirical, based on appearance, cytology or surface culture, rather than deep tissue culture. It was hypothesized that cultures obtained from deep tissue would yield different results than predicted by surface culture and cytology, and that isolates from ALD have unpredictable susceptibility patterns showing resistance to antibiotics routinely administered for canine pyoderma. Biopsies were obtained from 31 lesions and submitted for aerobic, anaerobic and fungal culture, and histopathological evaluation. Surface aerobic culture and susceptibility and cytology were obtained for comparison in 22 dogs. Skin scrapings and dermatophyte culture were performed. Bacteria were isolated in 30 of 31 cases. Staphylococcus intermedius was isolated in 58% of deep cultures. Twenty per cent of deep isolates were methicillin-resistant Staphylococcus species. Forty-eight per cent of cases yielded organisms defined as multidrug resistant on deep culture. Only 57% and 55% of bacteria isolated from tissue culture were sensitive to amoxicillin-clavulanic acid and cefazolin, respectively. Cytology and superficial cultures did not correlate well with deep cultures. Surface culture predicted deep tissue isolates in eight of 22 cases. Microsporum gypseum was isolated from one dog. Histopathological features included acanthosis, follicular elongation, lymphoplasmacytic dermal inflammation, folliculitis, furunculosis, perihidradenitis, hidradenitis and vertical streaking fibrosis. Lesions associated with ALD warrant tissue bacterial cultures as the majority of cases yielded positive growth of bacteria differing from superficial culture and often resistant to empirical drugs.
Sources of Funding
This project was funded by a grant from the American Canine Kennel Club Foundation.
Conflict of Interest
No conflict of interest has been declared.
Introduction
Acral lick dermatitis (ALD), also called acral lick granuloma or acral pruritic nodule,1 is a common and often frustrating disease characterized by compulsive licking of the lower portion of a limb and development of a firm, proliferative, ulcerative and alopecic plaque (Fig. 1).1–4 Although there are reports of ALD-like lesions in various species, including cattle, zoo and exotic species, and humans,5–8 the disease is primarily seen in middle aged or older dogs. Previously reported predisposed breeds include the doberman pinscher, great dane, Labrador retriever, Irish setter, golden retriever and German shepherd.1–3 In the current literature, ALD is most commonly categorized as a primary behavioural dermatosis or a manifestation of obsessive-compulsive disorder.9,10 However, licking and associated lesions decreased or ceased in only 21–63% of dogs treated with behaviour-modifying medications,11–13 suggesting that behavioural disorders are not the only aetiology or contributing factor. Acral lick dermatitis may result from underlying diseases such as hypersensitivity reactions, bacterial or fungal disease, demodicosis, previous trauma, joint disease, neoplasia or neuropathy.1–4 Secondary deep pyoderma can perpetuate clinical signs or disease.
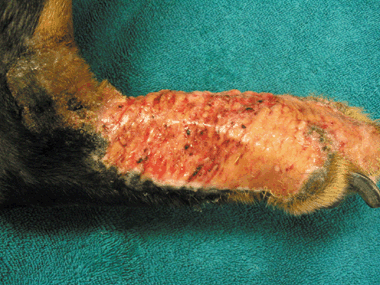
Right distal limb of a doberman demonstrating the ulceration, alopecia, erythema, exudation and proliferation associated with acral lick dermatitis.
Although ALD is one of the top 10 most common skin diseases in dogs,14 minimal data regarding its microbiology and histopathology have been published. There are several articles on ALD and its treatment as a manifestation of obsessive-compulsive disorder,11–13,15–19 and also textbook chapters outlining histopathological features based on pathologists’ experience.1,4 However, to the investigators’ knowledge, there is only one published article on 11 dogs that includes incidental descriptions of culture and histopathological findings.11 However, the main purpose of that study was evaluation of the response of naltrexone as a treatment for ALD, and although staphylococci and streptococci were isolated from 50% of cases, no antibiotic susceptibility profiles or detailed histopathological features were reported.
Acral lick lesions are often associated with deep bacterial furunculosis,1,2 and hence treatment failure may occur if antibiotic therapy is based on empirical selection, isolation of bacteria from the surface of the lesion, or too short a duration of therapy. Moreover, multiple antibacterial therapies combined with inappropriate duration of therapy have been shown to contribute to the selection of drug resistant bacteria.20–23 Multidrug-resistant and methicillin-resistant bacteria are emerging problems in veterinary medicine and result in delayed therapeutic resolution and the potential for zoonotic transmission of methicillin-resistant staphylococci.23–26 Inappropriate use of antibiotics may contribute to multidrug resistance, therefore more basic information on the microbiology of ALD is needed.
The purposes of this prospective study were to determine the species of bacterial and fungal pathogens commonly present in the superficial and deep dermal tissue of canine acral lick lesions, to evaluate the antimicrobial susceptibility patterns of the aerobic bacteria isolated, and to characterize the histopathological features. This should enable testing of the hypotheses that surface culture may not predict the species or antimicrobial susceptibility of bacterial isolates from deep tissue culture and whether deep tissue isolates from dogs with ALD have susceptibility patterns resistant to antibiotics routinely used for canine pyoderma.
Materials and methods
Animals
Thirty-one client-owned dogs with historical and clinical findings consistent with ALD were enrolled. Dogs of any age, sex or breed with a firm, proliferative, eroded to ulcerated and alopecic plaque clinically consistent with ALD on either the distal fore- or hindlimbs that had been present and persistent for at least 3 weeks were included. Dogs were excluded if oral and/or topical antibiotics, antifungals, nonsteroidal anti-inflammatory drugs or antihistamines had been administered within the previous 7 days and also if they had received topical, oral or injectable steroids within the previous 7 days, or 3 and 6 weeks, respectively; these latter dogs were subsequently enrolled following drug withdrawal for a sufficient period to meet inclusion criteria.
Owners were asked to complete a questionnaire to provide information on name, age, sex and breed of the dog, disease duration, previous therapies instituted for the treatment of ALD, and any previous pertinent medical history. Each client signed a form of informed consent acknowledging the procedures performed and risks involved in the study.
Diagnostic protocol
All cases received physical and dermatological examinations and recommendations for further diagnostic evaluation of potential underlying disorders, including atopy, food hypersensitivity or behavioural consultation. A clinical diagnosis of ALD was made based on the history of a self-induced lesion(s) and the presence of one or more firm, proliferative, eroded to ulcerated, alopecic plaque(s) involving any of the distal limbs.
Keratin and hair were collected using the Mackenzie toothbrush technique27 as well as hair plucks. The collected material was inoculated on both dermatophyte test medium (DTM) and Sabouraud's Agar utilizing Dermduet® culture plates (Bacti-Laboratory, Mountain View, CA, USA). The cultures were monitored for fungal growth daily for 21 days. Speciation of mycelial colonies was performed visually at ×10 and ×40 magnification.
Surface cytology for infectious organisms was undertaken using impression smears and scalpel blade technique, whereby a scalpel blade is gently scraped across the lesion and the sample smeared on a microscope slide. Samples were heat-fixed, stained with Diff-Quick® (Baxter Healthcare Co., McGraw Park, IL, USA), and evaluated microscopically at ×10 and ×100 magnification. The presence of bacterial and yeast organisms and white blood cells was recorded.
Skin scrapings for the presence of Demodex spp. in the acral lick lesions were obtained using mineral oil and evaluated at ×10 magnification.
Superficial skin cultures were not performed in the first nine dogs enrolled in the study. The study protocol was amended to include superficial culture for the remaining 22 dogs, from which a swab was obtained from the surface of the acral lick lesion using a BactiSwab containing Amies gel (Remel, Lenexa, KS, USA) and shipped to the Clinical Microbiology Laboratory (CML) at the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania for aerobic bacterial culture.
Samples for tissue culture and histopathology: The dogs were sedated with medetomidine 5–8 µg kg−1 intravenously (Domitor®, 1 mg mL−1) (Pfizer Animal Health, Exton, PA, USA), and reversed with atipamezole HCl 25 mg kg−1 intramuscularly (Antisedan, 5 mg mL−1) (Pfizer Animal Health). Propofol was titrated intravenously to achieve a sedative effect in place of medetomidine in dogs with medical conditions in which medetomidine may be contraindicated or in dogs undergoing general anaesthesia for a separate medical procedure. An injection of lidocaine HCl 2% (Vedco Inc., St. Joseph, MO, USA) was administered subcutaneously to provide local anaesthesia. Using 6-mm surgical biopsy punches (Miltex, York, PA, USA), two skin samples were obtained from each dog, one of which was submitted for histopathology, and the other for aerobic, anaerobic and fungal cultures. The skin samples were taken from a nonulcerated central area of the lesions. The cutaneous site selected for culture was clipped and surgically scrubbed. After removal of the specimen, the epidermis was aseptically dissected from the dermis using a number 10 surgical scalpel to obtain only deep dermal tissue for homogenization and culture. Dermal tissue was transported in BBL Port-A-Cul® tubes (Becton Dickinson, Sparks, MD, USA) to the CML. The tissue was macerated in a sterile Petri dish prior to aerobic, anaerobic and fungal culture. For aerobic culture, a portion of the macerated tissue was inoculated on trypticase soy agar containing 5% sterilized sheep blood, MacConkey agar and CNA (colistin, nalidixic acid) agar and incubated overnight at 35 °C. For anaerobic culture, a portion of the macerated tissue was inoculated on CDC, CDC with phenylethyl alcohol agar and Brucella agar plates and incubated at 35 °C in an anaerobic chamber. For fungal culture, the macerated tissue was inoculated on a Sabouraud dextrose agar slant and incubated at room temperature for 21 days. Identification and antibiotic susceptibility of bacteria isolated by aerobic culture were generated by an automated system (MicroScan Walkaway 40, Dade Behring, West Sacramento, CA, USA). Staphylococci that exhibited resistance to oxacillin were confirmed as methicillin resistant by demonstrating the presence of the penicillin-binding protein, PBP2′, which is encoded by the mecA gene.
In each case, a separate skin sample was obtained and preserved in 10% formalin. These were routinely processed, sectioned and stained with haematoxylin and eosin (H&E) and subjectively evaluated by a board-certified veterinary pathologist (DL) blinded to the gross clinical severity of the lesion and to the presence or absence of infectious organisms cultured. The degree of acanthosis, spongiosis, hypergranulosis, keratosis, follicular elongation, folliculitis/furunculosis, the types of inflammatory cells present, and sebaceous and epitrichial gland changes were all subjectively scored using the scale 0 = absent; 1 = mild; 2 = moderate; and 3 = severe. Additionally, the presence of visible infectious organisms (bacteria, fungal hyphae or arthrospores) was recorded.
Results
Table 1 illustrates the clinical presentation and findings of the 31 dogs. The age of onset was estimated by owners and ranged from 1 to 12 years of age with a median of 4 years. The duration of clinical symptoms ranged from 2 months to 5 years. Although not statistically significant (P = 0.3), a trend for females over males was identified. Twenty-one of the dogs were spayed females (68%), eight were castrated males (26%), and two were intact males (6%). The breeds were four doberman pinschers (13%), two (6%) each of Labrador retriever, golden retriever, Weimaraner, Jack Russell terriers, one (3%) each of German shepherd, Chihuahua, Hungarian greyhound, Irish setter, Australian cattle dog, Queensland heeler, pit bull, German short-haired pointer, great dane, and vizsla, and nine (29%) mixed breeds. Lesion location(s) included: 13 (42%) solely involving the left carpal/metacarpal region, six (19%) solely involving the right carpal/metacarpal region, three (10%) involving the right tarsal/metatarsal region, one (3%) involving the left tarsal region, two (7%) involving both the right and the left carpal/metacarpal regions, one (3%) involving the left and right carpal/metacarpal and left tarsal regions, two (7%) involving the right carpal/metacarpal and right tarsal/metatarsal regions, and three (10%) involving all four limbs. Seventy-seven per cent of the dogs had been treated with one or more oral antibiotics for ALD as reported by the owners.
| Canine | Sex | Age of onset | Duration | Prior oral antibiotic therapy | Prior topical antibiotic therapy | Cytology | Superficial bacteria cultured | Deep bacteria cultured | Dermatophyte cultured |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MN | 5 years | 1 year | Cephalexin | None | Cocci, rods, neutrophils | Not performed | Enterobacter cloacae † S. intermedius | – |
| 2 | MN | 3.5 years | 6 months | Amoxicillin | Silver sulfadiazine, bacitracin, neomycin, polymixin B ointment | Cocci, neutrophils, RBC | Not performed | S. intermedius * | – |
| 3 | FS | 3 years | 4 years | Cephalexin, cefpodoxime | Mupirocin | Cocci, neutrophils | Not performed | Enterobacter sp. † | – |
| 4 | FS | 2 years | 5 years | Cephalexin | Silver sulfadiazine, triple antibiotic ointment | – | Not performed | S. xylosus * | – |
| 5 | FS | 3 years | 3 years | None | None | Cocci | Not performed | MRSI* | – |
| 6 | MN | 1 year | 2 years | Cefpodoxime | None | Cocci | Not performed | S. intermedius | – |
| 7 | FS | 6 years | 2 years | Cephalexin, cefpodoxime | None | Cocci | Not performed | MRSI* | – |
| 8 | FS | 4 years | 2 years | Cephalexin, enrofloxacin | Bacitracin, neomycin, polymixin B ointment | Cocci, neutrophils | Not performed | S. intermedius | – |
| 9 | FS | 2 years | 3 years | Cephalexin | None | Cocci, neutrophils, RBC | Not performed | S. intermedius Clostridium | – |
| 10 | FS | 5 years | 2 months | None | None | Cocci, neutrophils | S. intermedius Pseudomonas aeruginosa | S. intermedius | – |
| 11 | FS | 7 years | 5 years | None | None | Cocci, neutrophils | No growth | S. intermedius | – |
| 12 | MI | 7.5 years | 8+ months | Cephalexin | Bacitracin, neomycin, polymixin B ointment | Cocci, neutophils, eosinophils | MRSI* | MRSI* | – |
| 13 | FS | 12 years | 8+ months | None | Bacitracin, Neomycin, Polymixin B ointment | Cocci, neutrophils | S. intermedius | S. intermedius | Microsporum gypseum |
| 14 | MN | 10.5 years | 6 months | None | Various topicals | Cocci | Micrococcus sp. | S. intermedius | – |
| 15 | FS | 9 years | 1 year | Cephalexin, amoxicillin/ clavulanic acid | None | Cocci | S. intermedius * | Enterobacter cloacae † S. intermedius | – |
| 16 | MN | 6 years | 2 years | Amoxicillin | Neomycin sulfate, gentamicin | – | No growth | No growth | – |
| 17 | FS | 3 years | 6 months | None | Gentamicin sulfate | Cocci | S. intermedius | Pseudomonas aeruginosa | – |
| 18 | FS | 3.5 years | 3 years | Amoxicillin/clavulanic acid, enrofloxacin, cefpodoxime | None | – | No growth | S. intermedius | – |
| 19 | FS | 4.5 years | 3 years | Cephalexin, cefpodoxime, amoxicillin/clavulanic acid | Gentamicin sulfate | Cocci | MRSA* | MRSA* | – |
| 20 | MI | 1 year | 1 year | Cefadroxil, amoxicillin/ clavulanic acid, clindamycin | Bacitracin, neomycin, polymixin B ointment | Cocci, neutrophils | S. intermedius | S. intermedius | – |
| 21 | MN | 3 years | 1 year | Cephalexin | None | Cocci | MRSS* P. aeruginosa | MRSS* P. aeruginosa | – |
| 22 | FS | 4 years | 2 years | None | Bacitracin, neomycin, polymixin B ointment | Cocci | S. aureus | S. aureus * | – |
| 23 | FS | 1 year | 2 years | None | None | – | S. intermedius | S. intermedius | – |
| 24 | FS | 8 years | 1 year | Cephalexin | None | Cocci | MRSS* | MRSS* | – |
| 25 | FS | 2.5 years | 6 months | Cephalexin | Bacitracin, neomycin, polymixin B ointment | – | S. intermedius | S. intermedius * | – |
| 26 | FS | 6 years | 5 years | Amoxicillin, cephalexin, amoxicillin/clavulanic acid | Gentamicin sulfate, panalog | Cocci, neutrophils | S. intermedius | S. intermedius | – |
| 27 | FS | 4 years | 4 years | Amoxicillin | Various topicals | Cocci | S. intermedius | No growth | – |
| 28 | FS | 9 years | 2 months | Yes – unknown drug | Bacitracin, neomycin, polymixin B ointment | Cocci, rods, neutrophils, macrophages | S. intermedius | S. intermedius | – |
| 29 | FS | 4 years | 1 year | Amoxicillin/clavulanic acid | Gentamicin | Cocci, neutrophils, RBC | MRS sciuri*P. fluorescens | MRSI* Acinetobacter baumani | – |
| 30 | MI | 6 years | 4 years | Cephalexin, cefpodoxime enrofloxacin, orbifloxacin | Bacitracin, neomycin, polymixin B ointment | Cocci, neutrophils, RBC | Proteus mirabilis | P. aeruginosa MRSI* MRS sciuri* | – |
| 31 | FS | 5 years | 2 years | Cephalexin | Gentamicin, neomycin sulfate | Cocci | S. intermedius | S. intermedius | – |
- * Organisms resistant to ≥ 3 drug classes used to treat organism.
- † Highly resistant microorganism according to Dutch guidelines29.
- FS, female, spayed; MRSA, methicillin-resistant Staphylococcus aureus; MRSI, methicillin-resistant S. intermedius; MRSS, methicillin-resistant staph schleiferi; MN, male, neutered; RBC, red blood cell.
No Demodex organisms were identified on any of the 31 dogs upon microscopic evaluation (×10) of skin scrapings from the affected regions. Microsporum gypseum was cultured from a single affected dog. No growth was present on any of the deep tissue fungal cultures performed on the submitted tissue, including the case with identified M. gypseum.
Cytological evaluation
Cytological evaluation revealed coccoid and rod bacteria in 26 of 31 (84%) and two of 31 (6%) cases, respectively.
Superficial culture
Surface bacterial cultures were not performed in the first nine dogs enrolled; the initial study protocol focused only on description of the deep bacterial cultures. The study protocol was amended to include surface culture for comparison with deep culture results for the remaining 22 dogs. Aerobic bacterial cultures of the superficial skin resulted in positive growth from 19 of 22 dogs (86%). The resultant isolates for these cases are presented in Table 1. Staphylococcus intermedius, including methicillin-resistant strains (11 of 22 cases), and Pseudomonas spp. (three of 22 cases) were the predominate isolates cultured (Table 2, column 1). Methicillin-resistant staphylococcal organisms were identified in five of 22 dogs (23%).
Deep tissue culture
Aerobic and anaerobic culture of deep tissue resulted in bacterial growth from 29 of 31 cultures (94%), with resultant isolates for each case presented in Table 1. Staphylococcus intermedius (including methicillin-resistant strains), Pseudomonas spp. and Enterobacter spp. were isolated from 21 of 36 (58%), three of 36 (8%) and three of 36 (8%) cases, respectively (Table 2, column 2). Methicillin-resistant staphylococcal organisms were identified in eight of 31 dogs (26%) and represented 25% (nine of 36) of all deep tissue isolates. The susceptibility results of deep tissue isolates are summarized in Table 3. In addition to the methicillin-resistant staphylococcal isolates, an additional four individual methicillin-sensitive staphylococcal isolates, obtained from deep cultures, were resistant to three or more drug classes28 that are often used to treat staphylococcal infections (amoxicillin-clavulanic acid, cefazolin, trimethoprim sulfamethoxazole, clindamycin, erythromycin, fluoroquinolones). Three Enterobacter species isolated were highly resistant microorganisms (HRMO) according to guidelines29 in use at the CML. Thus, 48% of the dogs yielded one or more deep tissue isolates resistant to multiple antibiotic drug classes; 52% of all deep tissue isolates were resistant to multiple antibiotic drug classes. Ninety-one percent of the deep bacterial isolates demonstrated in vitro sensitivity to enrofloxacin, whereas only 57% and 54% were sensitive to amoxicillin-clavulanic acid and cefazolin, respectively (Table 3, column 1).
| Antibiotic | Total deep isolates susceptible* | Staphylococcal isolates susceptible* |
|---|---|---|
| Enrofloxacin | 32 (91.4%) | 26 (92.3%) |
| Trimethoprim sulfa | 31 (88.6%) | 27 (96.4%) |
| Marbofloxacin | 30 (85.7%) | 25 (89.3%) |
| Ciprofloxacin | 30 (85.7%) | 25 (89.3%) |
| Gentamicin | 29 (82.9%) | 22 (78.6%) |
| Vancomycin | 28 (80%) | 28 (100%) |
| Rifampin | 27 (77.1%) | 26 (92.3%) |
| Imipenem | 24 (68.6%) | 19 (67.9%) |
| Chloramphenicol | 23 (65.7%) | 22 (78.6%) |
| Clindamycin | 21 (60.0%) | 21 (75%) |
| Tetracycline | 21 (60%) | 20 (71.4%) |
| Amoxicilliln/clavulanic acid | 20 (57.1%) | 19 (67.9%) |
| Erythromycin | 20 (57.1%) | 28 (71.4%) |
| Cefazolin | 19 (54.3%) | 19 (67.9%) |
| Oxacillin | 19 (54.3%) | 19 (67.9%) |
| Ampicillin | 3 (8.6%) | 3 (10.7%) |
| Penicillin | 3 (8.6%) | 3 (10.7%) |
- * 36 total deep isolates and 28 Staphylococcus sp. isolates.
Cytological morphology and culture comparison
Superficial culture was not available for eight of the cases in which coccoid bacteria were noted on cytology. The morphology of the bacteria identified on surface cytology correlated with that of bacteria isolated from 14 of 22 (64%) of the superficial cultures and 17 of 31 (58%) of the deep cultures. In two of 22 cases (9%) with no bacterial growth on surface culture, coccoid bacteria were present on cytology. No bacteria were observed on cytology in two cases (9%) from which S. intermedius was cultured both superficially and deep. In four of 22 (18%) of the cases in which superficial culture was performed, bacterial rods were not detected by cytology, despite growth of Pseudomonas spp. (3) and Proteus mirabilis (1). Rods were noted on cytology in one case (5%) in which no rod-shaped bacteria were cultured either superficially or deep. No Malassezia organisms were noted on cytological evaluation.
Culture comparison
Combining the superficial and deep tissue results, bacterial growth was observed in 30 of 31 (97%) dogs. Twenty-two bacterial isolates were obtained from the 19 cases with positive growth on superficial cutaneous culture and 36 species were isolated from the 29 cases with positive growth on deep tissue culture, yielding a total of 58 positive bacterial isolates (Table 2). These were mainly staphylococcal species, especially S. intermedius, Enterobacter spp., and Pseudomonas spp.
For comparison of results from superficial and deep culture, isolates were considered different if the species isolated or the antibiotic susceptibility profile of isolates of the same species differed. Genotypic characterization was not performed on bacterial isolates to confirm that the same species isolated were separate strains. In eight of 22 cases (36%), the superficial and deep culture results agreed in both species and susceptibility patterns. In the remaining 14 cases, six (27%) exhibited different bacterial species, five (23%) had the same but with different susceptibility patterns, two (9%) were negative on superficial culture but positive on deep tissue culture, and one (5%) was negative on deep tissue culture but positive on superficial culture. Thus, only 36% of cases were in complete agreement with respect to species isolated and antibacterial susceptibility patterns from the deep and superficial cultures (Table 4).
| Number of cases, n= 22 | |
|---|---|
| Complete agreement | 8 (36%) |
| Differing isolates | 6 (27%) |
| Differing susceptibility patterns | 5 (23%) |
| Negative only on superficial culture | 2 (9%) |
| Negative only on deep culture | 1 (5%) |
Histopathology
The histopathological findings of the skin specimens from each of the 31 dogs are summarized in Table 5. The epidermis was completely absent in two cases, and irregular acanthosis was noted in the remaining 29. The epidermis was papillated in 48% of cases with epidermis present. Hyperkeratosis consisted of orthokeratotic hyperkeratosis (75%) or parakeratotic hyperkeratosis (65%) or a combination of both. Follicular changes were noted in the majority of cases and included infundibular hyperkeratosis (87%), hyperplasia (68%), follicular elongation (65%), furunculosis (32%), folliculitis (29%) and follicular dilatation (26%). The most prominent features affecting the adnexa included epitrichial gland epithelial cell hypertrophy (81%), epitrichial gland dilatation (71%) and sebaceous gland hyperplasia (45%). Epitrichial gland secretions were retained or inspissated in 81% of cases. Hidradenitis (Fig. 2) and epitrichial gland rupture (Fig. 3) were noted in 29% and 10%, respectively. In three cases, the epidermal hyperplasia and dermal fibrosis were so profound that the punch biopsy did not yield a sufficient depth of dermal tissue to enable full evaluation of the epitrichial glands. Dermal fibrosis was present both superficially (81%) and deep (45%), with vertical streaking (81%) often present in the superficial fibrosis. The dermal infiltrate was primarily lymphoplasmacytic (94% superficially, 74% deep) with the exception of multifocal granulomatous to pyogranulomatous infiltrate surrounding ruptured hair follicles. Additionally, the infiltrate surrounding the epitrichial glands varied from lymphoplasmacytic to primarily plasmacytic. Perihidradenitis was present in 90% of cases (Fig. 4). Bacteria were noted in the surface keratin, crust and within the follicular lumen in only five cases (16%). In the specimens in which bacteria were noted histopathologically, one had no growth on superficial culture, although S. intermedius was cultured from the deep tissue sample, one grew S. intermedius from both superficial and deep cultures, two of which were positive for methicillin-resistant S. intermedius on deep tissue culture (superficial not performed), and one was positive for methicillin-resistant S. schleiferi on both superficial and deep cultures. Gomori's methenamine silver stain was negative on the single case of M. gypseum.
| Mild | Moderate | Marked | Total | ||
|---|---|---|---|---|---|
| Epidermis | |||||
| Complete ulceration | 2 (6.5%) | 6.50% | |||
| Regular acanthosis | 2 (6.5%) | 6.50% | |||
| Irregular acanthosis | 11 (35.5%) | 12 (38.7%) | 4 (12.9%) | 87.10% | |
| Orthokeratotic | 10 (32.3%) | 14(45.2%) | 77.50% | ||
| Hyperkeratosis | |||||
| Parakeratotic | 8 (25.8%) | 12 (38.7%) | 64.50% | ||
| Hyperkeratosis | |||||
| Crust | 38.70% | ||||
| Hypergranulosis | 6 (22.6%) | 22.60% | |||
| Spongiosis | 11 (35.5%) | 1 (3.2%) | 38.70% | ||
| Papillosis | 9 (29.0%) | 5 (16.1%) | 45.10% | ||
| Follicles | |||||
| Infundibular hyperkeratosis | 18 (58.1%) | 9 (29.0%) | 87.10% | ||
| Follicular hyperplasia | 16 (51.6%) | 5 (16.1%) | 67.70% | ||
| Follicular elongation | 14 (45.2%) | 5 (16.1%) | 1 (3.2%) | 64.50% | |
| Follicular dilatation | 5 (16.1%) | 3 (9.7%) | 25.80% | ||
| Folliculitis | 6 (22.6%) | 1 (3.2%) | 1 (3.2%) | 29.00% | |
| Furunculosis | 5 (16.1%) | 3 (9.7%) | 2 (6.5%) | 32.30% | |
| Adnexa | |||||
| Sebacous gland hyperplasia | 12 (38.7%) | 1 (3.2%) | 1 (3.2%) | 45.10% | |
| Epitrichial gland hypertrophy | 13 (41.9%) | 12 (38.7%) | 80.60% | ||
| Epitrichial gland dilatation | 11 (35.5%) | 8 (25.8%) | 3 (9.7%) | 71.00% | |
| Epitrichial gland retained/inspissated secretions | 18 (58.1%) | 6 (19.4%) | 1 (3.2%) | 80.70% | |
| Epitrichial gland rupture | 2 (6.5%) | 1 (3.2%) | 9.70% | ||
| Hidradenitis | 7 (22.6%) | 2 (6.5%) | 29.10% | ||
| Dermis | |||||
| Superficial fibrosis | 11 (35.5%) | 14 (45.2%) | 80.70% | ||
| Vertical streaking | 19 (61.3%) | 6 (19.4%) | 80.70% | ||
| Deep fibrosis | 9 (29.0%) | 4 (12.9%) | 1 (3.2%) | 45.10% | |
| Dermal Infiltrate | |||||
| Superficial LPC perivascular | 19 (61.3%) | 6 (22.6%) | 2 (6.5%) | 90.40% | |
| Superficial neutrophilic perivascular | 1 (3.2%) | 3.20% | |||
| Middermal LPC | 3 (9.7%) | 2 (6.5%) | 1 (3.2%) | 19.40% | |
| Middermal pyogranulomatous | 1 (3.2%) | 1 (3.2%) | 6.40% | ||
| Deep LPC | 10 (32.3%) | 7 (22.6%) | 6 (19.4%) | 74.30% | |
| Perihidradenitis | 10 (32.3%) | 15 (48.4%) | 3 (9.7%) | 90.40% | |
| Perifolliculitis | 11 (35.5%) | 3 (9.7%) | 2 (6.5%) | 51.70% | |
- LPC, lymphoplasmacytic.
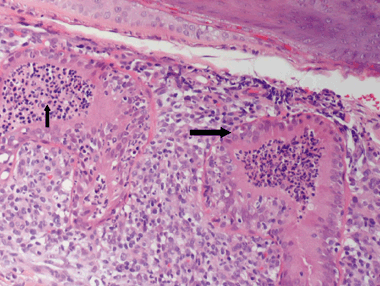
Photomicrograph depicting hidradenitis. Note the hypertrophy of the secretory epithelium of the epitrichial gland (large arrow) and the luminal infiltrate (small arrow). Haematoxylin and eosin, ×20.
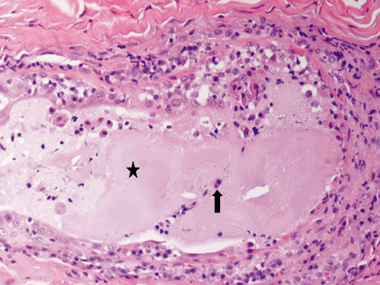
Photomicrograph depicting epitrichial gland rupture. Note the large pool of epitrichial glandular secretions (star) admixed with infiltrating inflammatory cells (arrow). Haematoxylin and eosin, ×4.

Photomicrographs depicting perihidradenitis: (a) Epitrichial glands are mildly dilated; several contain inspissated secretions (large arrow). Note the moderate leucocytic infiltrate surrounding the glands (small arrow). Haematoxylin and eosin (H&E), ×4 (b) higher magnification of Figure 4 (a) demonstrating the predominantly plasmacytic periglandular infiltrate (arrow). H&E, ×20.
Discussion
To the authors’ knowledge, this is the first study providing a detailed description of the microbiology and histopathology of canine ALD.
The trend for a predilection for females affected with ALD contradicted previous reports where either a male or no sex predilection has been reported.2,30 However, this was not significant. The median age of onset, 4 years, was younger than a previous report of 5 years of age or older.2 The left carpal region was involved in the majority of dogs, a trend consistent with previous reports.11,31
The presence of Micrococcus spp., Proteus mirabilis, and two Pseudomonas spp. on superficial and not on deep tissue culture may be attributed to contamination as these are common oral bacterial species. Surface culture may therefore not be indicative of deep bacterial infection.
The results support those of a previous study,11 where S. intermedius was the predominant species isolated on deep tissue culture, and Pseudomonas spp., Proteus spp., and Clostridium spp. were found, but differed in failing to detect Streptococcus spp., Escherichia coli, Actinobacillus spp. and S. epidermidis. Enterobacter spp. and various methicillin-sensitive and -resistant staphylococcal species were, however, isolated in this study but not in the previous report. The resistance patterns of the various deep tissue isolates identified cannot be compared as these were not reported in the previous study.
Antimicrobial resistance, and more specifically methicillin-resistance, has become an increasing concern in veterinary medicine, as resistance can hinder successful treatment and methicillin-resistant organisms can pose a risk of zoonosis.23–26 In this study, bacteria resistant to multiple classes of antimicrobials were isolated from the deep tissue of 15 of 31 (48%) dogs; 26% of them yielded a methicillin-resistant staphylococcal species. The majority of the dogs had been treated with one or more oral antibiotics for ALD, and antibiotic use is known to select for multidrug resistant and methicillin-resistant organisms.20,22,32 Interestingly, however, three of the dogs with no antibiotic treatment prior to culture displayed organisms resistant to three or more of the drug classes often used to treat staphylococcal infections (one S. intermedius, one S. aureus, and one methicillin-resistant S. intermedius).
Empirical therapy is frequently implemented in the management of canine pyoderma because the susceptibility pattern of S. intermedius, the most common isolate associated with this condition has been described as ‘stable’.2,33 The susceptibility profiles of deep tissue isolates indicate a relatively high level of resistance to cefazolin and amoxicillin-clavulanic acid, but, by comparison, a high level of susceptibility to the fluoroquinolones and trimethoprim sulfamethizole. Thus, if empirical therapy was being implemented in the treatment of ALD, in this study population, a fluoroquinolone or potentiated sulphonamide would more likely result in therapeutic success than cefalexin or amoxicillin-clavulanic acid. However, fluoroquinolone exposure has been shown to be a risk factor for the development of methicillin-resistant S. aureus (MRSA),34 and indiscriminate use may create a selective advantage for methicillin resistance.35
The cytological morphology of the bacteria exhibited a poor correlation with the superficial and deep tissue culture results, completely agreeing with the superficial isolates in only 14 of 22 cases (64%). Less agreement was noted with results of deep tissue culture; only a 55% (17 of 31 cases) correlation. Additionally, isolates obtained from superficial culture were in poor agreement from those obtained by deep tissue culture. Only eight of 22 cases (36%) had superficial isolate(s) in complete agreement in respect to both species isolated and susceptibility profiles with isolates cultured from deep tissue. Thus, surface cytology and superficial culture often did not reflect the deep bacterial infection present, indicating that superficial examination by cytology and culture is not a good indicator of deep bacterial infection.
The histopathological features previously described for ALD include moderate to marked, often papillated, acanthosis with occasional neutrophilic serocellular crusts, erosion or ulceration with exudation; orthokeratotic to parakeratotic hyperkeratosis; superficial dermal fibrosis, often in a vertical streaking pattern; thickened and elongated follicles; a perivascular, perifollicular or diffuse dermal infiltrate of lymphocytes, neutrophils, macrophages and plasma cells; often plasmacytic perihidradenitis; folliculitis and furunculosis.2,4 The results confirm these features as common findings in the histopathology of ALD; however, other features not previously described were identified. Specifically, numerous changes involving the epitrichial glands including dilatation, hypertrophy, retained and inspissated secretions, perihidradenitis and hidradenitis, and occasionally glandular rupture. Although perihidradenitis has been reported as a histopathological finding in ALD, in this study, all cases in which epitrichial glands were observed exhibited some degree of perihidradenitis. Additionally, to the authors’ knowledge, there has been no previous report of hidradenitis or epitrichial gland rupture. Excluding the three cases where the skin specimens submitted were insufficiently deep to evaluate the epitrichial glands histopathologically, 32% and 11% of cases exhibited hidradenitis and epitrichial gland rupture, respectively. A limitation of this study was the subjective analysis of the degree of severity of the histopathological features and the descriptive finding demonstrating greater than expected involvement of the epitrichial glands warrants future study, which should include standardized morphometric analysis of features of interest. Why the epitrichial glands appear to be a focus of inflammation in ALD is unknown. One possible explanation is that deep fibrosis contributes to ductal obstruction, resulting in dilatation, hidradrenitis, and eventual rupture, although this does not explain the presence of perihidradenitis and hidradenitis in all the cases, as deep fibrosis was only detected in 45% of them. Hidradenitis and perihidradenitis may contribute to the pruritus associated with ALD, perpetuating the itch cycle. Hidradenitis has been reported to be consistently associated with bacterial folliculitis, furunculosis or a combination of both, even if follicular inflammation was absent histologically.2,36 In this study, only one of the nine cases in which hidradenitis was noted had histopathological features of folliculitis or furunculosis. However, all were culture positive on deep tissue culture. This finding supports a previous demonstration36 of the presence of hidradenitis associated with bacterial infection, even if in the absence of follicular inflammation.
One possible explanation for the negative deep tissue fungal culture in case 13, despite a positive dermatophyte culture, is that an isolated skin sample may miss infected areas. Additionally, although spores of several species of dermatophyte can penetrate the follicle to the level of the Adamson's fringe,2,37 it is possible that the dermatophyte infection in this case involved only superficial structures such as stratum corneum or superficial follicles, which would have been excised along with the epidermis while obtaining the deep tissue cultures. During the follow up of case 13, the ALD lesion did not resolve until antifungal therapy was instituted despite 2 weeks of appropriate antibiotic therapy based on antibiotic susceptibility. This individual case emphasizes the importance of performing a dermatophyte culture to rule out a dermatophytic infection as a primary or concurrent aetiology for ALD.
In conclusion, deep bacterial infection was present in 29 of 31 (94%) of clinical cases of ALD. Many organisms, including Staphylococcal spp. and Enterobacter spp., cultured from the acral lick lesions, were resistant to multiple drug classes or were methicillin-resistant staphylococcal species. Bacterial culture of ALD lesions is warranted, especially if empirical therapy is unsuccessful. Additionally, there is poor correlation between the organisms cultured from the surface of lesions and those from deep tissue. Organisms isolated on surface culture may be oral contaminants or superficial colonies unrepresentative of deep bacterial infection. Culture of the deep tissue is recommended for selection of long-term antibiotic therapy. Although dermatophytosis was uncommon, dermatophyte culture should also be performed to eliminate the possibility of fungal skin infection. Histopathology is beneficial in eliminating other underlying aetiologies that can mimic the clinical presentation of ALD such as neoplasia, foreign body reactions, fungal kerion reactions and other primary infections, including leishmaniasis.38 Although not specifically addressed in the course of this study, identification and treatment of the underlying cause of ALD (allergic, behavioural, neuropathic, orthopaedic, etc.), in combination with appropriate antibiotic therapy based on deep tissue culture, are necessary to effectively treat this complicated, multifactorial disorder.
Acknowledgements
Many thanks to the Apothecary Shop for shipping the cultures and to the microbiology laboratory staff at the Matthew J. Ryan Veterinary Hospital of the University of Pennsylvania. Photomicrographs were provided by Sharon M. Dial, of the University of Arizona. Statistical analysis was provided by Dan Gingerich, of Stolle Milk Biologics, Inc.
References
Résumé Le but de cette étude était d’étudier les données microbiologiques et histopathologiques de la dermatite de léchage chez le chien (ALD). Les données microbiologiques sont peu rapportées dans la littérature récente. Si une infection est notée, le traitement antibiotique est souvent empirique, en se basant sur l’aspect clinique, la cytologie ou la culture de surface plutôt qu’en profondeur. Il a été suspecté que les cultures obtenues à partir de prélèvements profonds pourraient présenter des résultats différents que ceux des prélèvements de surface et que les isolements d’ALD auraient des antibiogrammes peu prédictibles avec des résistances aux principaux antibiotiques utilisés en routine pour les pyodermites du chien. Des biopsies ont été obtenues de 31 lésions et soumises à culture aérobie, anaérobie, culture fongique et examen histopathologique. Des cultures en aérobiose de prélèvements de surface avec antibiogramme, et des cytologies ont été obtenues pour comparaison chez 22 chiens. Des raclages et des cultures fongiques ont été réalisées. Des bactéries ont été isolées chez 30/31 chiens. Staphylococcus intermedius a été isolé pour 58% des cultures profondes, 25% des isolements étant methicillin-resistant. 48% des cas ont montré des organismes définis comme polyrésistants en culture profonde. Seulement 57% et 55% des bactéries isolées étant sensibles à respectivement l’ amoxicilline-acide clavulanique et la cefazoline. Ni la cytologie ni la culture de surface ne corrélaient avec les cultures profondes. La culture de surface corrélait avec la culture profonde pour seulement 8/22 cas. Microsporum gypseum a été isolé dans un cas. Les examens histopathologiques ont montré une acanthose, une élongation des follicules pileux, une inflammation lymphoplasmocytaire dermique, une folliculite, une furonculose, une périhidradénite, une hidradénite et une fibrose verticale. Les lésions d’ALD nécessitent une culture bactériologique car la majorité des cas présente une croissance bactérienne différente de celle observée en surface et souvent résistante aux antibiotiques usuels.
Resumen El objetivo de este estudio fue investigar las características microbiológicas e histopatológicas de las dermatitis acral por lamido canina (ALD). Las características microbiológicas de la ALD han sido poco descritas en la literatura científica. Si se descubre una infección generalmente de trata de forma empírica, basándose en la apariencia, citología o cultivo superficial, en lugar de cultivo profundo. Formulamos una hipótesis que implicaba que los cultivos obtenidos de tejido profundo darían resultados diferentes que los indicados por el cultivo superficial y la citología, y que los aislados de ALD tienen susceptibilidad impredecible mostrando resistencia a antibióticos administrados de forma rutinaria para pioderma canina. Se obtuvieron biopsias de 31 lesiones y se remitieron para cultivo aeróbico, anaeróbico y fúngico así como para examen histopatológico. Se obtuvo cultivo aeróbico superficial con susceptibilidad y citología para comparar de 22 de los perros. Se realizaron raspados de piel y cultivo de dermatofitos. Se aislaron bacterias en 30/31 casos. Staphylococcus intermedius se aisló en un 58% de los cultivos profundos, 25% de los aislados eran especies de estafilococos resistentes a meticilina, 48% de los casos mostraron organismos definidos como resistentes a multiples antibióticos en cultivos profundos. Sólo un 57% y un 55% de las bacterias aisladas del cultivo de tejido fueron sensibles a amoxicilina-ácido clavulánico y a cefalozina, respectivamente. La citología y el cultivo superficial no se correlacionaron bien con los cultivos profundos. El cultivo superficial predijo los aislados profundos en 8/22 casos. Se aislóMicrosporum gypseum en un perro. Las características histopatológicas incluyeron acantosis, elongación de los folículos, inflamación dermal linfoplasmacítica, foliculitis, furunculosis, perihidradenitis, hidradenitis, y fibrosis con orientación vertical. Las lesiones asociadas con ALD necesitan cultivo bacteriano ya que la mayoria de los casos presentaron crecimiento bacteriano diferente del cultivo superficial y a menudo con resistencia a tratamientos empíricos.
Zusammenfassung Das Ziel dieser Studie war es die mikrobiologischen und histopathologischen Charakteristika der caninen akralen Leckdermatitis (ALD) zu untersuchen. Die mikrobiologischen Merkmale der ALD sind in der aktuellen Literatur schlecht beschrieben. Wenn eine Infektion vorliegt, so wird die antimikrobielle Selektion meist empirisch durchgeführt, eher basierend auf dem äußeren Erscheinungsbild, der Zytologie oder einer oberflächlichen Kultur als auf einer Gewebekultur der tieferen Schichten. Es wurde die Hypothese aufgestellt, dass Kulturen, die von tiefen Geweben angelegt werden, unterschiedliche Ergebnisse erbringen als oberflächliche Kulturen und Zytologie und dass Isolate von ALD ein unvorhersagbares Empfindlichkeitsmuster sowie eine Resistenz gegen routinemäßig für canine Pyodermie verabreichte Antibiotika aufweisen. Es wurden Hautbiopsien von 31 Veränderungen genommen und für aerobe und anaerobe Kultur und Pilzkultur, sowie für eine histopathologische Evaluierung eingeschickt. Im Vergleich dazu wurden oberflächliche aerobe Kulturen und Antibiogramme sowie eine zytologische Untersuchung bei 22 Hunden durchgeführt. Hautgeschabsel und Pilzkulturen wurden gemacht. Es wurden bei 30/31 Fällen Bakterien isoliert. Staphylococcus intermedius wurde bei 58% der tiefen Kulturen isoliert. Bei 25% der tiefen Isolate handelte es sich um eine Methicillin-resistente Staphylococcus Spezies. Bei 48% der Fälle wurden Organismen gefunden, die nach tiefer Gewebekultur als multi-drug resistent eingestuft wurden. Nur 57% bzw. 55% der Bakterien, die aus den tiefen Geweben isoliert wurden, waren empfänglich für Amoxicillin/Clavulansäure bzw. Cefazolin. Die Zytologie und die Oberflächenkulturen korrelierten schlecht mit den tiefen Gewebekulturen. Bei 8/22 Fällen wurden durch die Oberflächenkultur die Isolate aus den tiefen Geweben prognostiziert. Bei einem Hund wurde Microsporum gypseum isoliert. Die histopathologischen Charakteristika umfassten Acanthose, follikuläre Elongation, lymphoplasmazelluläre dermale Entzündung, Follikulitis, Furunkulose, Perihydradenitis, Hydradenitis und eine vertikale streifenartige Fibrose. Bei Läsionen, die bei ALD vorkommen, sind bakterielle Gewebekulturen gerechtfertigt, da die Mehrzahl der Fälle ein positives Bakterienwachstum ergaben, welches sich von den oberflächlichen Kulturen unterschied und häufig auf empirisch verabreichte Medikamente resistent war.





